Hi (Haemophilus influenza) fusion protein as well as construction method and application thereof
一种流感嗜血杆菌、融合蛋白的技术,应用在疫苗产品开发领域,能够解决HiD相对分子量小、免疫原性弱等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Preparation of Hin47-HiD fusion protein
[0073] (1) Construction of Hin47-HiD expression vector
[0074] Lifetechnology company synthesized Hin47-HiD fusion gene, wherein the selected linker is G4S (GGGGS), the fusion gene each includes a molecule of Hin47 and a molecule of HiD, and its front end is Hin47, and the back end is HiD; the designed enzyme cutting site point, the front is NdeI, and the back is BamHI, wherein the amino acid sequence of Hin47 is the sequence shown in Sequence Listing 1, the amino acid sequence of Linker is the sequence shown in Sequence Listing 2, and the amino acid sequence of HiD is the sequence shown in Sequence Listing 2. For the sequences shown in List 3, see Table 1 below for details.
[0075] Table 1 Hin47-HiD fusion protein amino acid sequence
[0076]
[0077]
[0078] It can be seen from Table 1 that Hin47-HiD contains 790aa and has a molecular weight of 87KD.
[0079] After the fusion gene is double-digested with ...
Embodiment 2
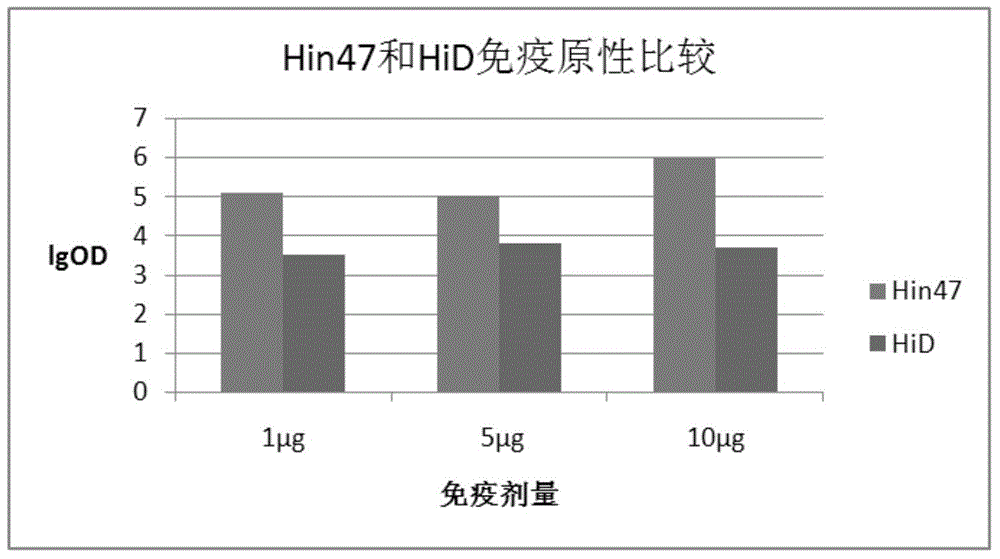
[0088] Example 2 Study on Immunogenicity of Hin47-HiD Fusion Protein
[0089] Hin47-HiD fusion protein was used to immunize BALB / C mice to study its immunogenicity. At the same time, Hin47 and HiD were used as controls to immunize three times with an interval of two weeks. Whole blood was collected 14 days after the last immunization to detect the antibody level in serum.
[0090] The immunization doses of Hin47 and HiD protein antigens in the control group were 1ug, 5ug and 10ug, while the effective doses of protein monomers in the fusion protein immunization group were 1ug, 5ug and 10ug.
[0091] Hin47 and HiD antigens were used to immunize mice with three doses of 1ug, 5ug and 10ug, and the antibody titers of the three immune sera were detected with the corresponding antigens. image 3 ,
[0092] Statistical Analysis:
[0093] P(Hin471ug:HiD1ug)=0.000<0.01
[0094] P(Hin475ug:HiD5ug)=0.000<0.01
[0095] P(Hin4710ug:HiD10ug)=0.000<0.01
[0096] The results showed that: at...
Embodiment 3
[0111] The preparation of embodiment 3Hia and Hib conjugate vaccine
[0112] The preparation of Hia polysaccharide and Hib polysaccharide-protein conjugates adopts the same test method. Specifically, after the capsular polysaccharide is added to CDAP for 30s, 0.2 MTEA is added to adjust the pH to 9.5 to activate the polysaccharide, and the activation lasts for 2.5 min. After activation, adjust the pH to 9.0, then add Hin47-HiD fusion protein according to the glycoprotein ratio of 1:1, react at room temperature for 1 hour, and overnight at 4°C, then add 2M glycine to terminate the reaction. After dialysis to remove the added reagents, the conjugate was separated and purified on CL-4B gel.
[0113] The gel separation results of Hia conjugate CL-4B are shown in Figure 6 .
[0114] The gel separation results of Hib conjugate CL-4B are shown in Figure 7 .
[0115] Table 2 Quality inspection results of Hia and Hib conjugate vaccines
[0116]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com