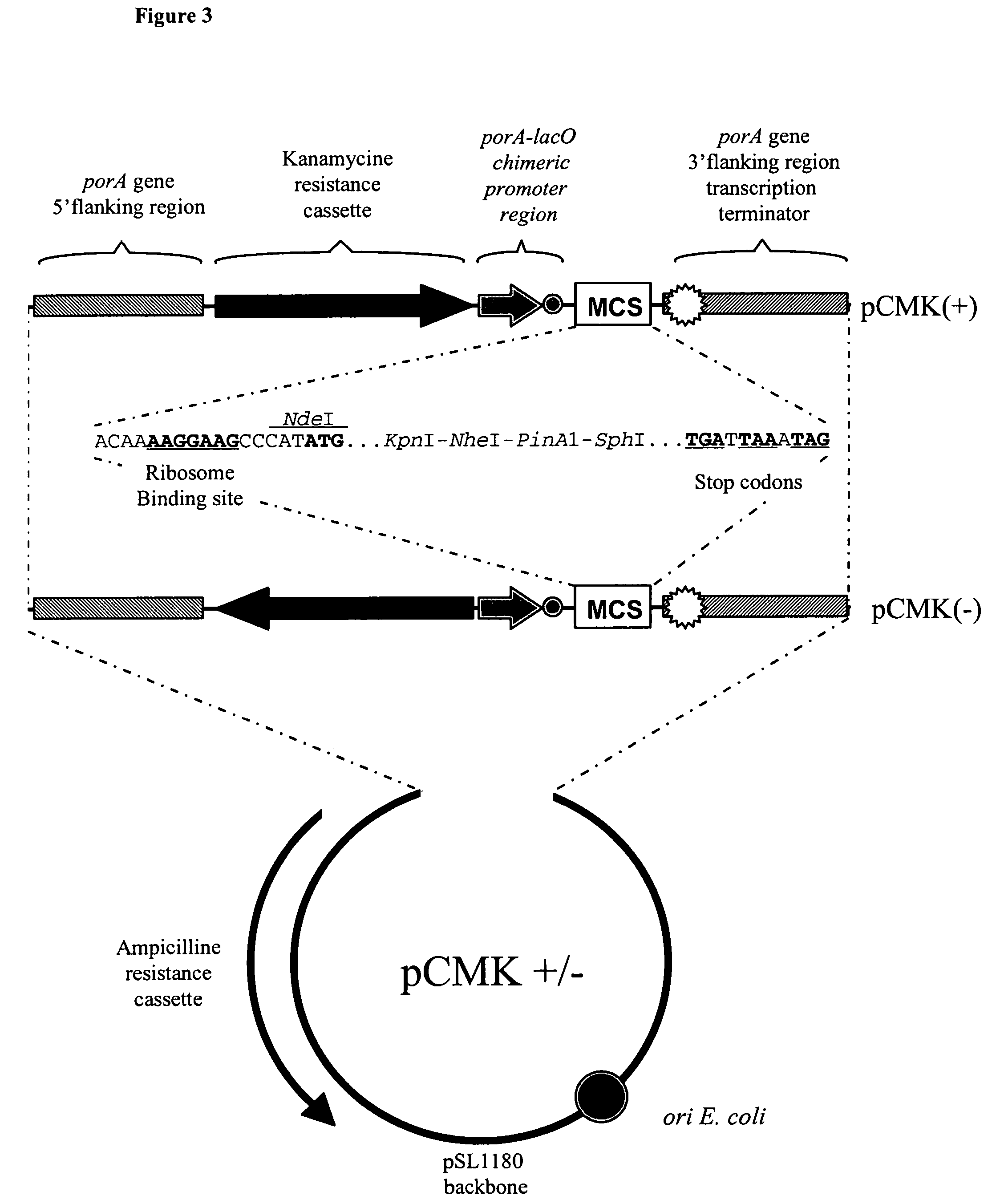
Patents
Literature
176 results about "Haemophilus influenzae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Haemophilus influenzae (formerly called Pfeiffer's bacillus or Bacillus influenzae) is a Gram-negative, coccobacillary, facultatively anaerobic pathogenic bacterium belonging to the Pasteurellaceae family. H. influenzae was first described in 1892 by Richard Pfeiffer during an influenza pandemic.
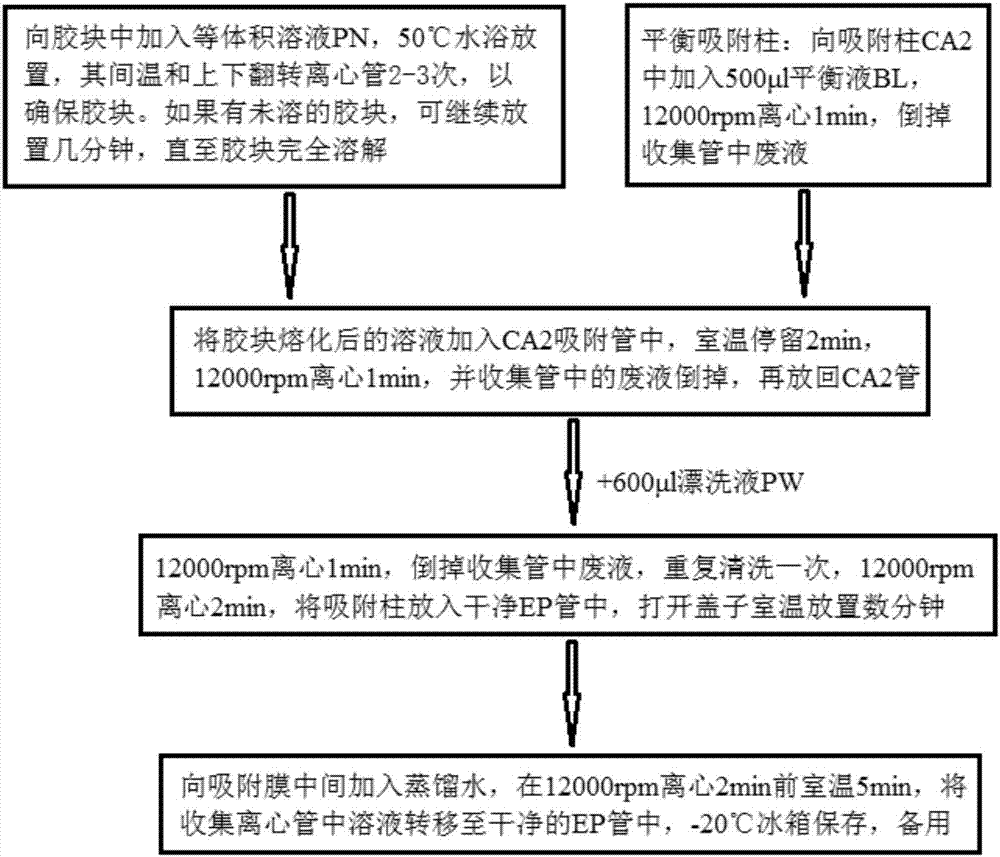
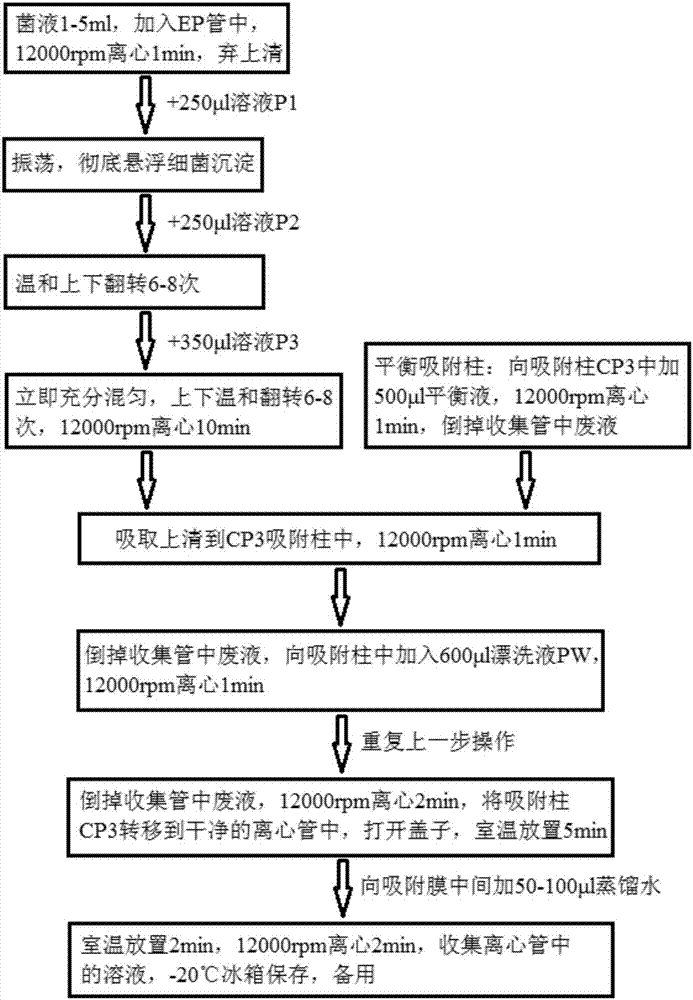
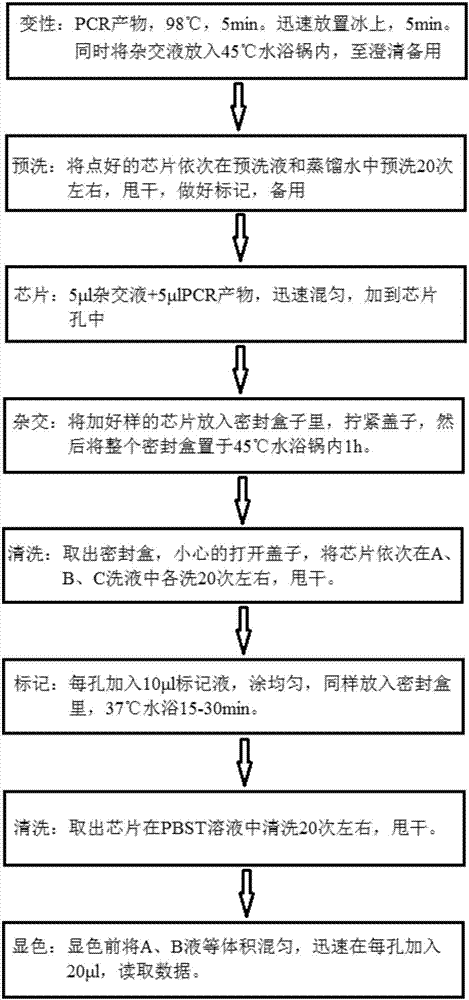
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
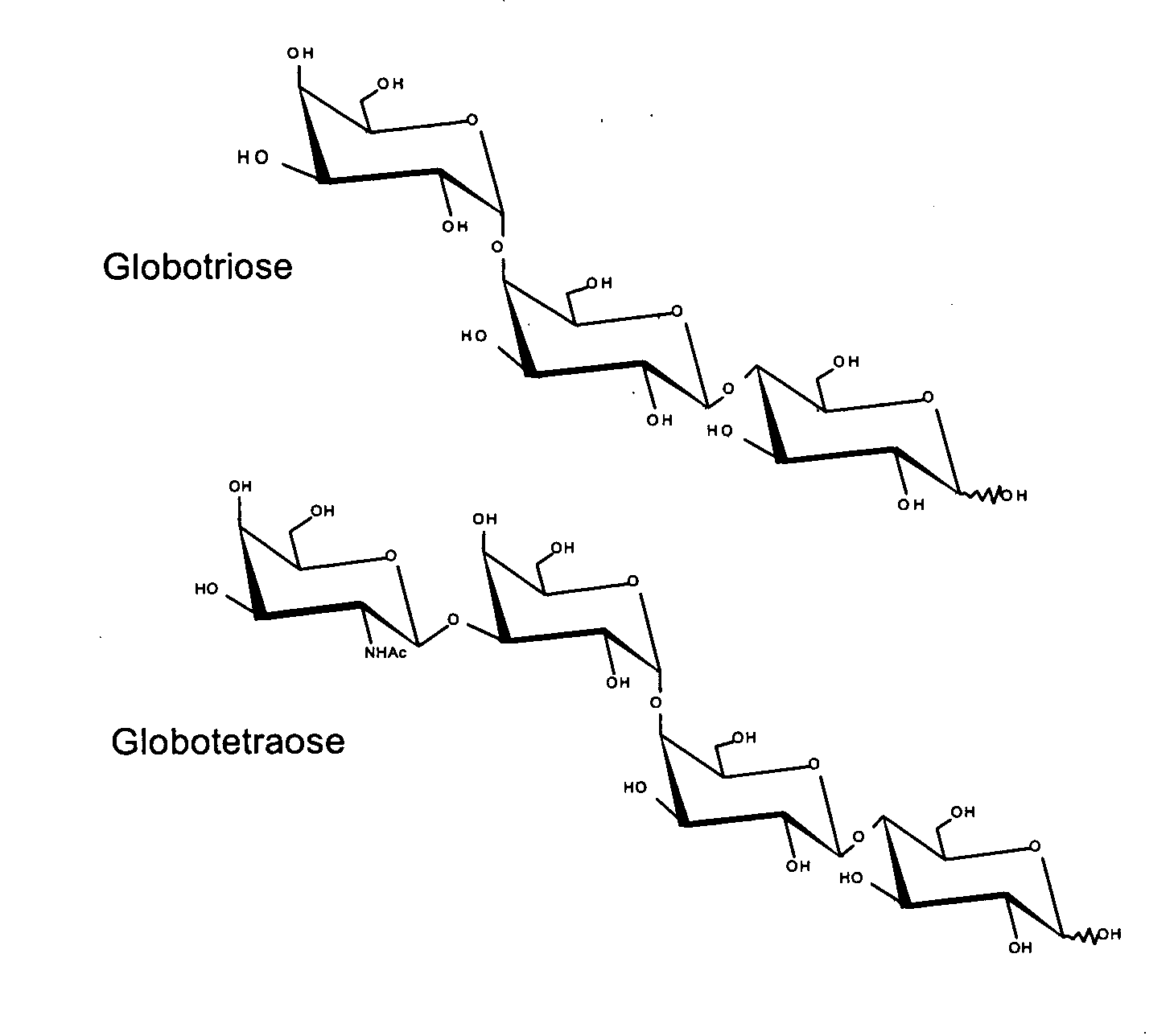
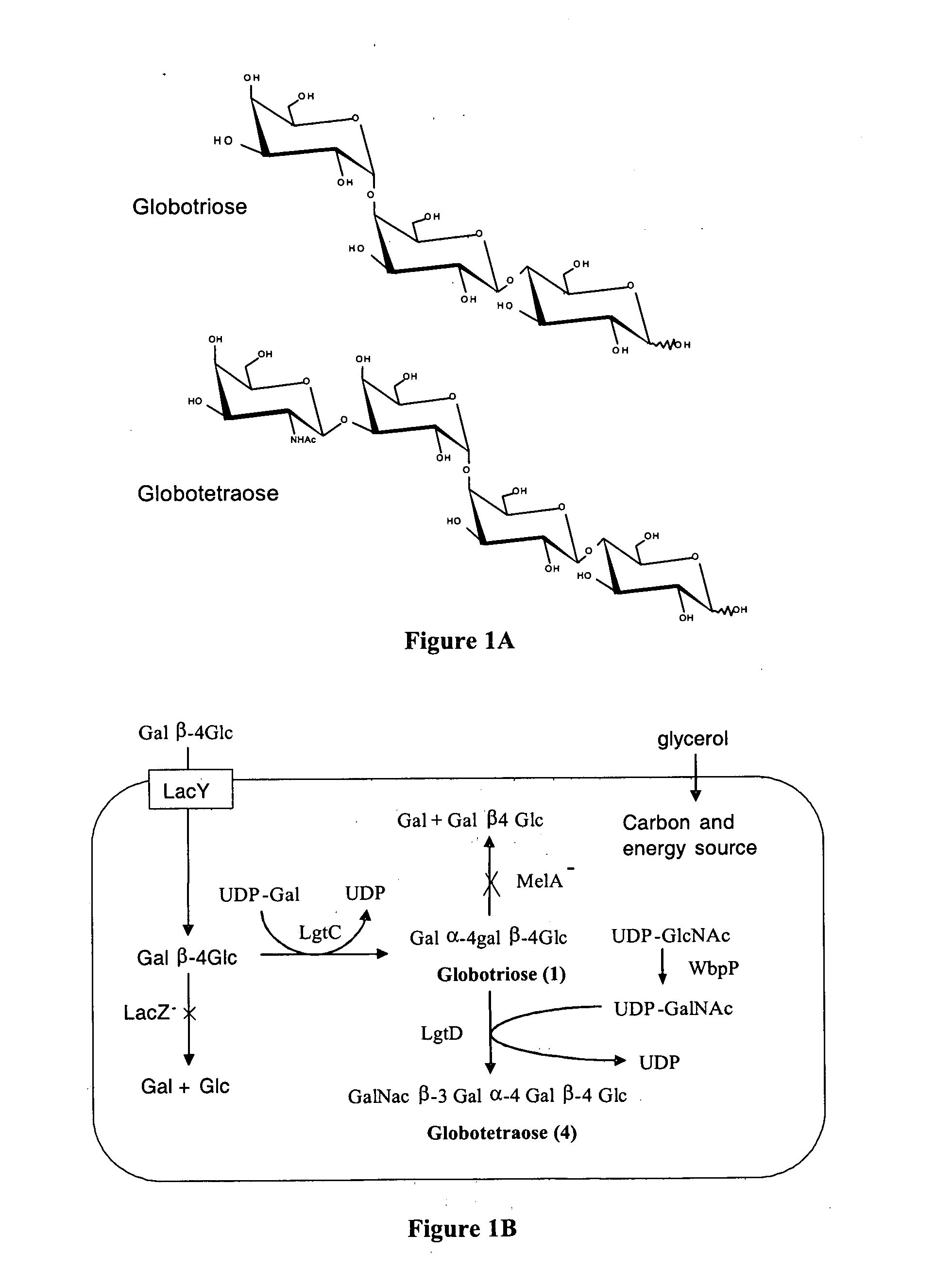
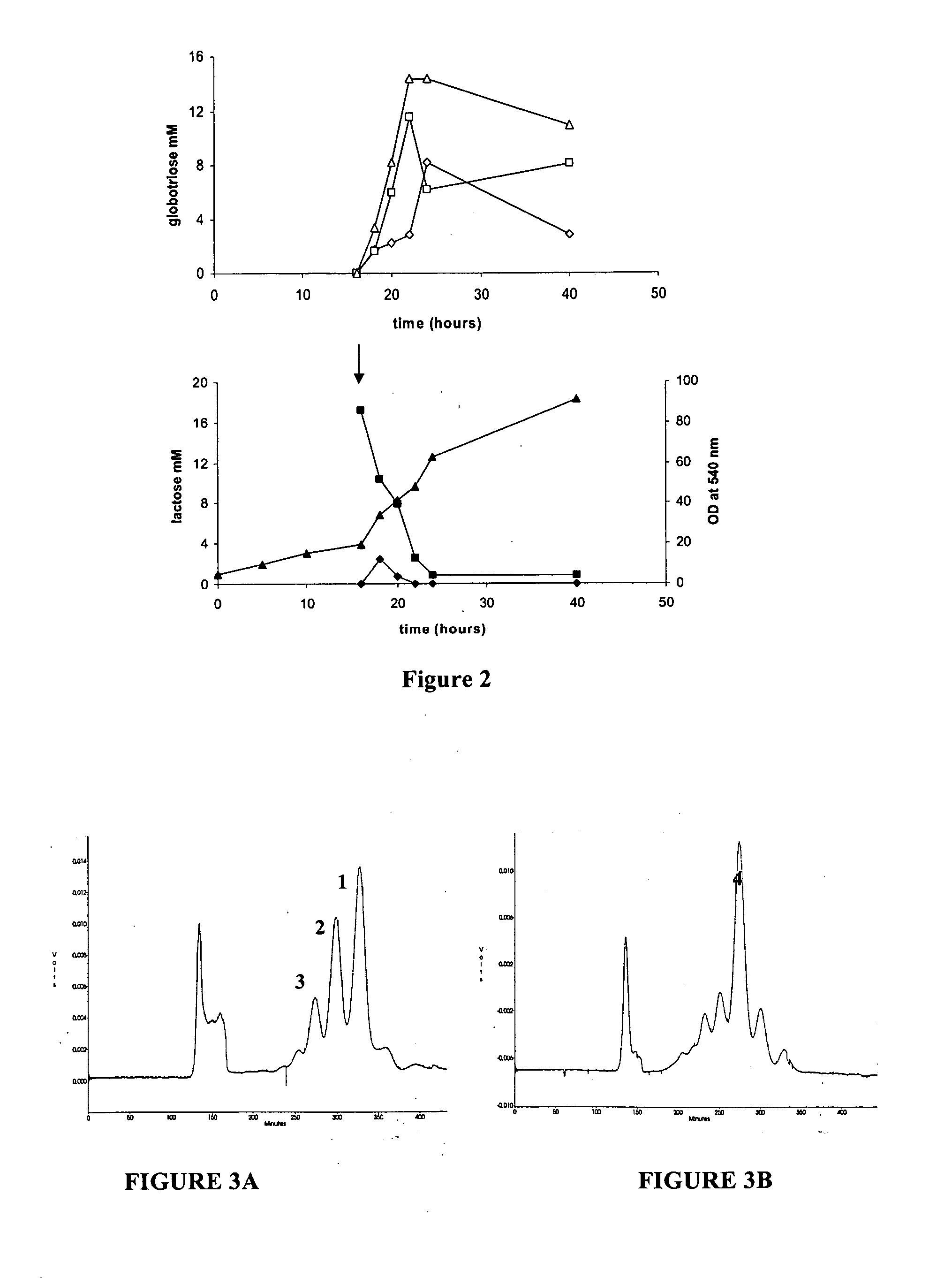
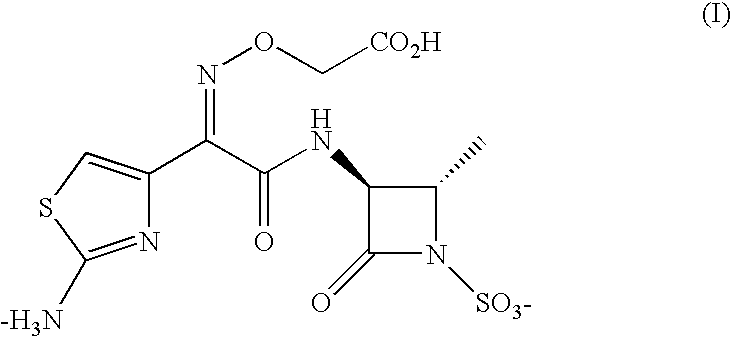
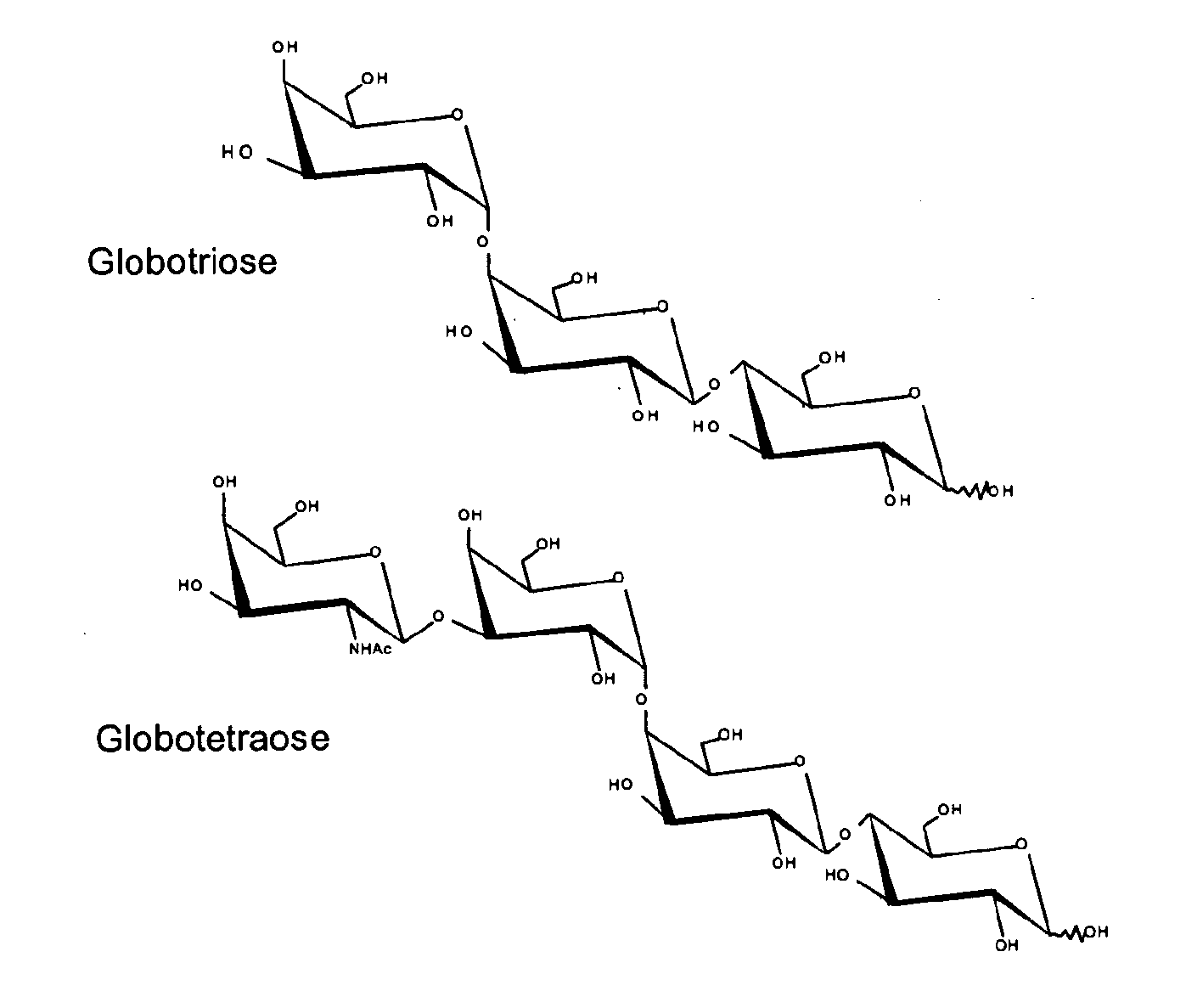
Production of globosides oligosaccharides using metabolically engineered microorganisms
The present invention relates to the large scale in vivo synthesis of globosides oligosaccharides; especially globotriose, globotetraose and globopentaose, which are the carbohydrate portions of globotriosylceramide (Gb3Cer), globotetraosylceramide (Gb4Cer) and globopentaosylceramide (Gb5Cer) respectively. It also relates to high yield production of potential anticancer vaccines of the globo-series glycosphingolipids, including the Globo-H. It also relates to the use of the glycosyltransferase encoded by the lgtD gene from Haemophilus influenzae as a beta1,3 galactosyl transferase to catalyze the transfer of a galactose moiety from UDP-Gal to an acceptor bearing the terminal non reducing structure GalNAcbeta-3-R to form the Galbeta-3GalNAcbeta-3-R structure
Owner:CENT NAT DE LA RECHERCHE SCI
Inhalable aztreonam aerosol for treatment and prevention of pulmonary bacterial infections
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam, or a pharmaceutically acceptable salt thereof, delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
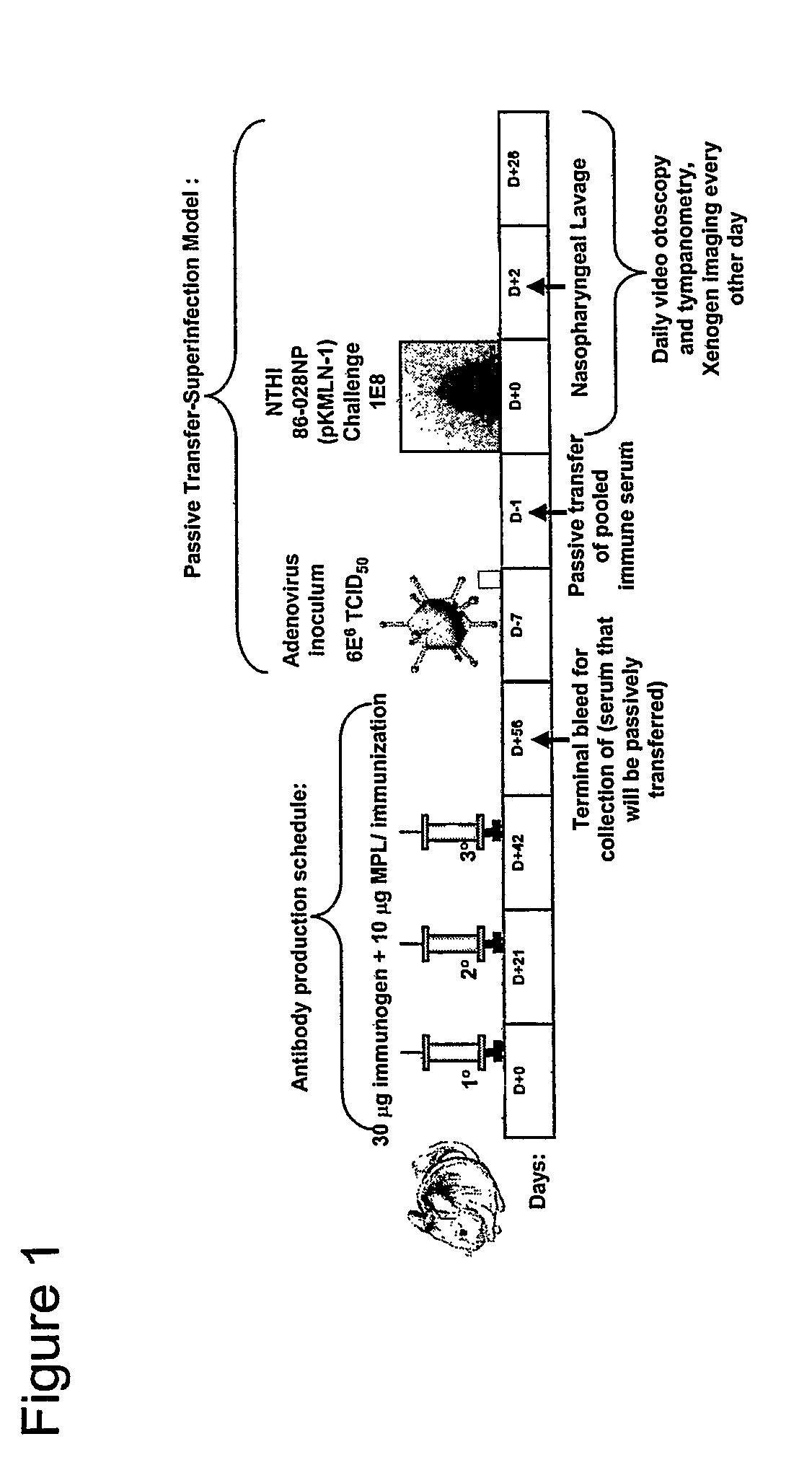
Genes of an otitis media isolate of nontypeable Haemophilus influenzae
InactiveUS8283114B2Good linkageEasy to modifyAntibacterial agentsSenses disorderBiotechnologyHaemophilus
The invention relates to the polynucleotide sequence of a nontypeable stain of Haemophilus influenzae (NTHi) and polypeptides encoded by the polynucleotides and uses thereof. The invention also relates to NTHi genes which are upregulated during or in response to NTHi infection of the middle ear and / or the nasopharynx.
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Polypeptide encoded by a nucleotide sequence of a nontypeable strain of Haemophilus influenzae genome
InactiveUS7241867B2Good linkageEasy to modifyAntibacterial agentsSenses disorderNucleotideHaemophilus influenzae
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +1
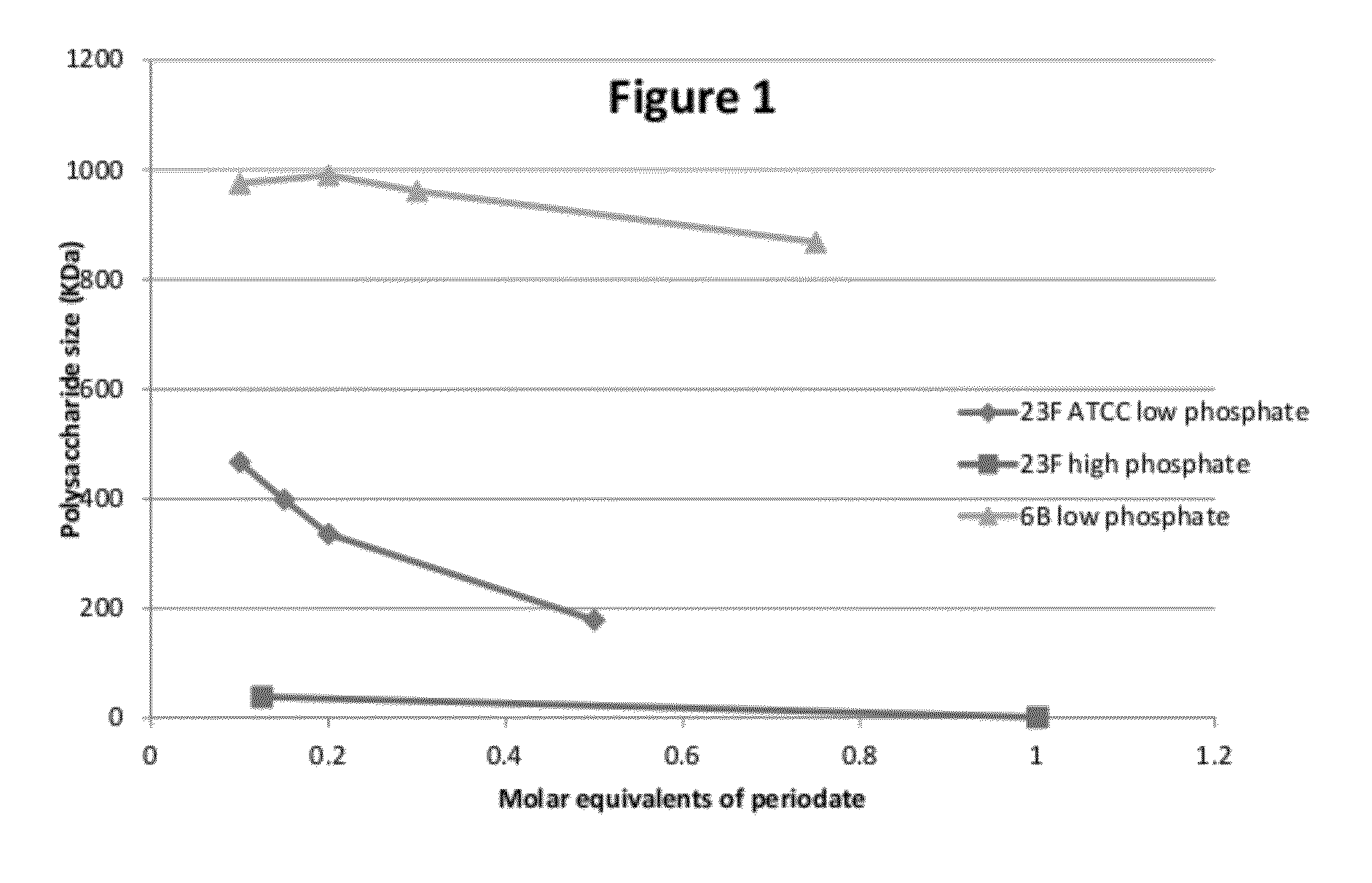
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS8753645B2Antibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Genes of an Otitis Media Isolate of Nontypeable Haemophilus influenzae
InactiveUS20110135646A1Good linkageEasy to modifyAntibacterial agentsOrganic active ingredientsBiotechnologyHaemophilus
The invention relates to the polynucleotide sequence of a nontypeable stain of Haemophilus influenzae (NTHi) and polypeptides encoded by the polynucleotides and uses thereof. The invention also relates to NTHi genes which are upregulated during or in response to NTHi infection of the middle ear and / or the nasopharynx.
Owner:NATIONWIDE CHILDRENS HOSPITAL +1
Nontypeable Haemophilus influenzae virulence factors
InactiveUS7306805B2Translation is prevented and reducedAvoid stickingAntibacterial agentsSenses disorderVirulent characteristicsOperon
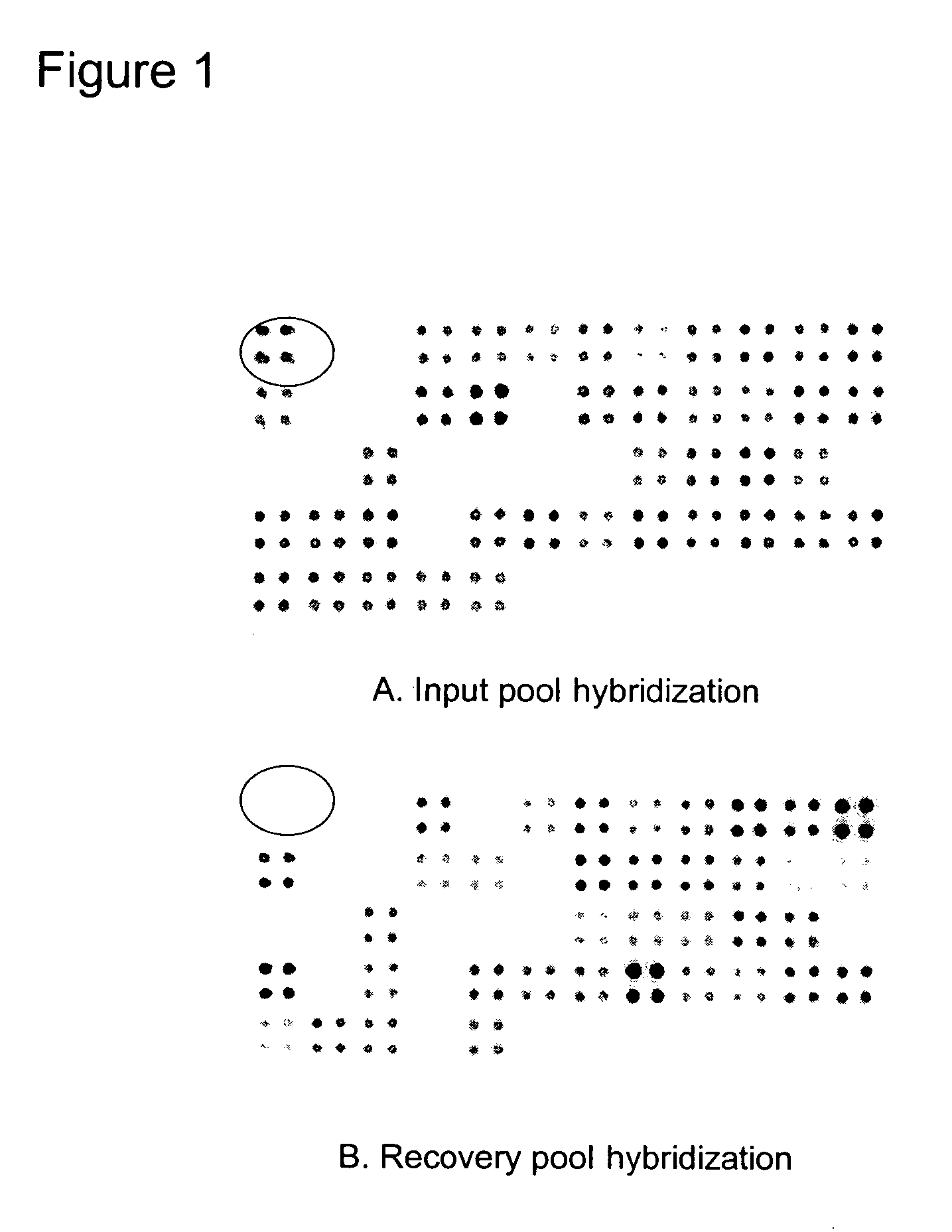
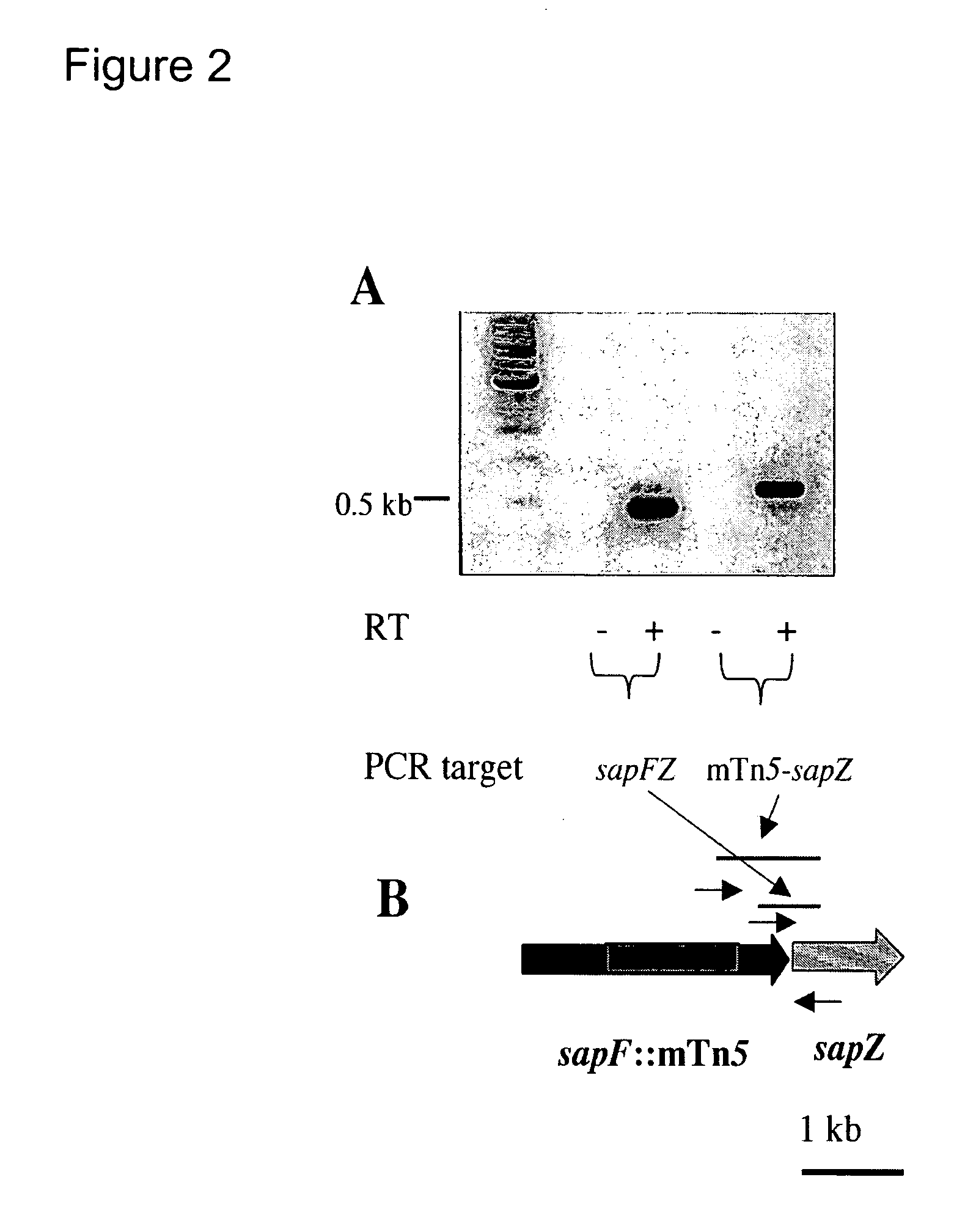
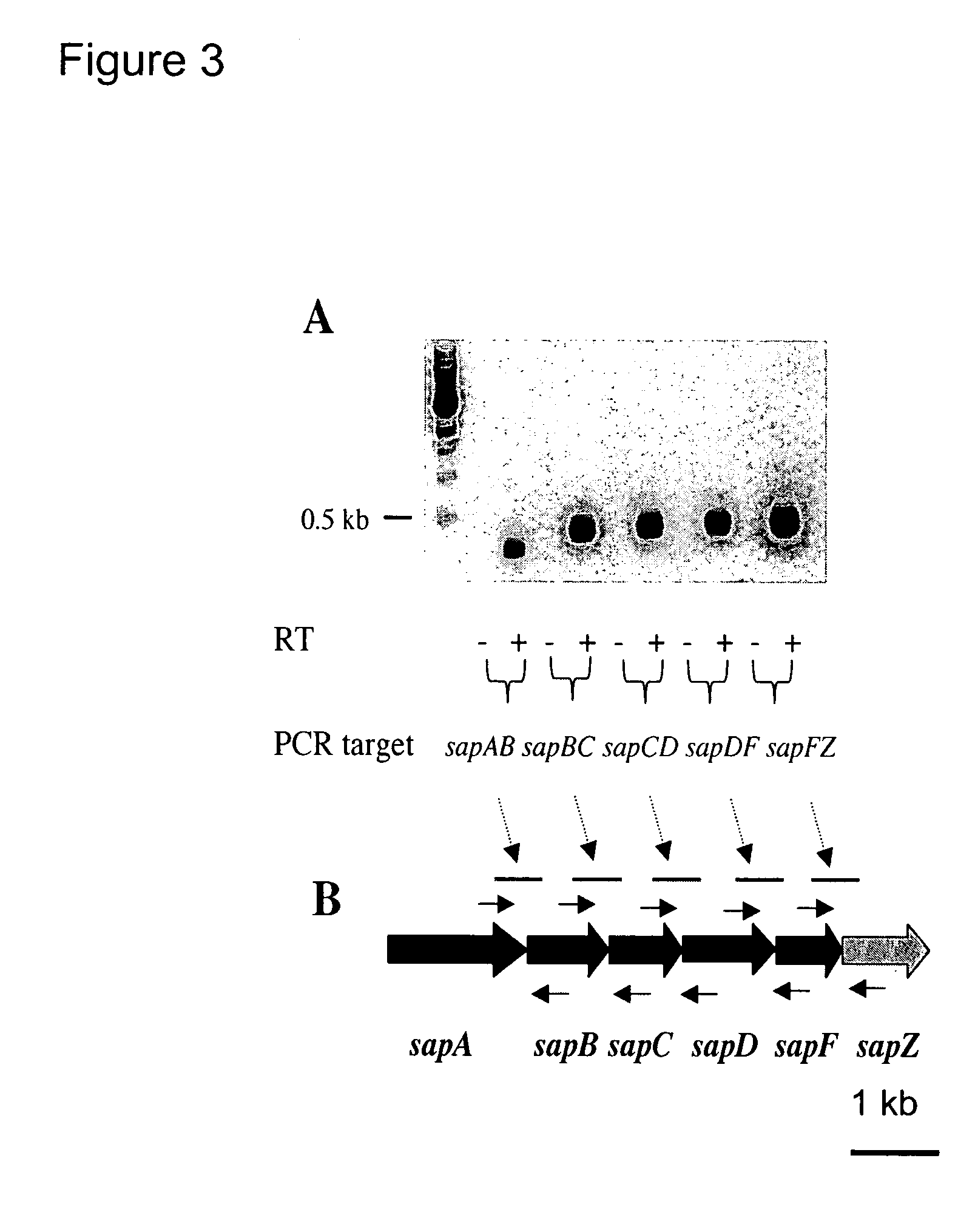
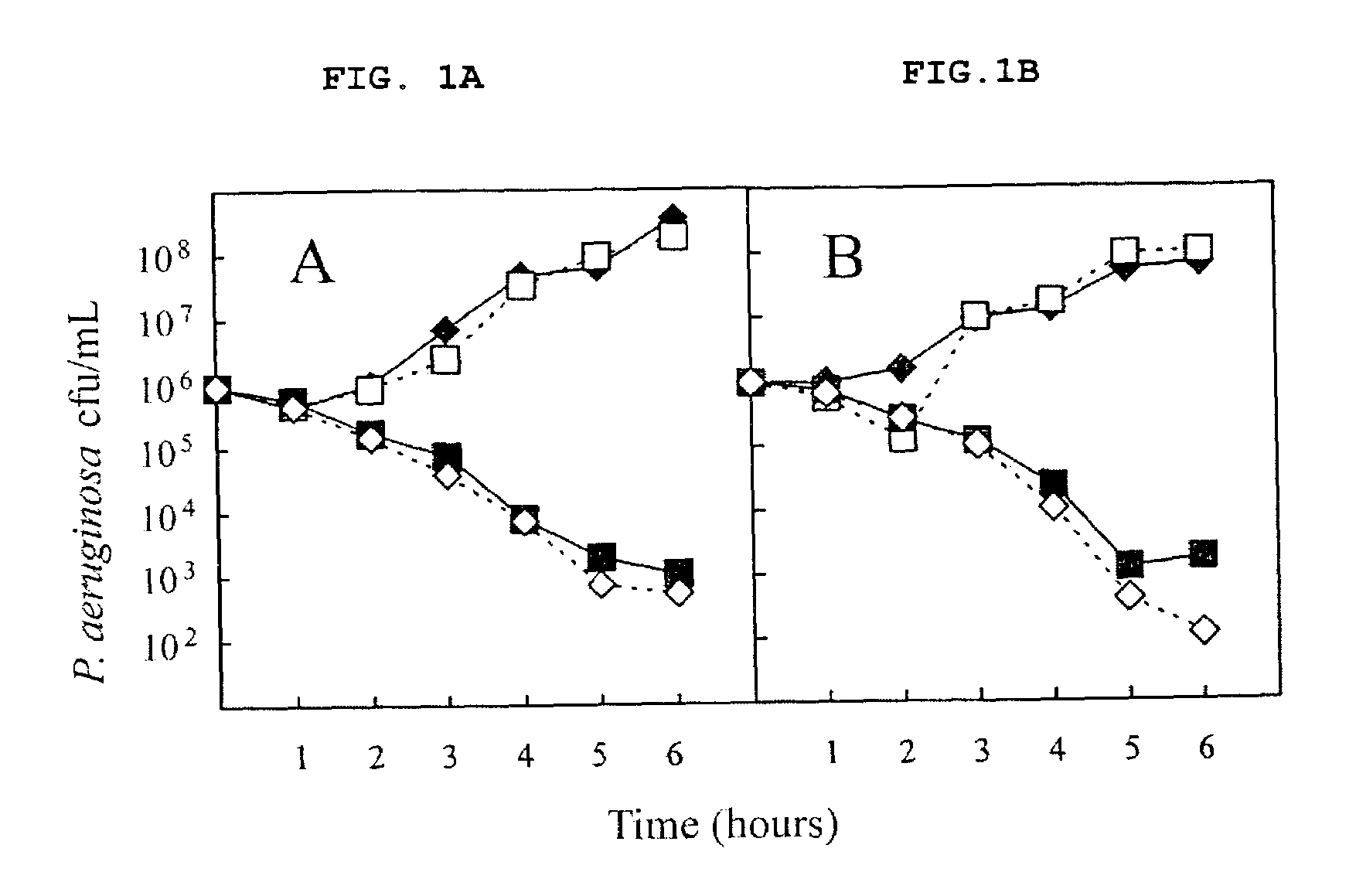
The invention relates to a mutation within the sap operon of an avirulent clone of a nontypeable strain of Haemophilus influenzae (NTHi). The invention also relates to the NTHi sap operon genes and the polypeptides encoded by these polynucleotide sequences. The invention also relates to a novel 110 kDa NTHi outer membrane protein and the polynucleotide that encodes this outer membrane protein. Methods of screening for NTHi infection, and treating and preventing NTHi related disorders are also contemplated.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Inhalable aztreonam lysinate formulation for treatment and prevention of pulmonary bacterial infections
InactiveUS7214364B2Improve stabilityHigh purityAntibacterial agentsPowder deliveryK pneumoniaeKlebsiella oxytoca
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam lysinate delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
Vaccine composition
InactiveUS20060216307A1UpregulationReducing lipid A toxicityAntibacterial agentsSenses disorderProtective antigenBacteroides
The present invention relates to an immuno-protective and non-toxic Gram-negative bleb vaccine suitable for paediatric use. Examples of the Gram-negative strains from which the blebs are made are N. meningitidis, M. catarrhalis and H. influenzae. The blebs of the invention are improved by one or more genetic changes to the chromosome of the bacterium, including up-regulation of protective antigens, down-regulation of immunodominant non-protective antigens, and detoxification of the Lipid A moiety of LPS.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Primer probe combination and kit for combined inspection of 15 kinds of respiratory tract infection pathogens
ActiveCN107937578ARapid combined detectionGuaranteed matchMicrobiological testing/measurementMicroorganism based processesStreptococcus pyogenesStaphylococcus aureus
The invention provides a pathogen inspection reagent, and concretely discloses a primer probe combination and a kit for combined inspection of 15 kinds of respiratory tract infection pathogens. By aiming at specific target sequences of the gene sequence conserved region of klebsiella pneumoniae, haemophilus influenzae, streptococcus pyogenes, staphylococcus aureus, escherichia coli, chlamydia pneumoniae, mycobacterium tuberculosis, stenotrophomonas maltophilia, baumanii, mycoplasma pneumoniae, enterococcus faecalis, ligionella pneumohpillia, streptococcus pneumoniae, bacillus pyocyaneus and mycobacterium abscessus, primers and probes which do not have mutual crossed reaction are designed, so that the problem that detection probes of different pathogens can easily generate mutual influenceor interference is solved; the combination and matching of different primers and probes are ensured; the goal of simultaneously performing efficient specific inspection at the same temperature can also be achieved.
Owner:西安九安生物技术有限公司
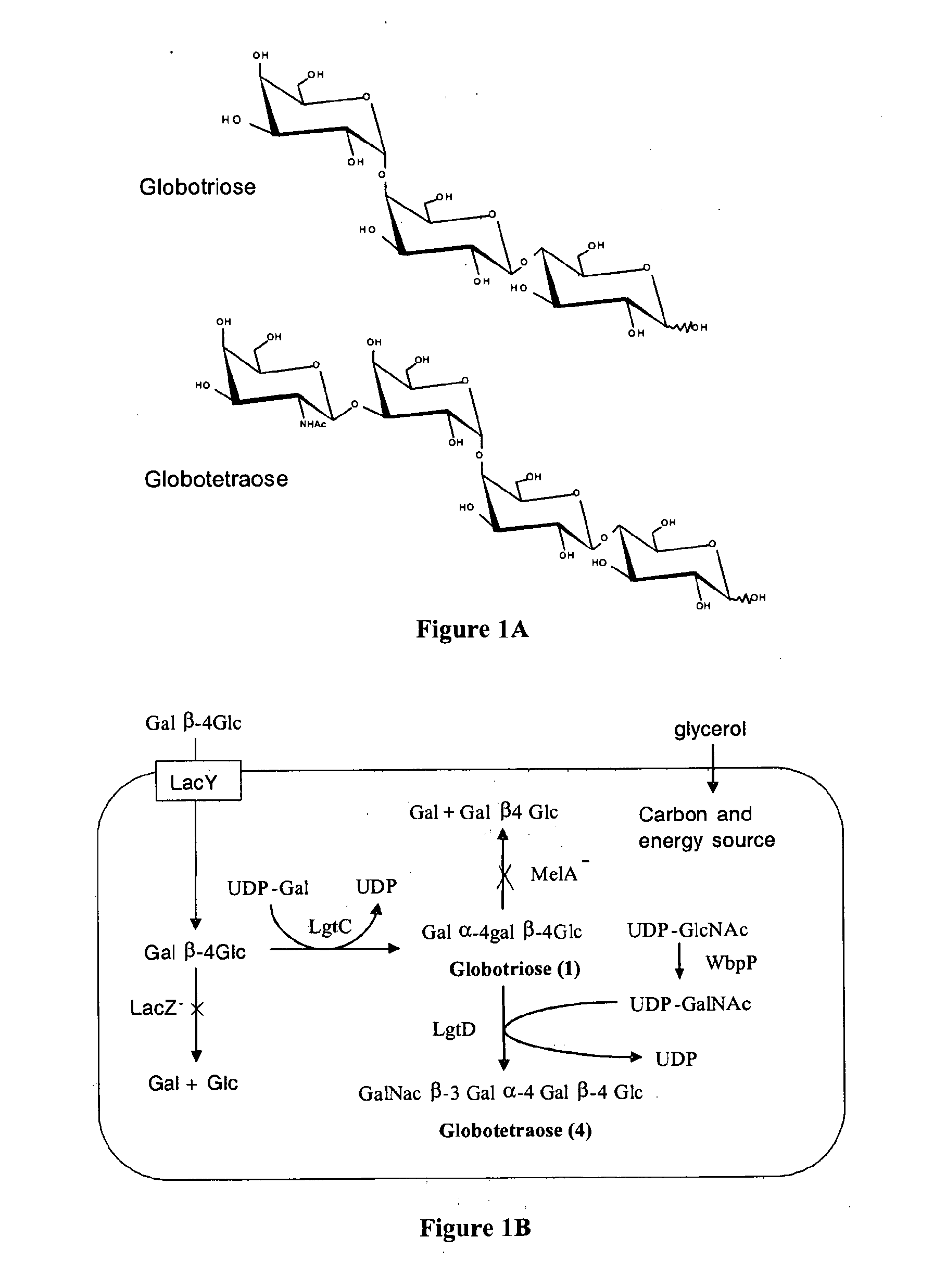
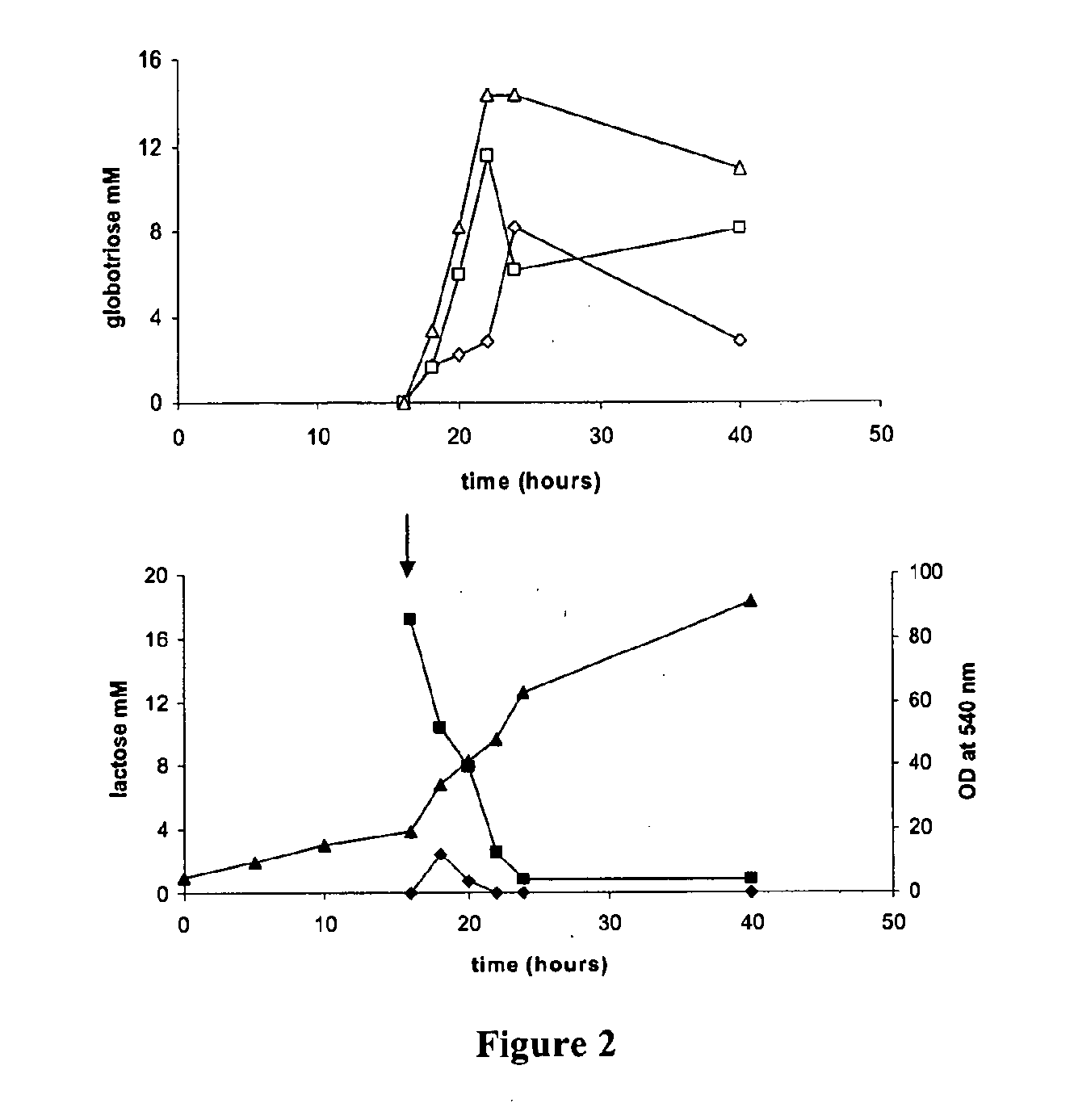
Production of globosides oligosaccharieds using metabolically engineered microorganisms
The present invention relates to the large scale in vivo synthesis of globosides oligosaccharides; especially globotriose, globotetraose and globopentaose, which are the carbohydrate portions of globotriosylceramide (Gb3Cer), globotetraosylceramide (Gb4Cer) and globopentaosylceramide (Gb5Cer) respectively. It also relates to high yield production of potential anticancer vaccines of the globo-series glycosphingo lipids, including the Globo-H. It also relates to the use of the glycosyltransferase encoded by the lgtD gene from Haemophilus influenzae as a β1,3 galactosyl transferase to catalyze the transfer of a galactose moiety from UDP-Gal to an acceptor bearing the terminal non reducing structure GalNAcβ-3-R to form the Galβ-3GalNAcβ-3-R structure
Owner:CENT NAT DE LA RECHERCHE SCI
Inhalable aztreonam lysinate formulation for treatment and prevention of pulmonary bacterial infections
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam lysinate delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
Method for detecting infectious disease pathogens and kit
ActiveCN101545010AThe detection process is fastImprove efficiencyMicrobiological testing/measurementMicroorganism based processesOrganismStreptococcus constellatus
The invention discloses a method for detecting infectious disease pathogens possibly existing in a biological sample. The infectious disease pathogens comprise chlamydia pneumoniae, haemophilus influenzae, mycoplasma pneumoniae, pneumoniae streptococcus and legionella pneumophila. The method comprises the following steps: expanding nucleic acid fragments of the biological sample, and detecting the nucleic acid fragments by using a probe. The invention also provides primers used for expanding and the probe used for detection. The invention also provides a kit comprising the primers. The method has the advantages of high sensitivity, strong specificity, simple operation and wide sample range, can simultaneously detect various infectious disease pathogens, and is suitable for early diagnosis of respiratory infectious diseases.
Owner:HAI KANG LIFE
Multiplex PCR kit for detecting bacterial meningitis pathogens
ActiveCN105734164AReduce usageReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesStaphylococcus aureus
The invention discloses a multiplex PCR kit for detecting bacterial meningitis pathogens. Six types of bacterial meningitis pathogens, namely streptococcus pneumoniae (SP), streptococcus agalactiae (GBS), haemophilus influenzae (HI), listeria monocytogenes (LM), staphylococcus aureus (SA) and neisseria meningitidis (NM) can be rapidly detected. The invention discloses a 'public primer'-mediated multiplex PCR method. The method concretely comprises the steps of constructing a plasmid template, screening a public primer, designing a multi-primer and carrying out multiplex PCR system detection; a two-step annealing temperature method is combined, so that specificity, sensibility and detection throughput of a multiplex PCR system are improved; the multiplex PCR method has a certain containment capability, detection targets can be continuously added, and the detection throughput of multiplex PCR can be beneficially improved. The multiplex PCR kit disclosed by the invention can be used for effectively detecting six types of common bacteria, has the advantages of high sensitivity, economy, high speed and simple operation and is beneficial to application and clinical diagnosis.
Owner:上海捷诺生物科技股份有限公司
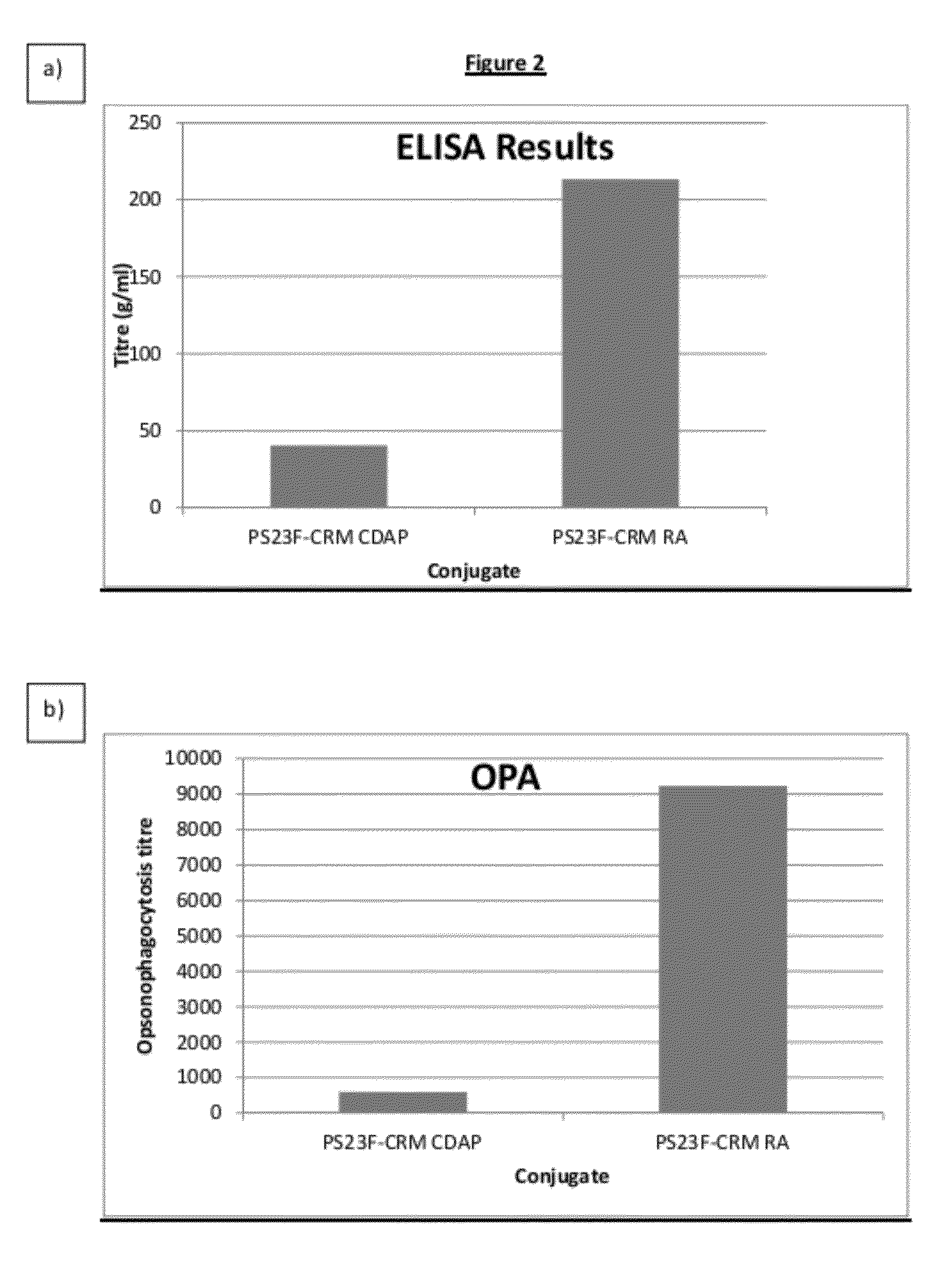
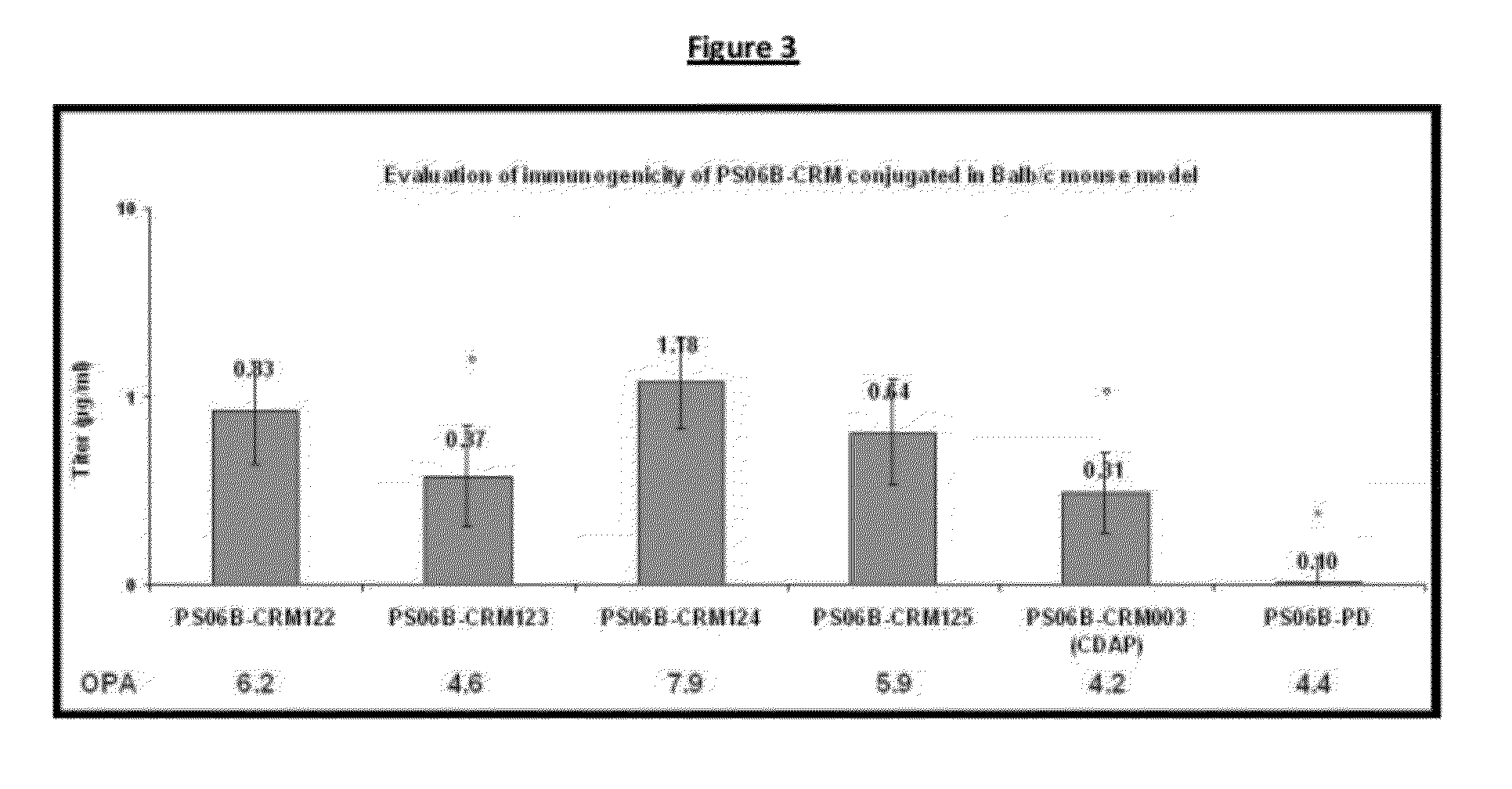
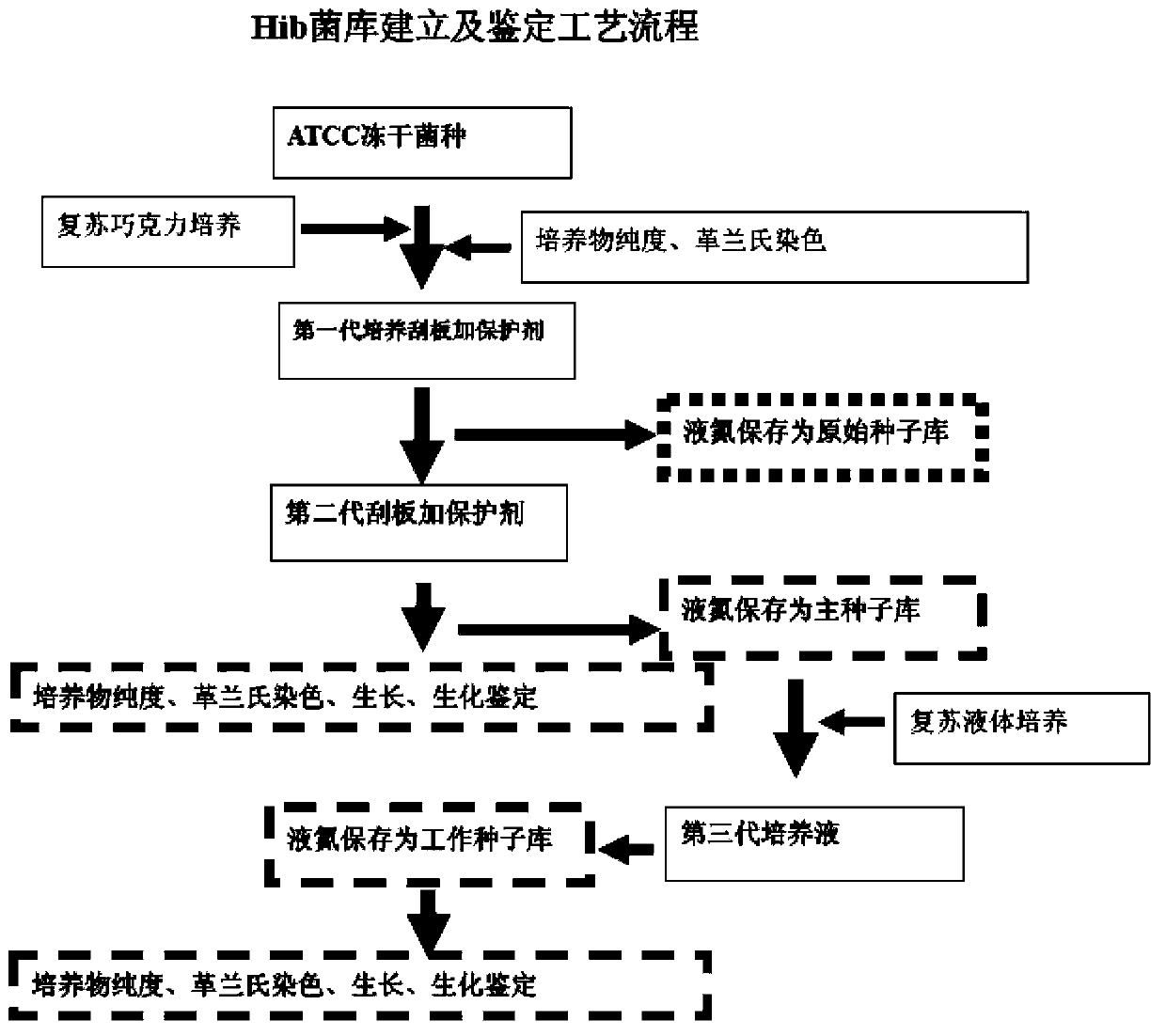
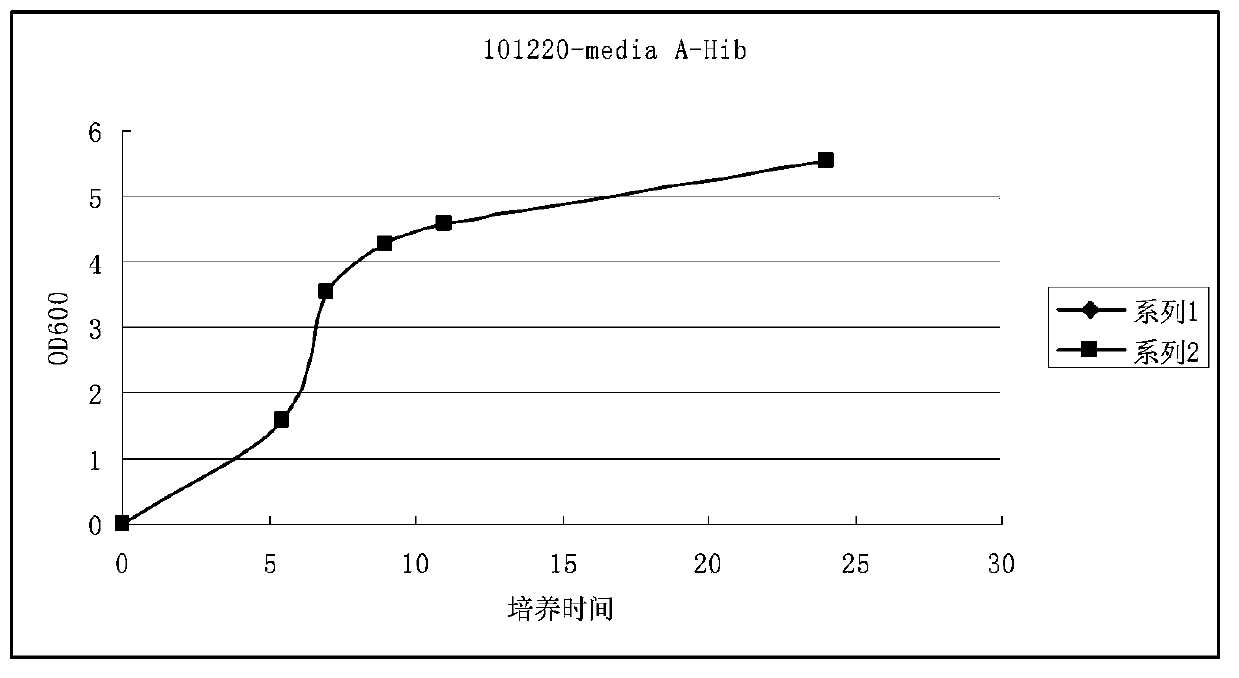
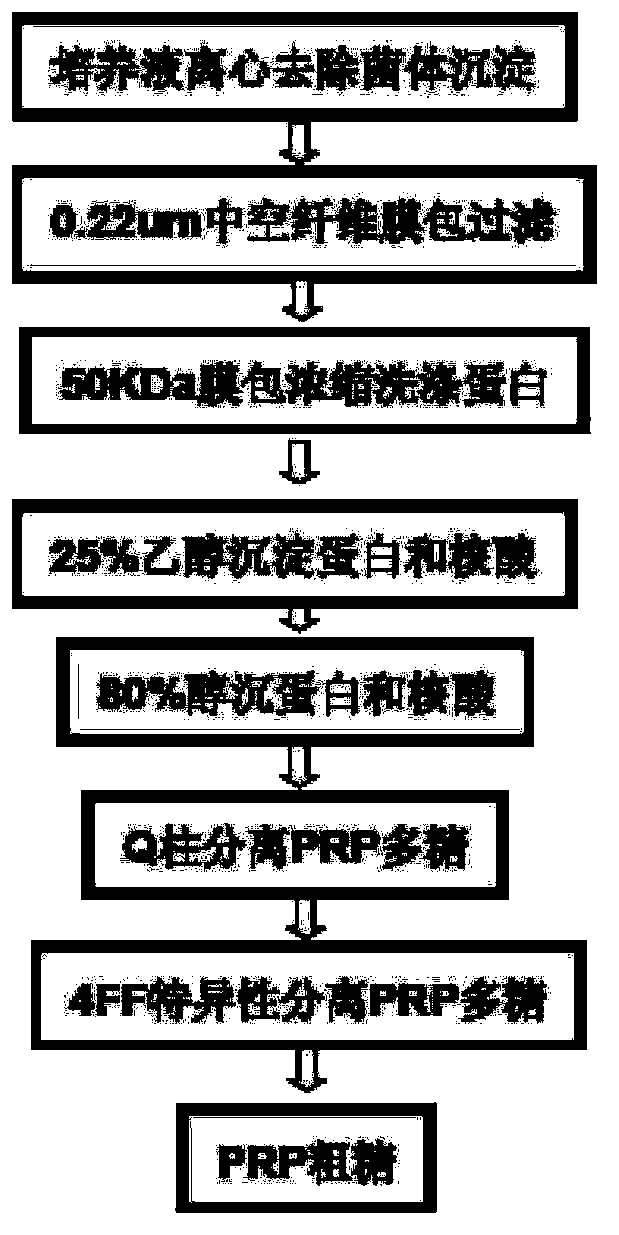
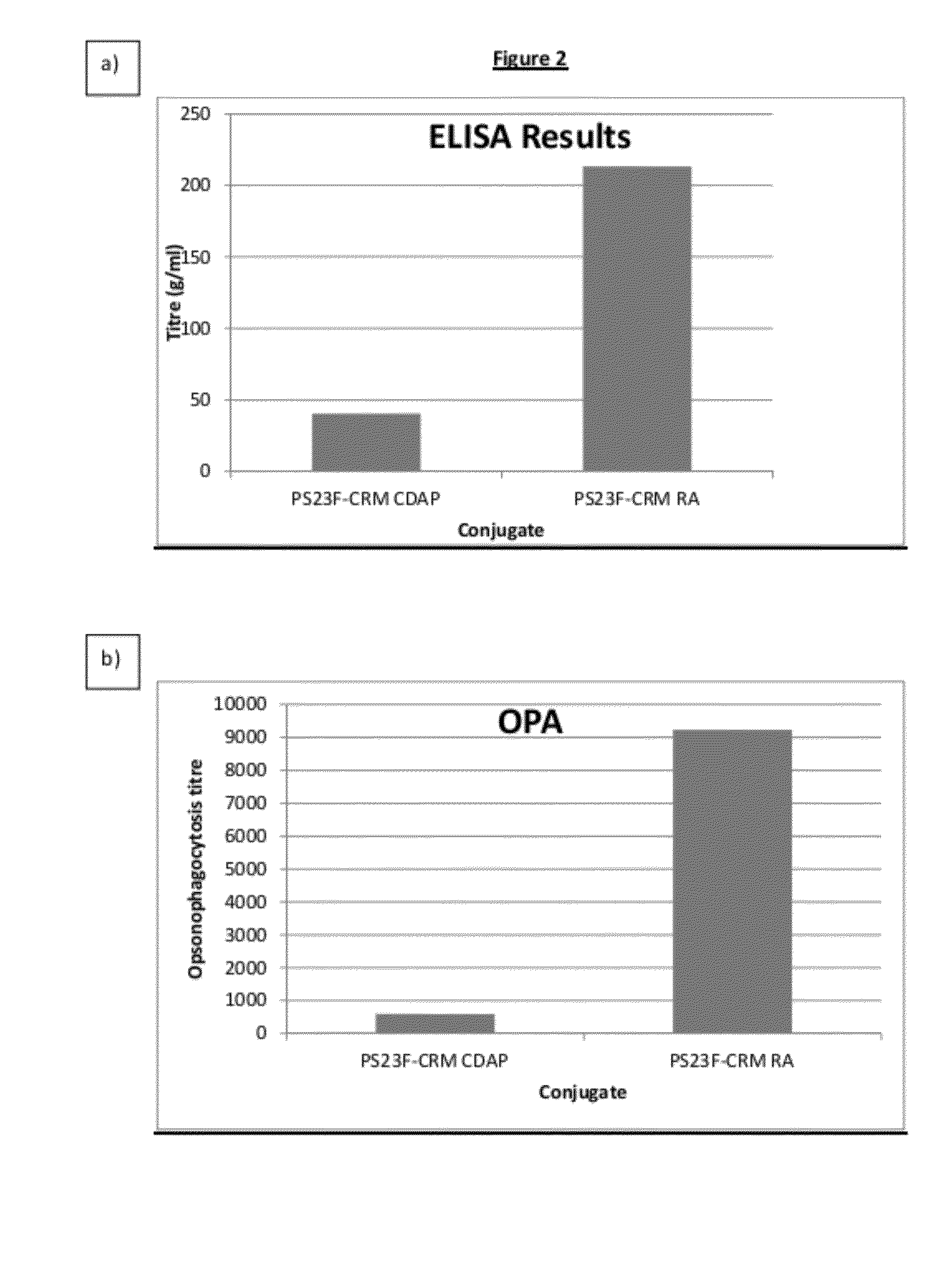
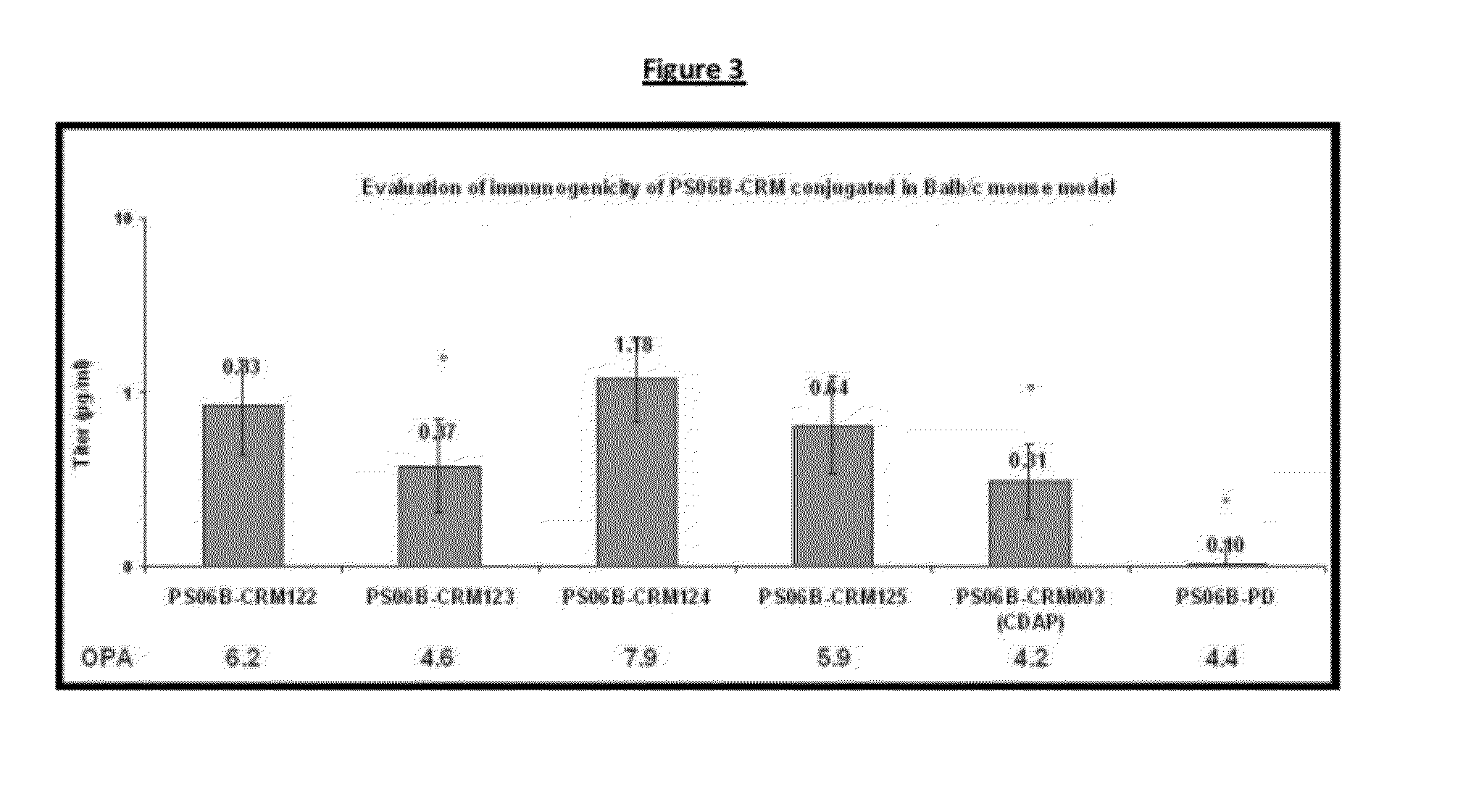
Haemophilus influenzac type B polysaccharide conjugate vaccine preparation method
The present invention discloses a Haemophilus influenzac type B polysaccharide conjugate vaccine preparation method, which comprises: A, preparing a Haemophilus influenzac type B polysaccharide extract (PRP), adopting ultrapure water or water for injection to dissolve the PRP polysaccharide at a room temperature until achieving a concentration of 20 mg / ml, adding CDAP according to a mass ratio of PRP to CDAP of 1, maintaining for 1.5 min, adjusting the pH value to 9.5 with 0.3 M NaOH, maintaining for 3 min, adding a solid ADH according to a mass ratio of ADH to the PRP polysaccharide of 4:1, adjusting the pH value to 9.5 with 0.3 M NaOH, maintaining for 1 h, and carrying out dialysis at a temperature of 2-8 DEG C with 0.2 M NaCl; and B, mixing the obtained PRP-AH and a 20 mg / ml TT solution according to a mass ratio of 1:2, adjusting the pH value to 5.0 with 0.1 M HCL, adding a solid EDAC according to a mass ratio of EDAC to PRP of 10:1, maintaining the pH value of 5.0 for 60 min at a room temperature, adjusting the pH value to 7.5 with 0.3 M NaOH, and carrying out a reaction for 30 min.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
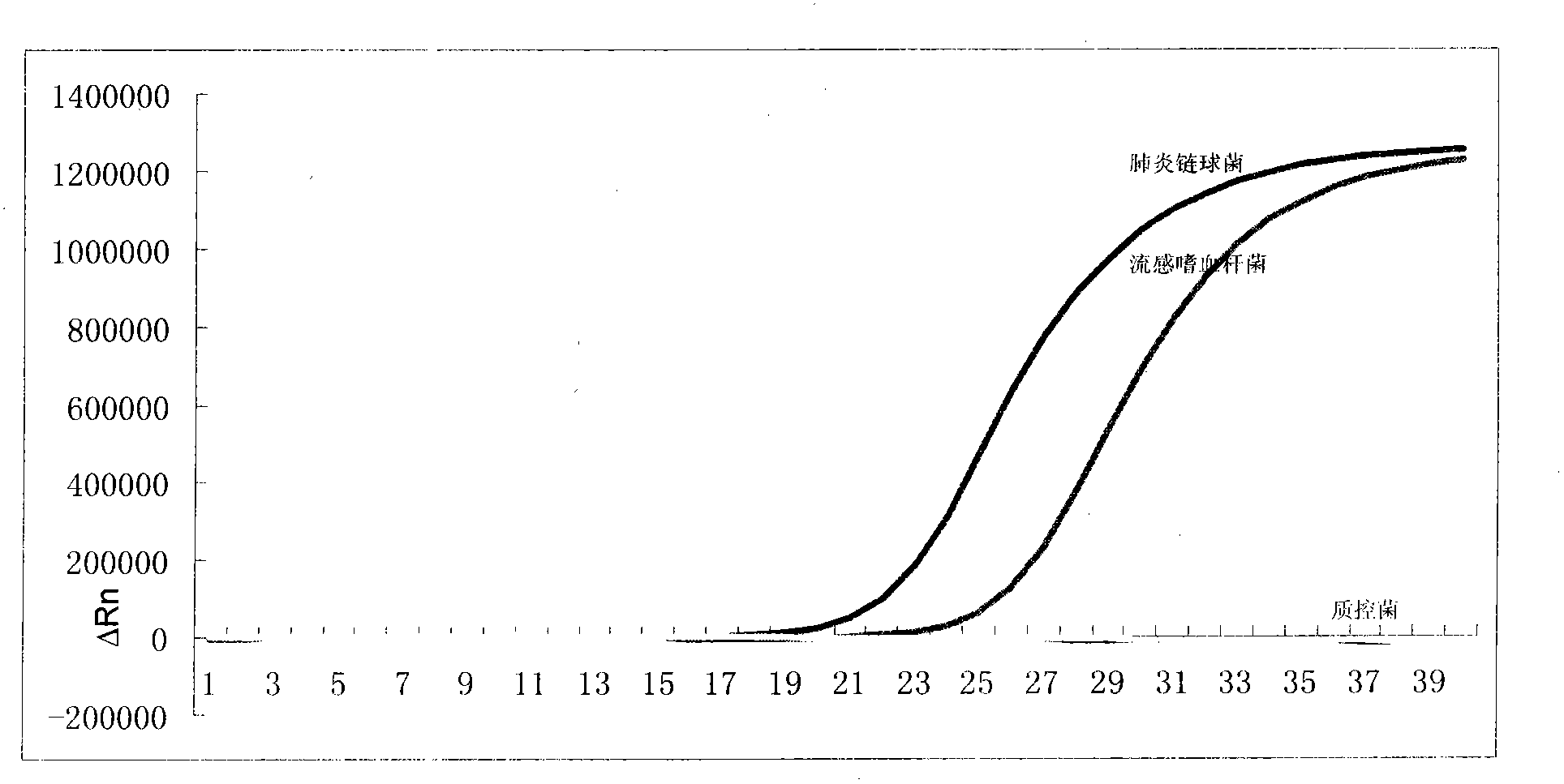
Primer and probe combination and kit for specifically detecting streptococcus pneumoniae and haemophilus influenzae
ActiveCN102660645AStrong specificityLow detection costMicrobiological testing/measurementFluorescence/phosphorescenceStreptococcus pneumoniaeDisease surveillance
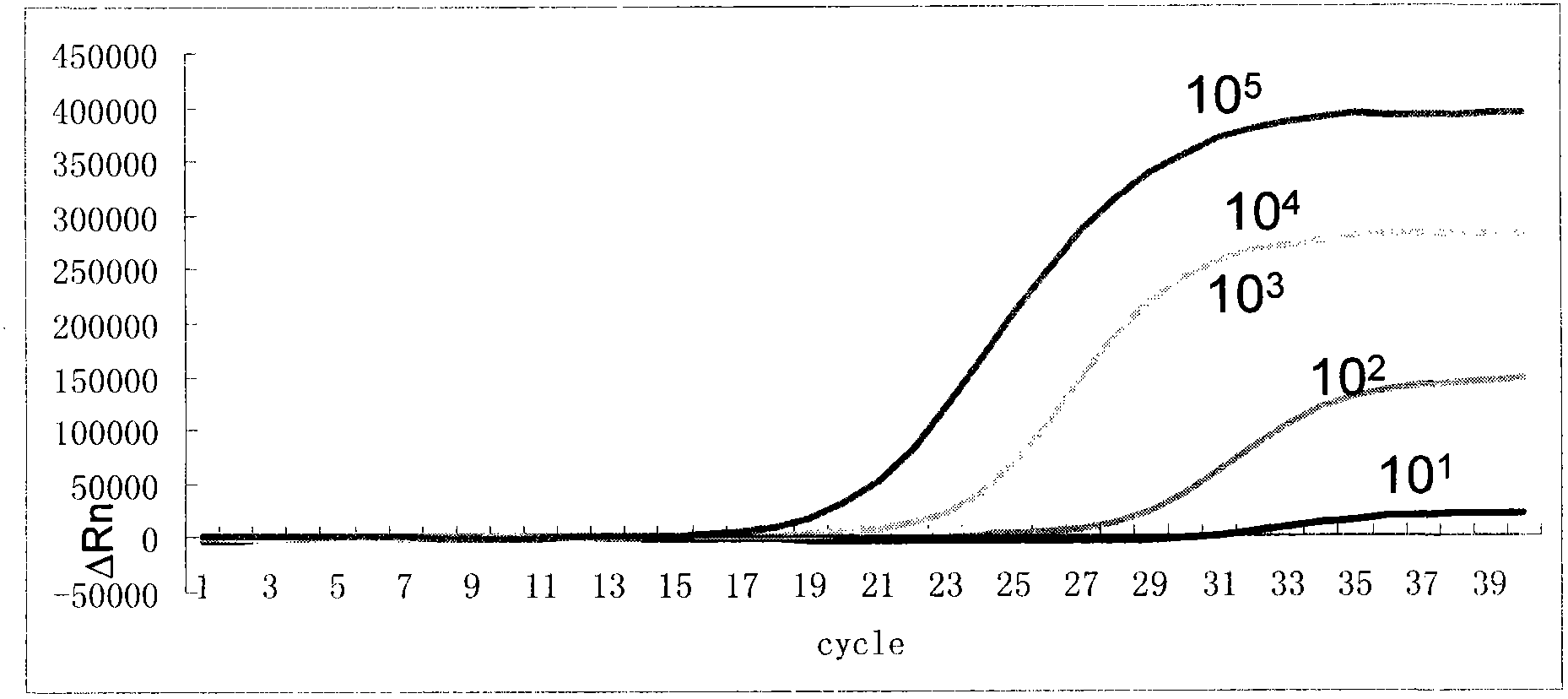
The invention relates to an oligonucleotide sequence combination for specifically detecting existence of streptococcus pneumoniae and haemophilus influenzae nucleic acids in samples by adopting fluorescent PCR (polymerase chain reaction) technology and a kit comprising the combination. The kit can sensitively detect and identify the streptococcus pneumoniae and haemophilus influenzae nucleic acids in the samples, has limits of detection of 20 copies per reaction system and has important application values in the fields such as disease surveillance and clinical diagnosis.
Owner:JIANGSU UNINOVO BIOLOGICAL TECH
Chimeric vaccine for haemophilus influenzae-induced disease
ActiveUS7811591B2Strong responseAssist in protein folding and/or antigen presentationAntibacterial agentsSenses disorderHaemophilus influenzaeRecombinant Chimeric Proteins
The invention described herein relates to a chimeric protein comprising the NTHi twitching pilus major subunit protein (PilA) presenting a portion of the NTHi OMP P5 protein. The invention provides for vaccine compositions comprising the recombinant chimeric protein and methods of eliciting an immune response using the recombinant chimeric proteins of the invention.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS20120321660A1Size of it can retainAntibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
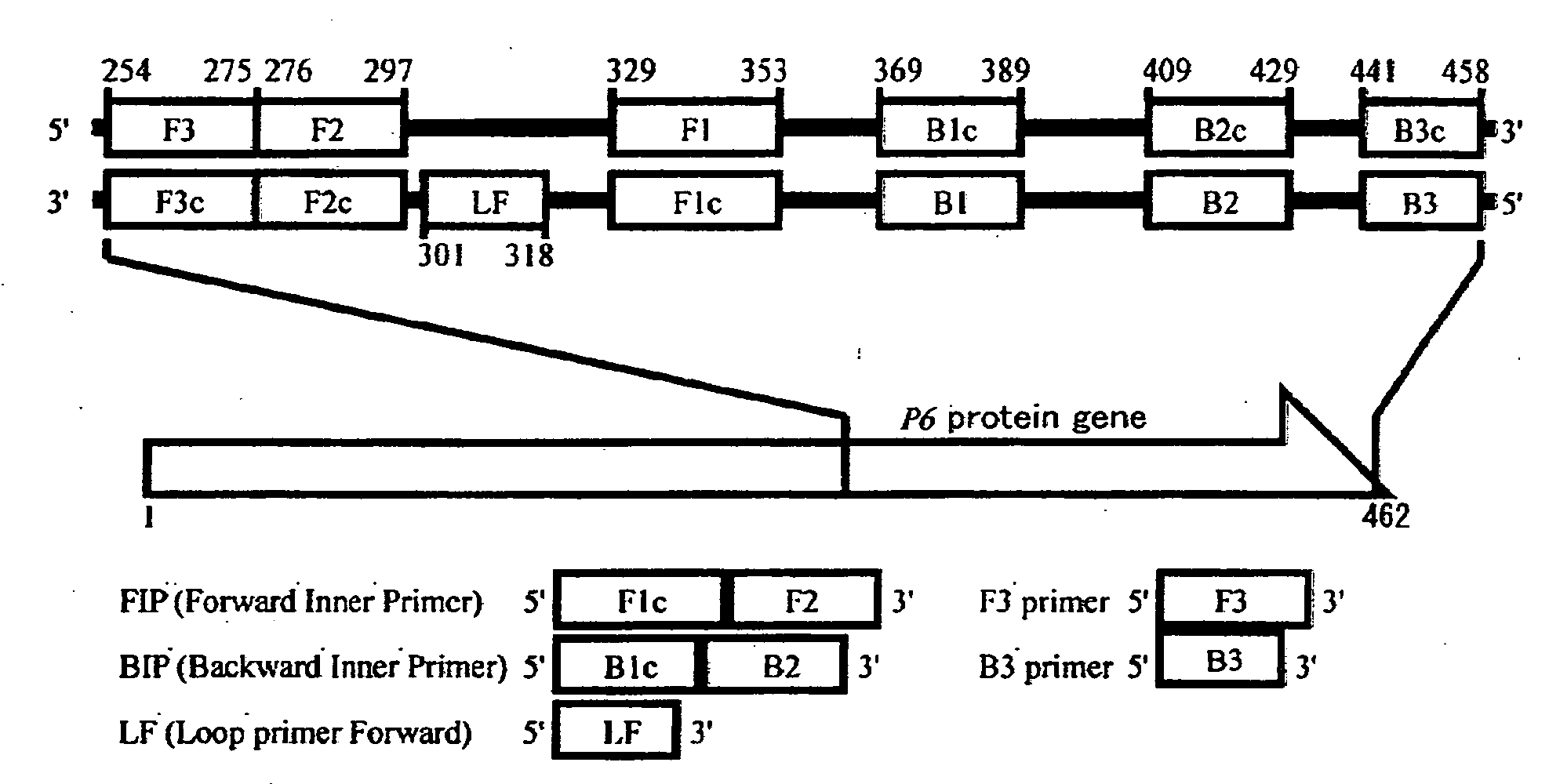
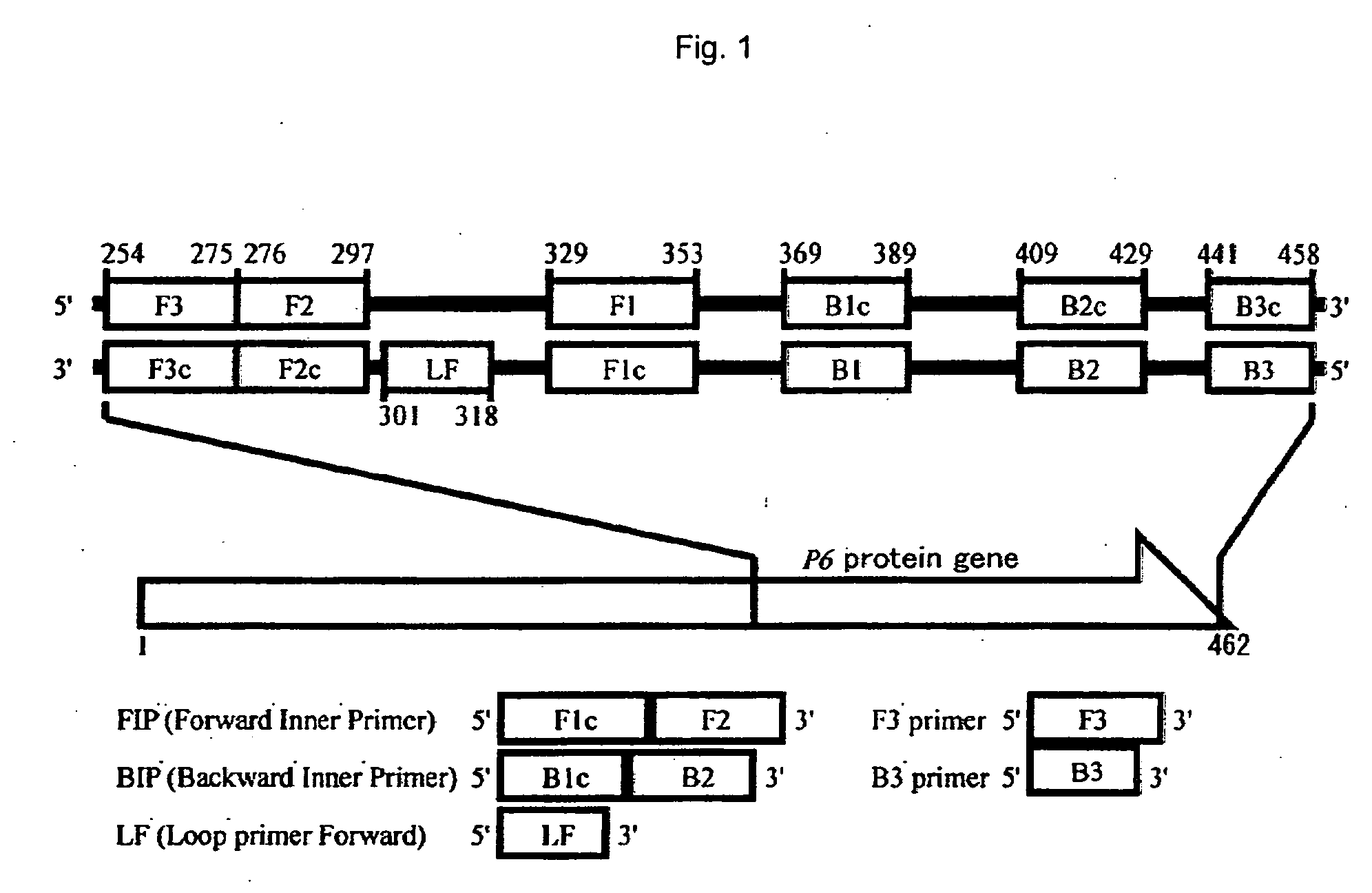
Method of detecting influenza bacillus, primer set for detection of influenza bacillus and kit for detection of influenza bacillus
InactiveUS20080305472A1Strong specificityQuick responseSugar derivativesMicrobiological testing/measurementHaemophilusSerotype
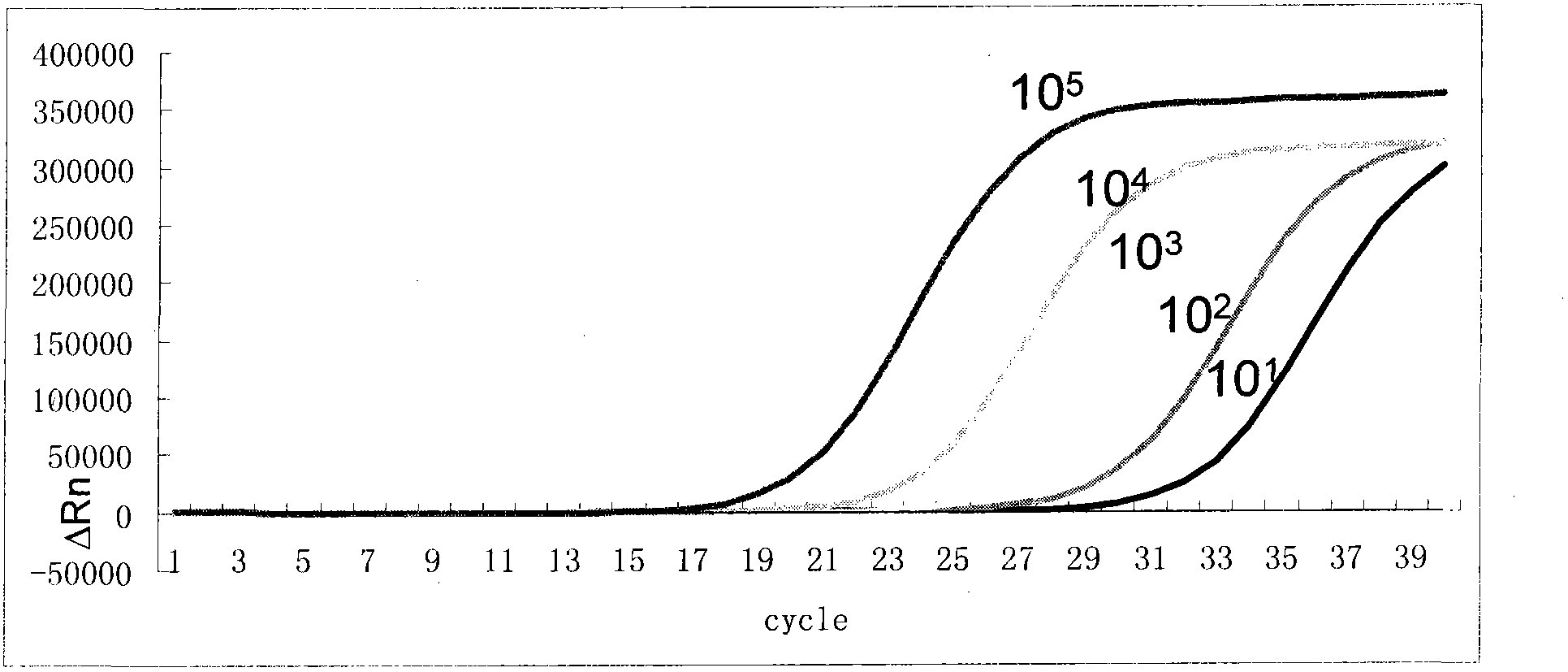
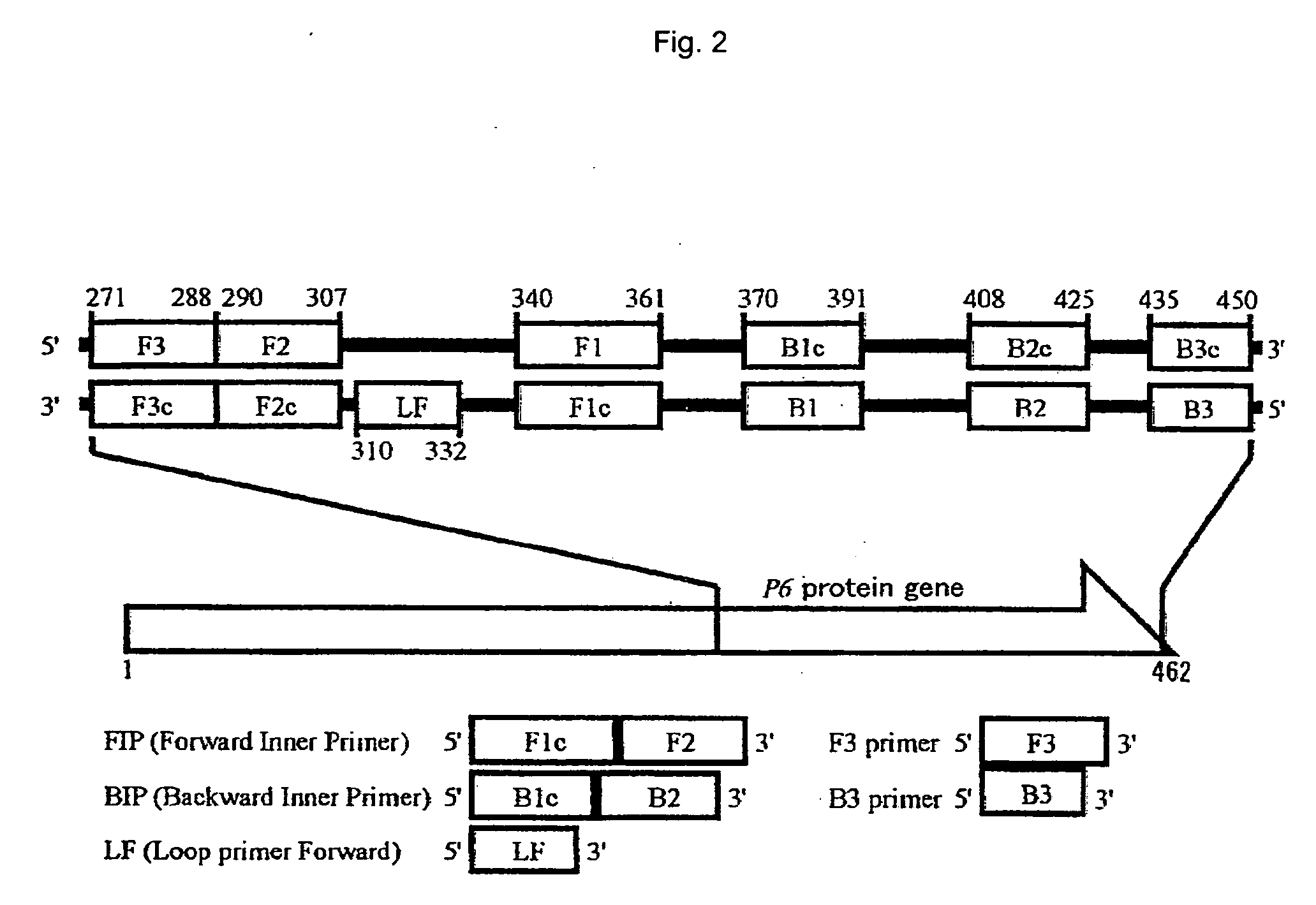
The present invention provides a method of detecting Haemophilus influenzae, which enables accurate and rapid detection of H. influenzae, a primer set for detecting H. influenzae, and a kit for detecting H. influenzae. Nucleic acid amplification is carried out using the DNA of H. influenzae as a template, and also using the LAMP primers as shown in SEQ ID NOS: 1 to 5 given as examples of the present invention. Thus, the presence or absence of the amplified product is detected. When primers having sequences that are complementary to the sequences as shown in SEQ ID NOS: 1 to 5 are used, such primers are excellent in terms of detection sensitivity and promptness of detection, as well as specificity. In addition, as another example of the present invention, nucleic acid amplification is carried out using LAMP primers as shown in SEQ ID NOS: 43 to 47, and the presence or absence of the amplified product is detected. Thereby, H. influenzae Type b can be distinguished from other capsular serotype and non-encapsulated type H. influenzae, and it can be detected rapidly, simply, and accurately.
Owner:NIHON UNIVERSITY
Method and kit for performing quick co-detection on anti-human Hi (Haemophilus influenzae) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling
ActiveCN104181301AFast separationIt has the effect of synergistic amplification of multiple signalsFluorescence/phosphorescenceSerum igeMagnetic bead
The invention discloses a method and a kit for performing quick co-detection on anti-human Hi (Haemophilus influenzae) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling. The kit consists of anti-human Hi antibody capturing nano magnetic beads with an anti-human Hi IgM and IgG antibody gathering function, anti-human IgM and IgG antibody nano probes labeled by multi-color quantum dots, quality control substances and a PBST buffering solution, wherein the quality control substances comprise a positive quality control substance and a negative quality control substance; the positive quality control substance is serum, in which anti-human Hi IgM and IgG antibodies of human Hi infected people are respectively positive; the negative quality control substance is serum, in which anti-human Hi IgM and IgG are respectively negative. The kit and the method have the advantages of simplicity, quickness and high sensitivity, and can be used for carrying out synchronous detection on the anti-human Hi IgM and IgG antibodies.
Owner:HUBEI UNIV OF TECH +1
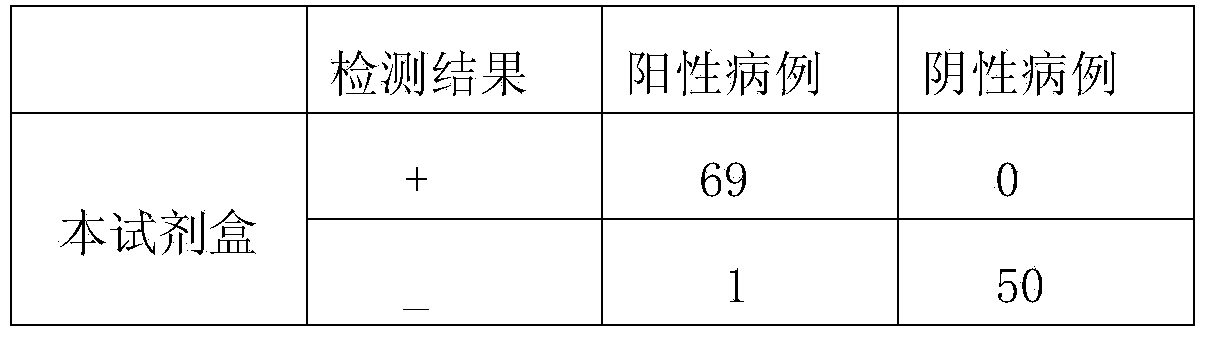
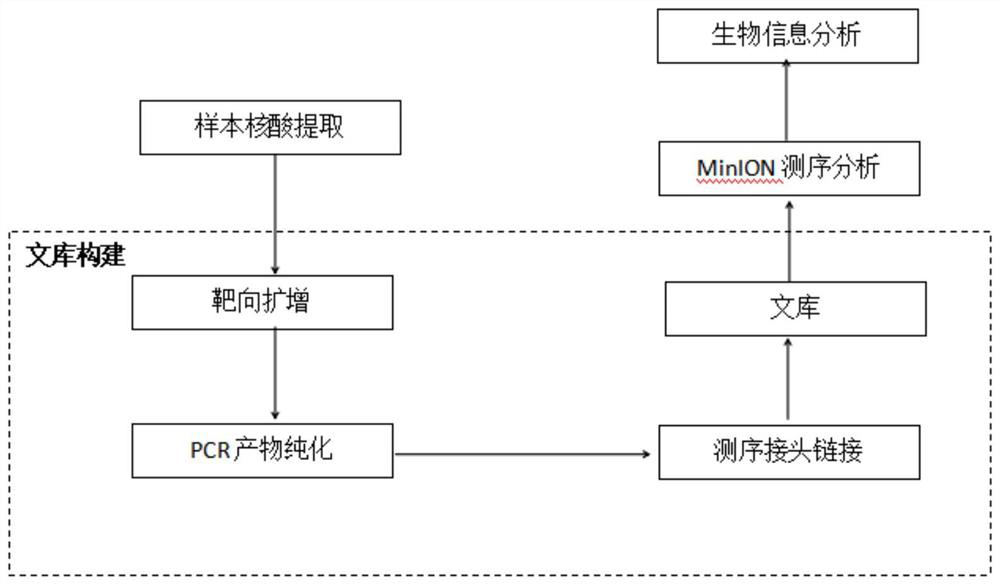
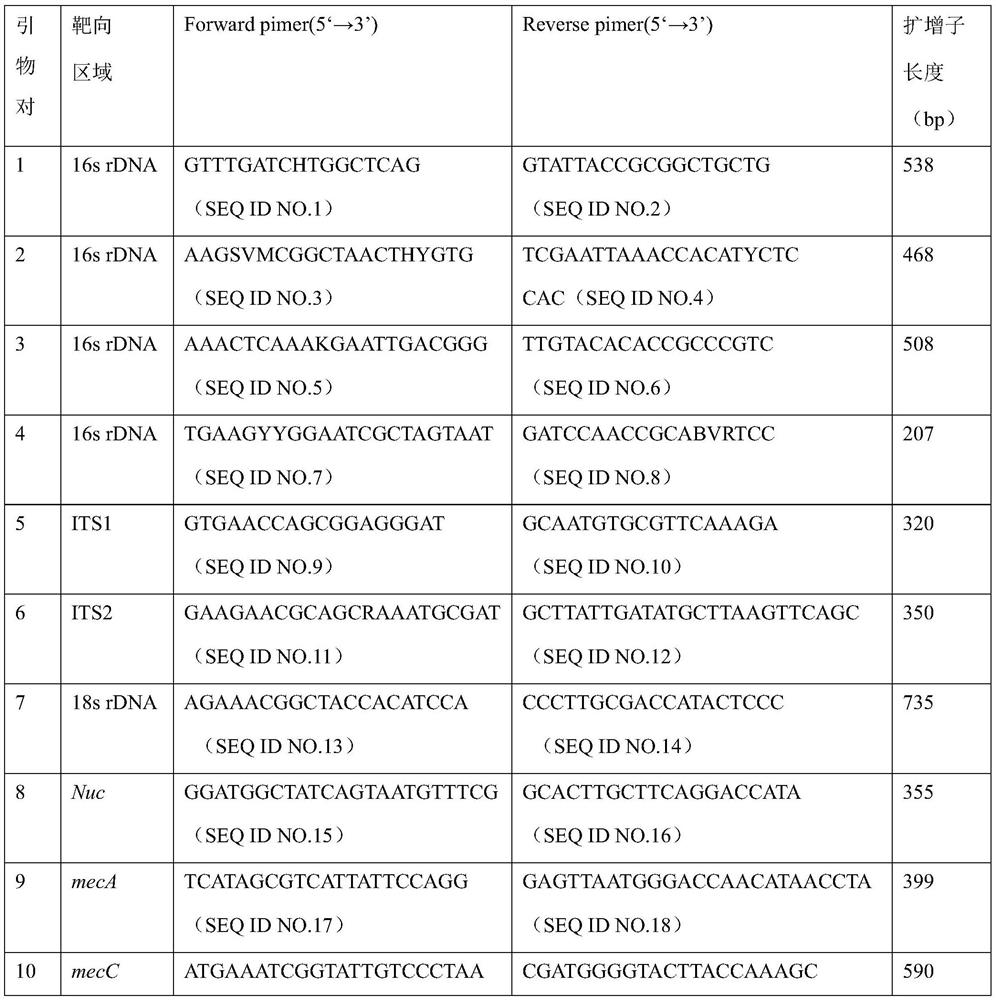
Primer group and kit for rapidly identifying respiratory tract microorganisms based on nanopore sequencing and application of primer group
ActiveCN112501268APrecise positioningImprove accuracyMicrobiological testing/measurementAgainst vector-borne diseasesPneumonitisEnterococcus
The invention discloses a primer group for detecting respiratory tract microorganisms based on a nanopore sequencing method. The nucleotide sequences of the primer group are as shown in SEQ ID NO:1-20. The microorganisms are streptococcus pneumoniae, staphylococcus aureus (resisting to methicillin), klebsiella pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, stenotrophomonas maltophilia, candida albicans, haemophilus influenzae, legionella pneumophila, enterococcus faecium, chlamydia psittaci, cryptococcus gattii, aspergillus fumigatus and pneumocystis jiroveci. According to the invention, sequencing optimization is carried out on different types of samples of the respiratory tract microorganisms, so that the detection method, the kit and the like are suitable for various typesof respiratory tract samples; and a targeted amplification method is adopted, so that the detection requirements of sputum, alveolar lavage and other sample types can be met. In addition, parallel sequencing can be performed, so that the flux of the detected samples is increased, the sequencing time is shortened, and the contradiction between the flux of detected pathogenic species and the cost and time is further relieved.
Owner:GUANGZHOU DARUI BIOTECH
Ceftizoxime sodium crystalline hydrate and preparation method and application thereof
InactiveCN101781316AReduce humidityEasy to makeAntibacterial agentsPowder deliveryJoint infectionsBacilli
The invention relates to a ceftizoxime sodium crystalline hydrate and a preparation method and application thereof. The ceftizoxime sodium crystalline hydrate has high storage stability, and is suitable for preparing medicaments for treating or preventing diseases such as infection of respiratory systems, liver and biliary systems, five sense organs and urinary tracts of human bodes or animals, infection of abdominal cavity, infection of pelvic cavity, ichorrhemia, infection of skin tissues, and infection of bones and joint caused by gram positive or negative bacteria, meningitis caused by streptococcus pneumonia or haemophilus influenza, and simple gonorrhea.
Owner:刘力
Primer combination for detecting PCR of Elizabethkingia meningoseptica
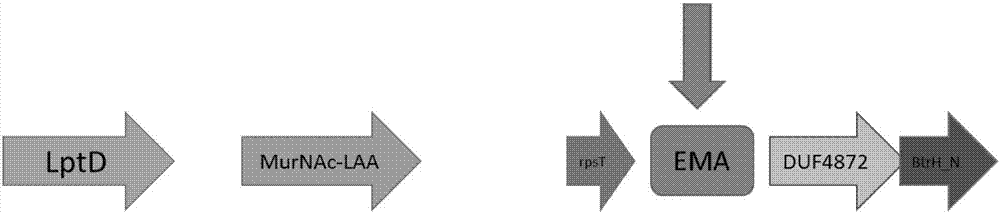
ActiveCN107083443AImprove featuresIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseStaphylococcus aureus
The invention discloses a primer sequence combination and a method for applying the primer sequence combination in PCR amplification. The amplification target in the method is specific fragments EMA inside the genome of Elizabethkingia meningoseptica, and the method shows excellent specificity and sensibility; and moreover, by using the method, the neisseria meningitidis, haemophilus influenzae, staphylococcus aureus, streptococcus pneumoniae, escherichia coli, listeria monocytogenes, mycoplasma pneumoniae, bordetella pertussis, klebsiella pneumoniae and mycobacterium tuberculosis and the other pathogenic bacteria which are likely to cause meningitis and diseases of the upper respiratory tract, and easy to be confused with the Elizabethkingia meningoseptica in an identification of bacteria, can be distinguished at one time. Additionally, the lower detection limit of the method can reach 10-4 ng per Mul.
Owner:ICDC CHINA CDC
Lavender cellulose fiber and preparation method thereof
PendingCN109267163AImprove antibacterial propertiesIncrease productivityMonocomponent cellulose artificial filamentCellulose/protein filament chemical after-treatmentStreptococcus pyogenesBreaking strength
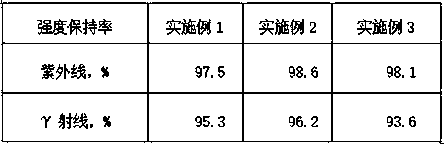
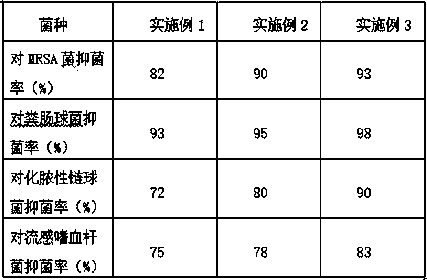
The invention provides lavender cellulose fiber. The lavender cellulose fiber is characterized in that the content of a lavender extract is 6.6-10.0%, the dry breaking strength is 3.7-4.5 cN / dtex andthe wet breaking strength is 2.5-3.2 cN / dtex. The invention further provides a preparation method for the lavender cellulose fiber. The preparation method comprises the steps of preparing a compound coordinating agent, preparing a modifying agent, carrying out mixing, carrying out spinning and carrying out after-treatment. According to the lavender cellulose fiber and the preparation method thereof, after a pure textile prepared from the lavender cellulose fiber is washed 20 times, the bacteriostasis rate of MRSA (Methicillin-resistant Staphylococcus aureus) is 82-93%, the bacteriostasis rateof enterococcus faecalis is 93-98%, the bacteriostasis rate of streptococcus pyogenes is 72-90%, and the bacteriostasis rate of haemophilus influenzae is 75-83%; and the lavender component is added into the fiber, so that the prepared fiber also has a part of functions of the fiber and has the effects of promoting cell regeneration, accelerating wound healing and ameliorating acne, abscess and eczema.
Owner:ZHONGKE TEXTILE RES INST QINGDAO CO LTD
Hi (Haemophilus influenza) fusion protein as well as construction method and application thereof
ActiveCN105175549AImproving immunogenicityAntibacterial agentsAntibody mimetics/scaffoldsConjugate vaccineHaemophilus
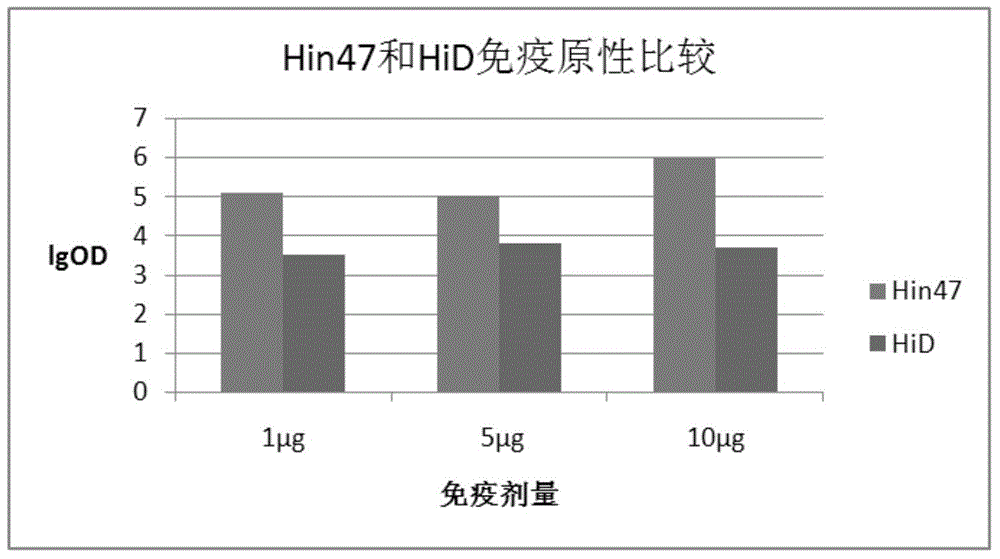
The invention provides haemophilus influenzae fusion protein as well as a construction method and an application thereof. According to the fusion protein, an HiD protein gene and an Hin47 protein gene form a fusion gene in a ratio of 1:1, and then a prokaryotic expression system is converted into a fusion gene expression system; the fusion protein can effectively enhance immunogenicity of single protein antigens of Hin47 and HiD, and as a conjugate vaccine protein carrier, Hin47-HiD can effectively enhance immunogenicity of polysaccharide antigens; a developed Hia and Hib divalent conjugate vaccine with fusion protein Hin47-HiD used as the conjugate vaccine protein carrier can prevent invasive and non-invasive Hi simultaneously.
Owner:CANSINO BIOLOGICS INC
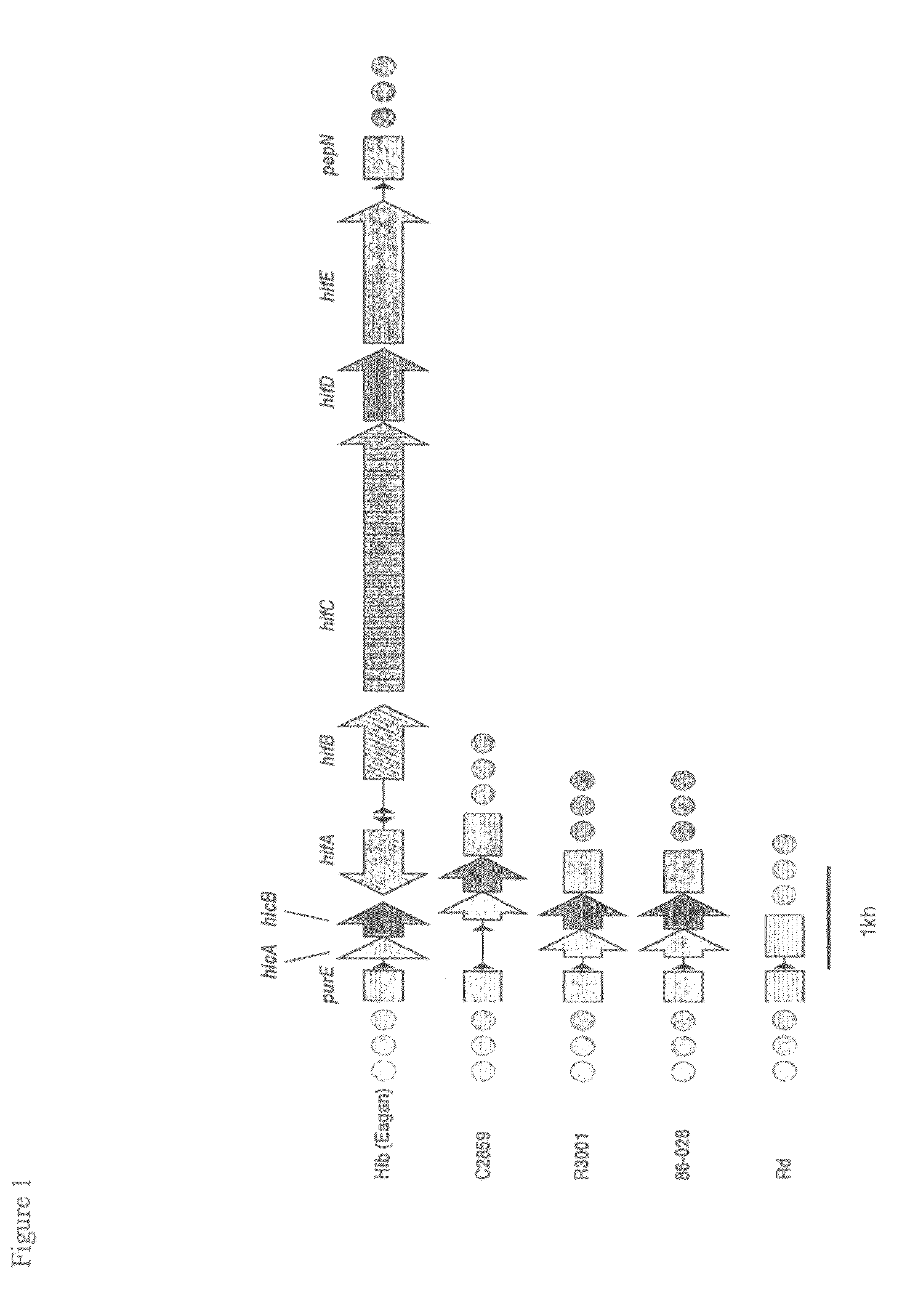
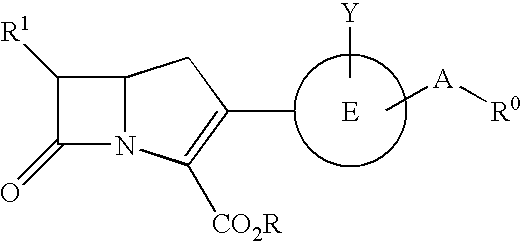
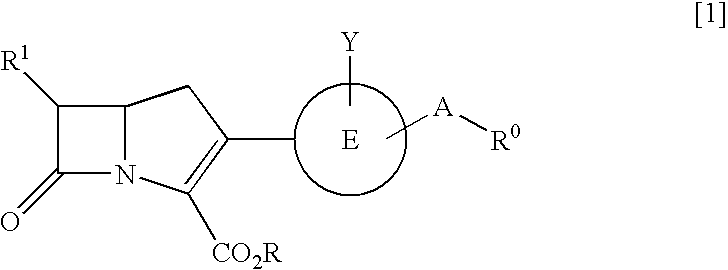
Carbapenem compounds
A compound or its pharmaceutically acceptable salt represented by the following formula:The invention is a carbapenem compound which has a potent antibacterial activity over a broad range of Gram negative and Gram positive bacteria, especially penicillin-resistant Streptococcus pneumoniae (PRSP) which has been isolated at an elevated frequency in recent years and thus causes a serious clinical problem, and Haemophilus influenzae which has acquired resistance against the existing β-lactam antibiotics over a wide scope due to penicillin-binding protein (PBP) mutations such as β-lactamase non-producing ampicillin-resistant (BLNAR) Haemophilus influenzae, and has excellent oral absorbability.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Haemophilus influenzae sialyltransferase and methods of use thereof
The present invention is directed to sialytransferases, such as SiaA sialytransferases isolated from Haemophilus influenzae. Further provided herein are methods for producing sialylated lipooligosaccharides, vaccines, and host cells and systems for the production of sialylated lipooligosaccharides.
Owner:UNIV OF IOWA RES FOUND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com