ACY W135 group meningococcal polysaccharide type-b haemophilus influenzae conjugate vaccine
A technology of Haemophilus influenzae and meningococcus, applied in the direction of antibacterial drugs, bacterial antigen components, antibody medical components, etc., to achieve the effect of preventing epidemic meningitis, reducing endotoxin content, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
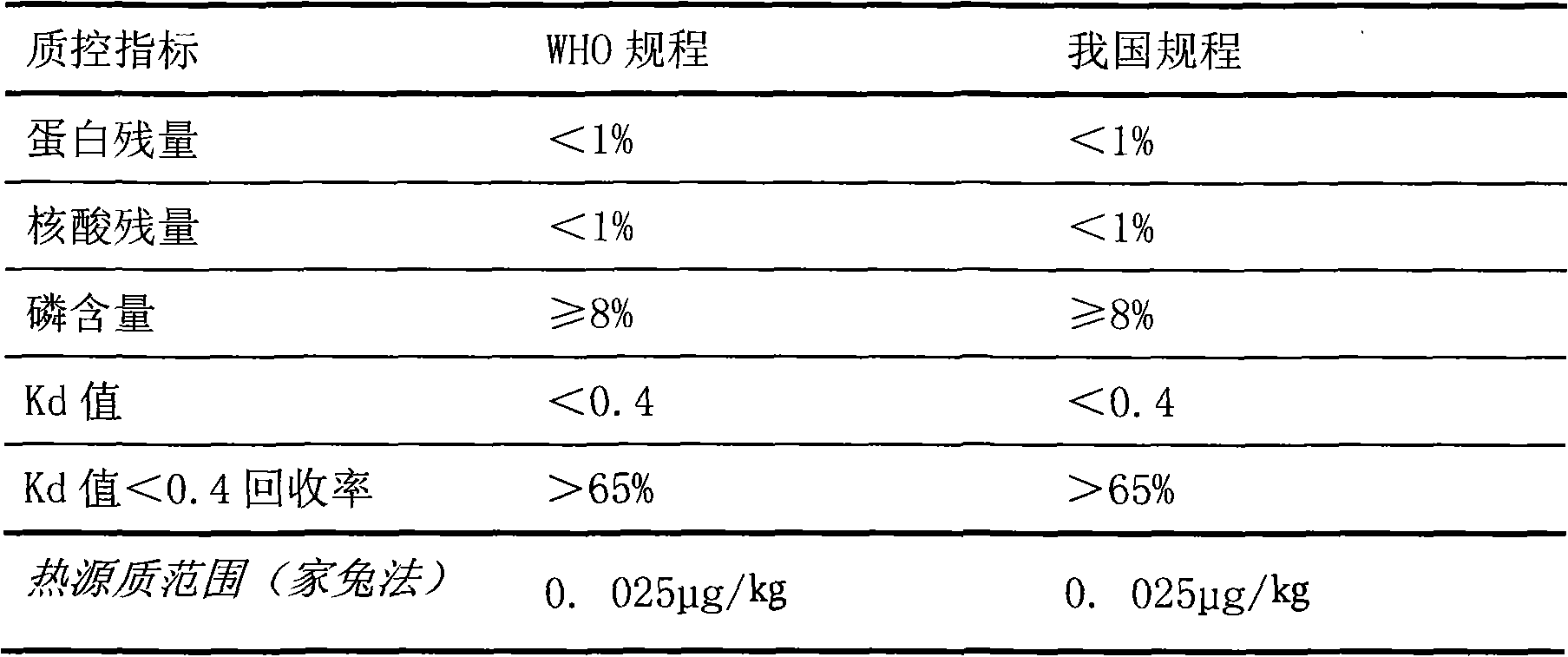
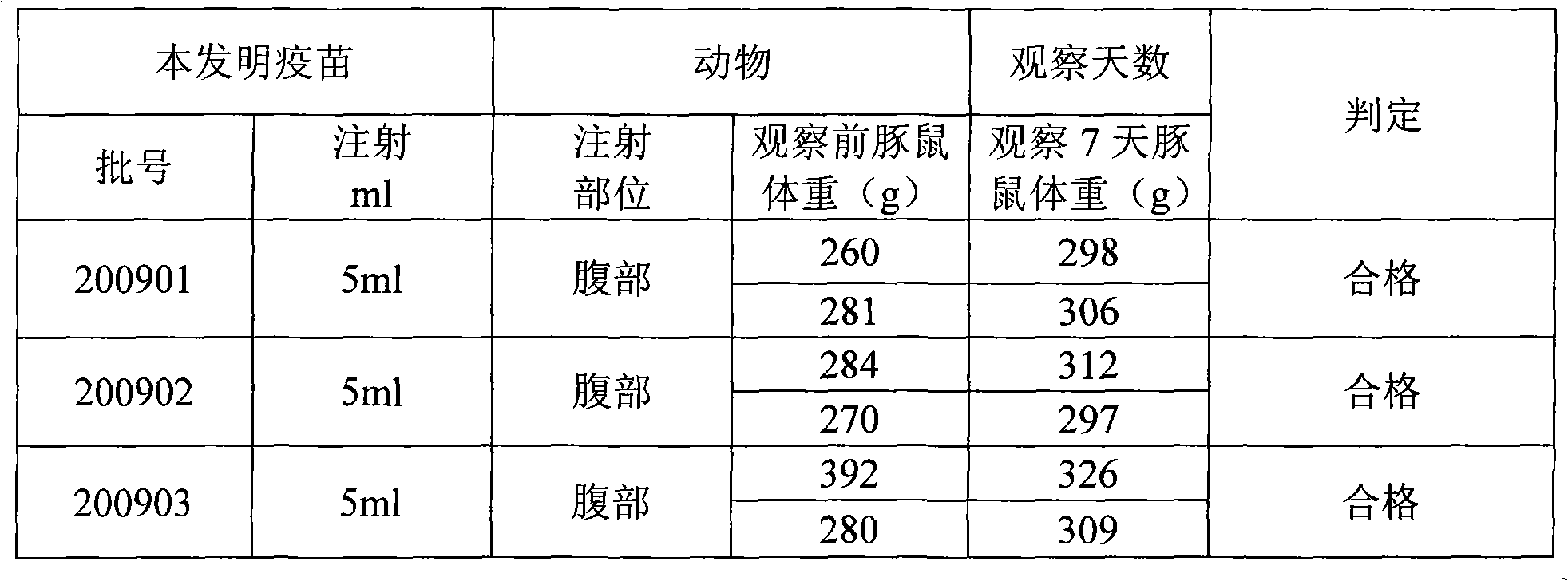
[0048] 200901 Batch ACYW 135 Preparation of Group Meningococcal Polysaccharide Vaccine-Haemophilus Influenza Type B Conjugated Vaccine
[0049] 1. Take group A meningococcus 29201 strain, C group meningococcus 29205 strain, Y group meningococcus 29028 strain, W 135 Group meningococcus 29037 strain, inoculated on ordinary agar medium containing 10% sheep blood, meningococcus does not grow at 25 ℃. Cultivate in a carbon dioxide environment at 35-37°C for 16-20 hours, grow smooth, moist, off-white colonies, the bacterial lawn is easy to remove, and appears a uniform suspension in physiological sodium chloride solution. Staining microscopic examination should show Gram-negative diplococci and monococci.
[0050]2. Use culture tanks for liquid culture. During the cultivation process, samples were taken for pure bacteria inspection, and smears were used for Gram staining microscopy. If any contaminated bacteria were found, they should be discarded. The culture was terminated at ...
Embodiment 2
[0060] 200902 Batch ACYW 135 Preparation of Group Meningococcal Polysaccharide Vaccine-Haemophilus Influenza Type B Conjugated Vaccine
[0061] 1. Take group A meningococcus 29201 strain, C group meningococcus 29205 strain, Y group meningococcus 29028 strain, W 135 Group meningococcus 29037 strain, inoculated on ordinary agar medium containing 10% sheep blood, meningococcus does not grow at 25 ℃. Cultivate in a carbon dioxide environment at 35-37°C for 16-20 hours, grow smooth, moist, off-white colonies, the bacterial lawn is easy to remove, and appears a uniform suspension in physiological sodium chloride solution. Staining microscopic examination should show Gram-negative diplococci and monococci.
[0062] 2. Use culture tanks for liquid culture. During the cultivation process, samples were taken for pure bacteria inspection, and smears were used for Gram staining microscopy. If any contaminated bacteria were found, they should be discarded. The culture was terminated at...
Embodiment 3
[0072] 200903 Batch ACYW 135 Preparation of Group Meningococcal Polysaccharide Vaccine-Haemophilus Influenza Type B Conjugated Vaccine
[0073] 1. Take group A meningococcus 29201 strain, C group meningococcus 29205 strain, Y group meningococcus 29028 strain, W 135 Group meningococcus 29037 strain, inoculated on ordinary agar medium containing 10% sheep blood, meningococcus does not grow at 25 ℃. Cultivate in a carbon dioxide environment at 35-37°C for 16-20 hours, grow smooth, moist, off-white colonies, the bacterial lawn is easy to remove, and appears a uniform suspension in physiological sodium chloride solution. Staining microscopic examination should show Gram-negative diplococci and monococci.
[0074] 2. Use culture tanks for liquid culture. During the cultivation process, samples were taken for pure bacteria inspection, and smears were used for Gram staining microscopy. If any contaminated bacteria were found, they should be discarded. The culture was terminated at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com