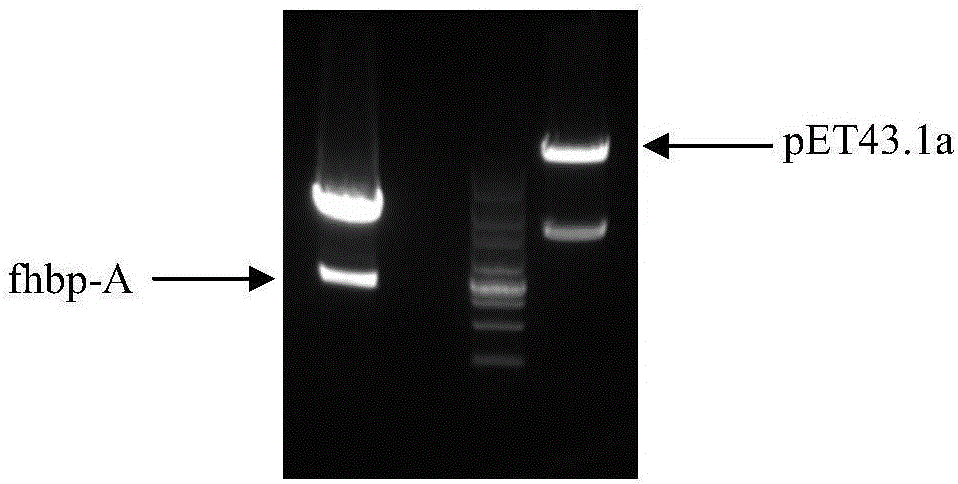
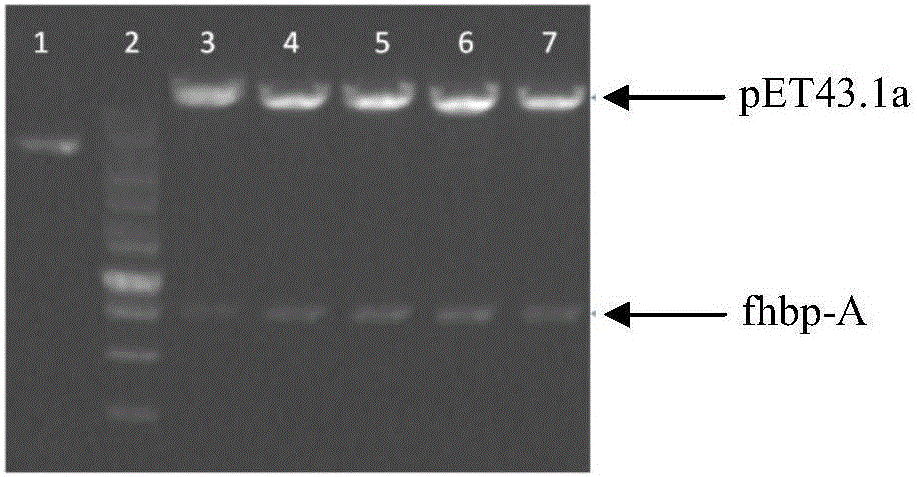
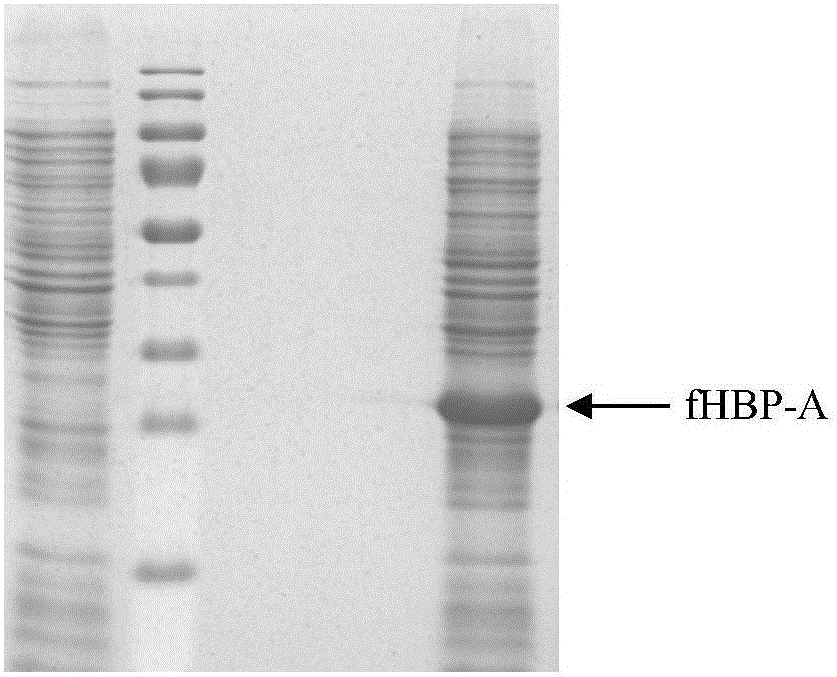
Group ABC meningococcus combined vaccine and preparing method thereof
A meningococcal and combined vaccine technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, vaccines, etc., can solve the problem of no bactericidal power, and achieve the effect of reducing the number of vaccinations and reducing the pain of vaccination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0066] The preparation of embodiment 1A group, C group polysaccharide:
[0067] After fermentation of group A and group C meningococci, the supernatant was collected by centrifugation in a disc centrifuge, and cetyltrimethylammonium bromide with a final concentration of 0.15% was added to precipitate the polysaccharide, and the polysaccharide precipitate was collected by centrifugation, and the final concentration was The precipitate was dissolved with 0.5 mol / L calcium chloride, and the nucleic acid was removed by ethanol precipitation with a final concentration of 25%, the supernatant was collected by centrifugation, and the polysaccharide was precipitated with cold ethanol with a final concentration of 75%. After dissolving the polysaccharide and treating it with 1-3% sodium deoxycholate, use GE Capto adhere and CaptoDEAE two kinds of gel medium chromatography in series to collect the flow-through peak, and filter the flow-through peak with a 30KD membrane bag ultrafiltratio...
Embodiment 2
[0068] The preparation of embodiment 2 carrier protein TT:
[0069]The fermentation culture was centrifuged in a disc centrifuge to collect the supernatant, after detoxification by 0.5% formaldehyde, 30KD ultrafiltration was used to remove impurities, 30% ammonium sulfate was used to precipitate TT, and Sephacryl S-300HR gel filtration chromatography was used to collect TT protein The monomer peak was concentrated by 30KD ultrafiltration to prepare TT stock solution. The detoxification process needs to be checked for detoxification, and the original solution needs to be checked for specific toxicity and toxicity reversal.
Embodiment 3
[0070] Preparation of embodiment 3 polysaccharide-protein conjugate (taking cyanogen bromide activation method as example):
[0071] 3.1 Polysaccharide activation:
[0072] Dissolve the polysaccharide to 5-15 mg / ml, adjust the pH value to about 10.8, add 1 / 10 (g / g) cyanogen bromide to the polysaccharide solution, and activate the polysaccharide for 8 minutes at room temperature in an alkaline environment with a pH value of 10.8.
[0073] 3.2 Polysaccharide derivation:
[0074] Add 0.5mol / L adipic dihydrazide at a ratio of 1:1 (volume ratio to activated polysaccharide), and react at room temperature for 50-70min. Cyanogen bromide and adipic dihydrazide were removed by ultrafiltration with a 30KD membrane bag to obtain polysaccharide derivatives.
[0075] 3.3 Polysaccharide protein binding:
[0076] Mix and stir the polysaccharide derivative and the carrier protein TT at a ratio of 1:1 (g / g), add carbodiimide at a ratio of carbodiimide: polysaccharide = 1:10, room temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com