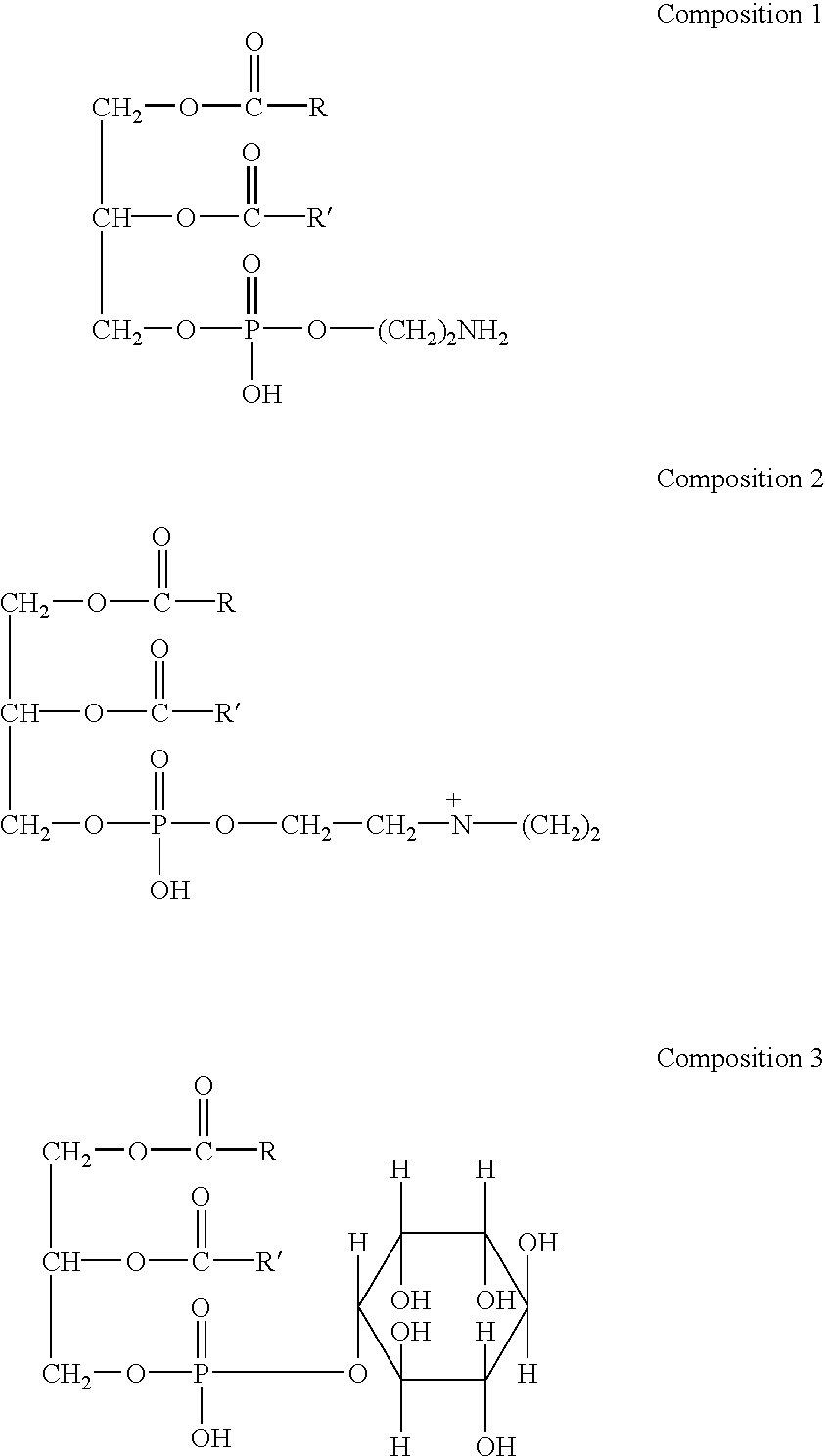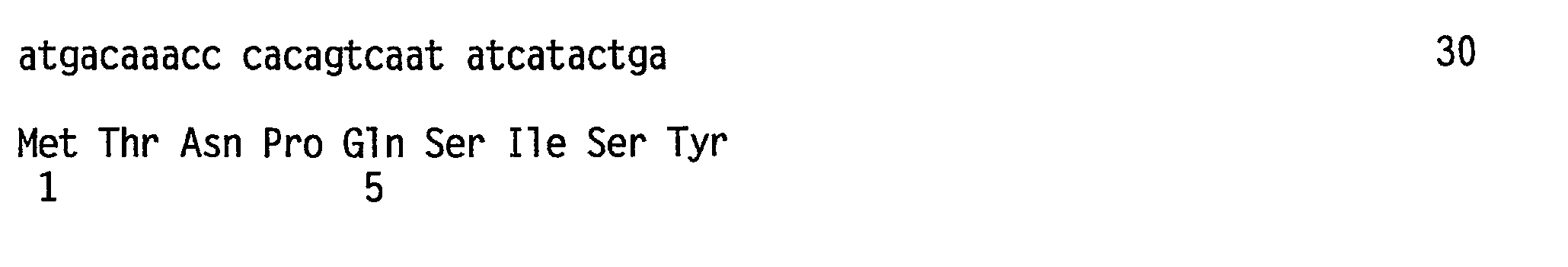Patents
Literature
613 results about "Protein amino acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Proteins are comprised of amino acids and more than one amino acid. For a protein to exist it takes a chain of various amino acids that are connected together in order to form the protein; in short: And it goes further than that: Try looking at each amino acid as part of a material list and protein as the end product.
In vivo incorporation of alkynyl amino acids into proteins in eubacteria
Owner:THE SCRIPPS RES INST
Rumen by-pass delivery system
InactiveUS20020127259A1Prevent oxidationEffective absorptionBiocideAnimal feeding stuffBiotechnologyNutrition
The present invention relates to a composition for the delivery of active agents that are protected from environmental oxidation and are resistant to ruminal fermentation degradation but not intestinal digestion and adsorption. The composition may contain biologically active ingredients such as for example, vitamins, minerals, proteins, amino acids, fatty acids, nutritional supplements, and pharmaceuticals together with natural and / or synthetic lecithin phospholipids.
Owner:ORTHOEFER FRAND T
Novel dipeptidyl peptidase iv (dp-iv) inhibitors as anti-diabetic agents
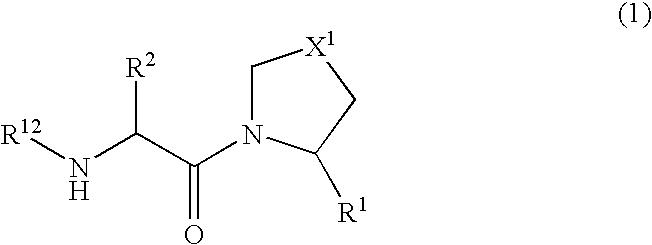
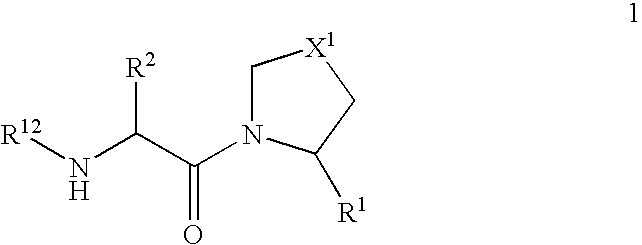
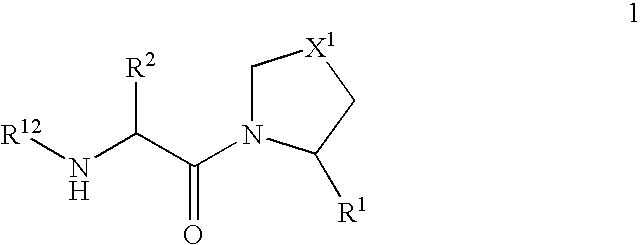
The present invention relates to a series of prodrugs of inhibitors of DP-IV with improved properties. The compounds can be used for the treatment of a number of human diseases, including impaired glucose tolerance and type II diabetes. The compounds of the invention are described by general formula (1); wherein R1 is H or CN; R2 is selected from CH2R5, CH2CH2R5 and C(R3)(R4)—X2—(CH2)aR5; R3 and R4 are each independently selected from H and Me; R5 is selected from CON(R6)(R7), N(R8)C(=0)R9, N(R8)C(═S)R9, N(R8)SO2R10 and N(R8)R10; R6 and R7 are each independently R11(CH2)b or together they are —(CH2)2-Z-(CH2)2— or CH2-o-C6H4-Z-CH2—; R8 is H or Me; R9 is selected from R11(CH2)b, R11(CH2)bO and N(R6)(R7); R10 is R11(CH2)b; R11 is selected from H, alkyl, optionally substituted aryl, optionally substituted aroyl, optionally substituted arylsulphonyl and optionally substituted heteroaryl; R12 is selected from H2NCH(R13)CO, H2NCH(R14)CONHCH(R15)CO, C(R16)═C(R17)COR18 and R19OCO; R13, R14 and R15 are selected from the side chains of the proteinaceous amino acids; R16 is selected from H, lower alkyl (C1-C6) and phenyl; R17 is selected from H and lower alkyl (C1-C6); R18 is selected from H, lower alkyl (C1-C6), OH, O-(lower alkyl (C1-C6)) and phenyl; R19 is selected from lower alkyl (C1-C6), optionally substituted phenyl and R20C(=0)OC(R21)(R22); R20, R21 and R22 are each independently selected from H and lower alkyl (C1-C6); Z is selected from a covalent bond, —(CH2)c—, —O—, —SOd— and —N(R10)—; X1 is S or CH2; X2 is O, S or CH2; a is 1, 2 or 3; b is 0-3; c is 1 or 2; and d is 0, 1 or 2.
Owner:FERRING BV
Compositions and methods for diagnosing and treating conditions, disorders, or diseases involving cell death
InactiveUS6277974B1Reduce diseaseMany timesBiocidePeptide/protein ingredientsNervous systemDisease cause
The present invention relates to compositions and methods for the treatment and diagnosis of conditions, disorders, or diseases involving cell death. The invention encompasses protective nucleic acids which, when introduced into a cell predisposed to undergo cell death or in the process of undergoing cell death, prevent, delay, or rescue the cell from death relative to a corresponding cell into which no exogenous nucleic acids have been introduced. The invention encompasses nucleic acids of the protective sequence, host cell expression systems of the protective sequence, and hosts that have been transformed by these expression systems, including transgenic animals. The invention also encompasses novel protective sequence products, including proteins, polypeptides and peptides containing amino acid sequences of the proteins, fusion proteins of proteins, polypeptides and peptides, and antibodies directed against such gene products. The invention further relates to target sequences, including upstream and downstream regulatory sequences or complete gene sequences, antibodies, antisense molecules or sequences, ribozyme molecules, and other inhibitors or modulators directed against such protective sequences, protective sequence products, genes, gene products, and / or their regulatory elements involved in cell death. The present invention also relates to methods and compositions for the diagnosis and treatment of conditions, disorders, or diseases, involving cell death, including, but not limited to, treatment of the types of conditions, disorders, or diseases, which can be prevented, delayed or rescued from cell death and include, but are not limited to, those associated with the central nervous system, including neurological and psychiatric conditions, disorders, or diseases, and those of the peripheral nervous system. Further, the invention relates to methods of using the protective sequence, protective sequence products, and / or their regulatory elements for the identification of compounds that modulate the expression of the protective sequence and / or the activity of the protective sequence product. Such compounds can be useful as therapeutic agents in the treatment of various conditions, disorders, or diseases involving cell death.
Owner:COGENT NEUROSCI
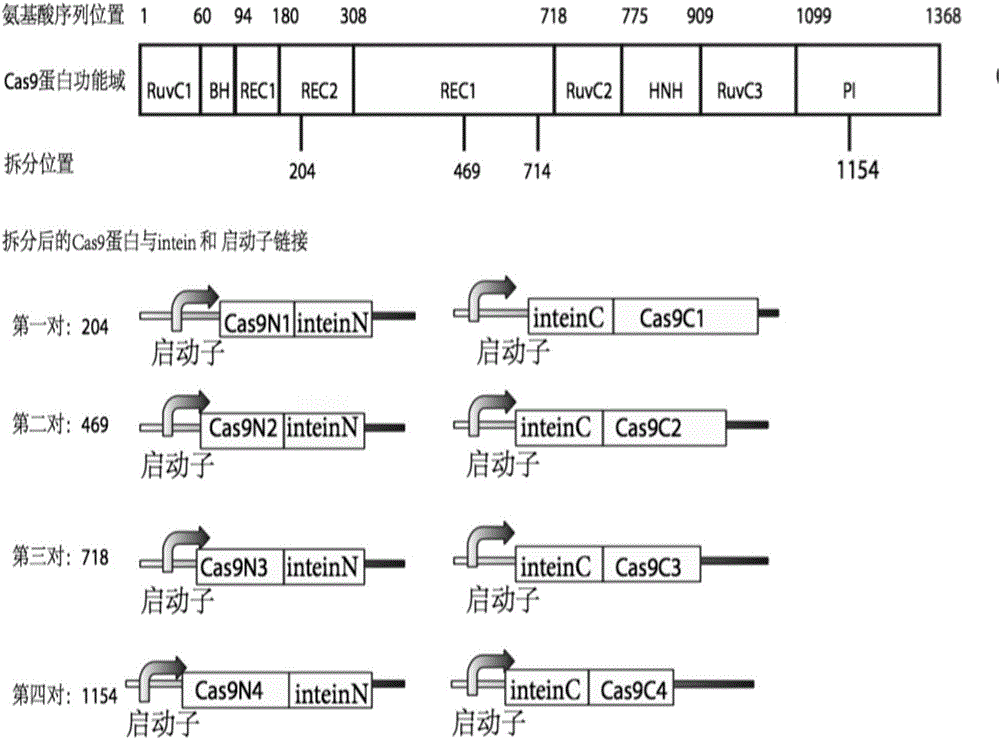
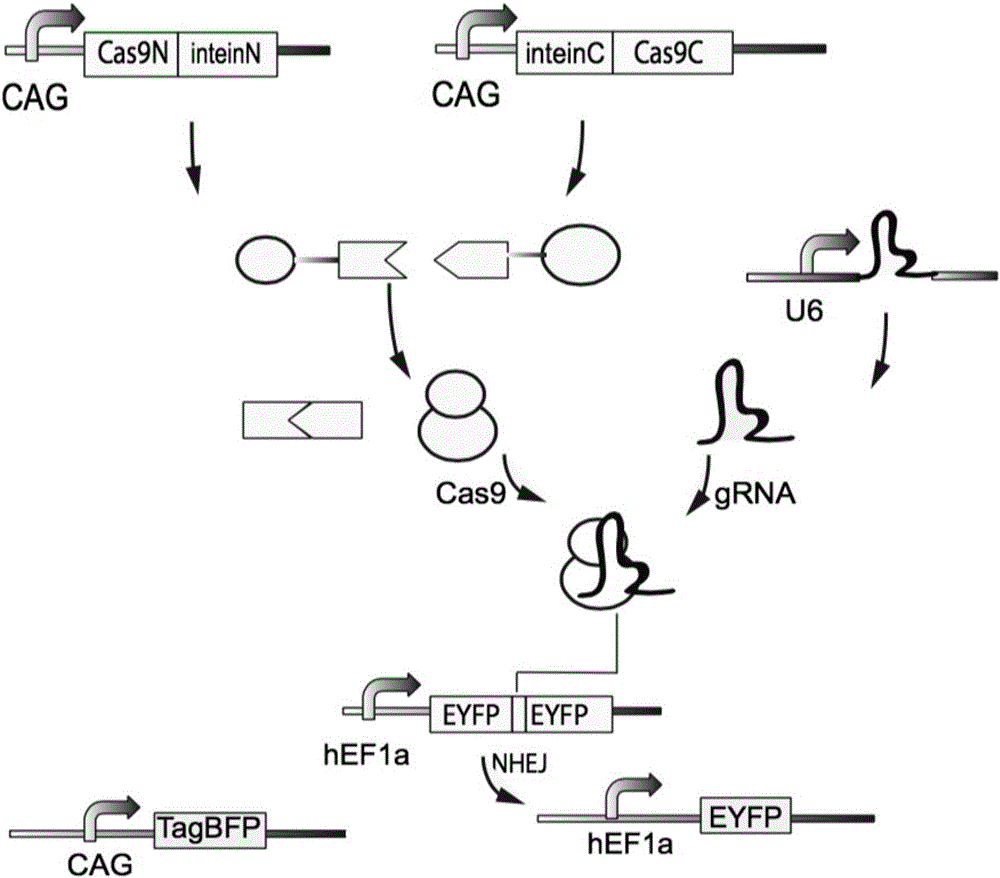
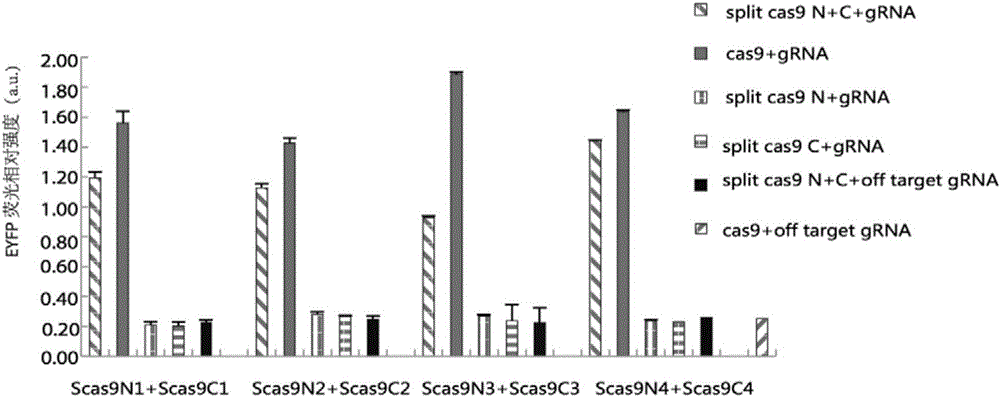
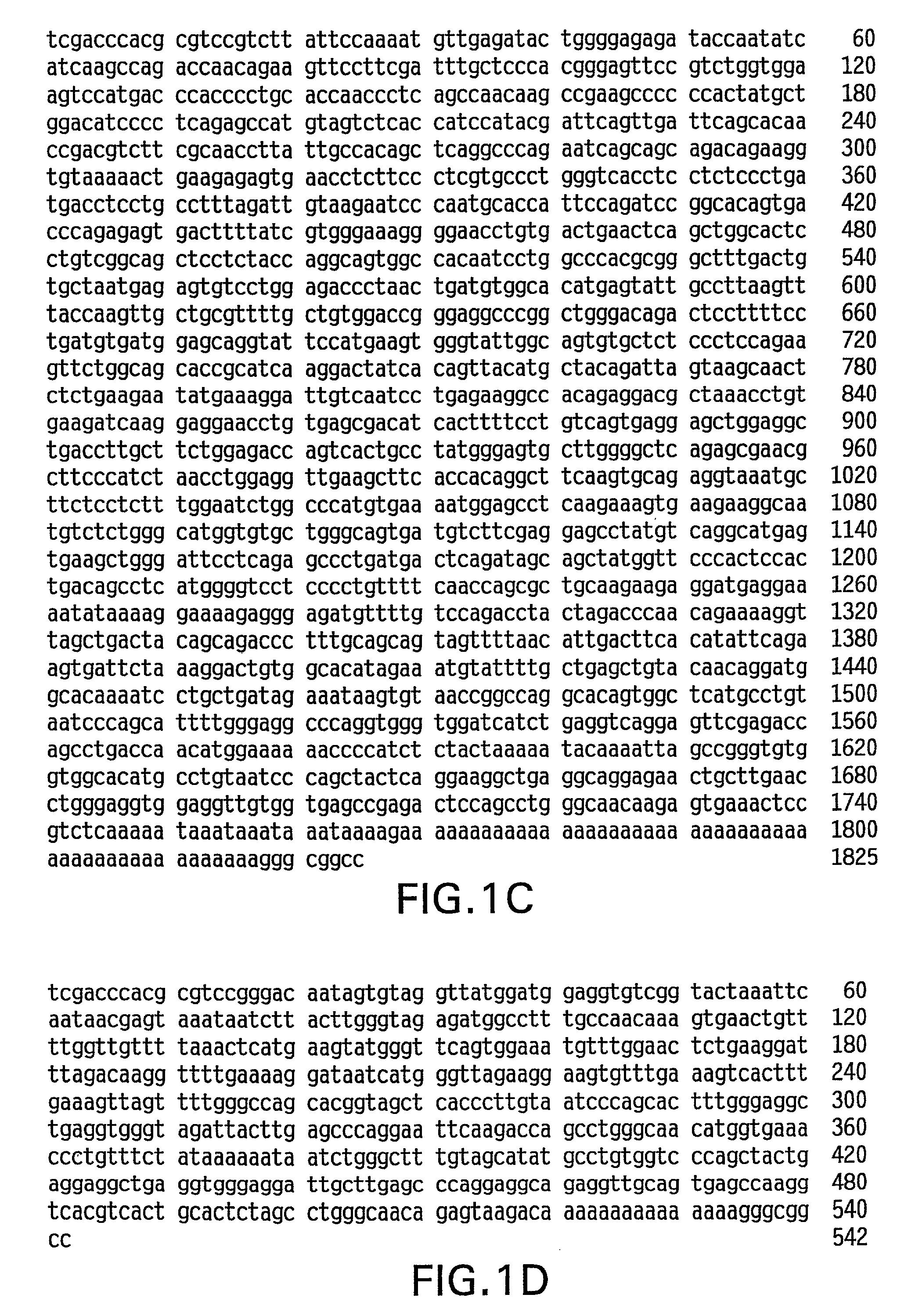
Method for carrying out gene editing and expression regulation by utilizing Cas splitting system
Owner:TSINGHUA UNIV
Compositions and methods for diagnosing and treating conditions, disorders, or diseases involving cell death
The present invention relates to compositions and methods for the treatment and diagnosis of conditions, disorders, or diseases involving cell death. The invention encompasses protective nucleic acids which, when introduced into a cell predisposed to undergo cell death or in the process of undergoing cell death, prevent, delay, or rescue the cell from death relative to a corresponding cell into which no exogenous nucleic acids have been introduced. The invention encompasses nucleic acids of the protective sequence, host cell expression systems of the protective sequence, and hosts that have been transformed by these expression systems, including transgenic animals. The invention also encompasses novel protective sequence products, including proteins, polypeptides and peptides containing amino acid sequences of the proteins, fusion proteins of proteins, polypeptides and peptides, and antibodies directed against such gene products. The invention further relates to target sequences, including upstream and downstream regulatory sequences or complete gene sequences, antibodies, antisense molecules or sequences, ribozyme molecules, and other inhibitors or modulators directed against such protective sequences, protective sequence products, genes, gene products, and / or their regulatory elements involved in cell death. The present invention also relates to methods and compositions for the diagnosis and treatment of conditions, disorders, or diseases, involving cell death, including, but not limited to, treatment of the types of conditions, disorders, or diseases, which can be prevented, delayed or rescued from cell death and include, but are not limited to, those associated with the central nervous system, including neurological and psychiatric conditions, disorders, or diseases, and those of the peripheral nervous system. Further, the invention relates to methods of using the protective sequence, protective sequence products, and / or their regulatory elements for the identification of compounds that modulate the expression of the protective sequence and / or the activity of the protective sequence product. Such compounds can be useful as therapeutic agents in the treatment of various conditions, disorders, or diseases involving cell death.
Owner:COGENT NEUROSCI
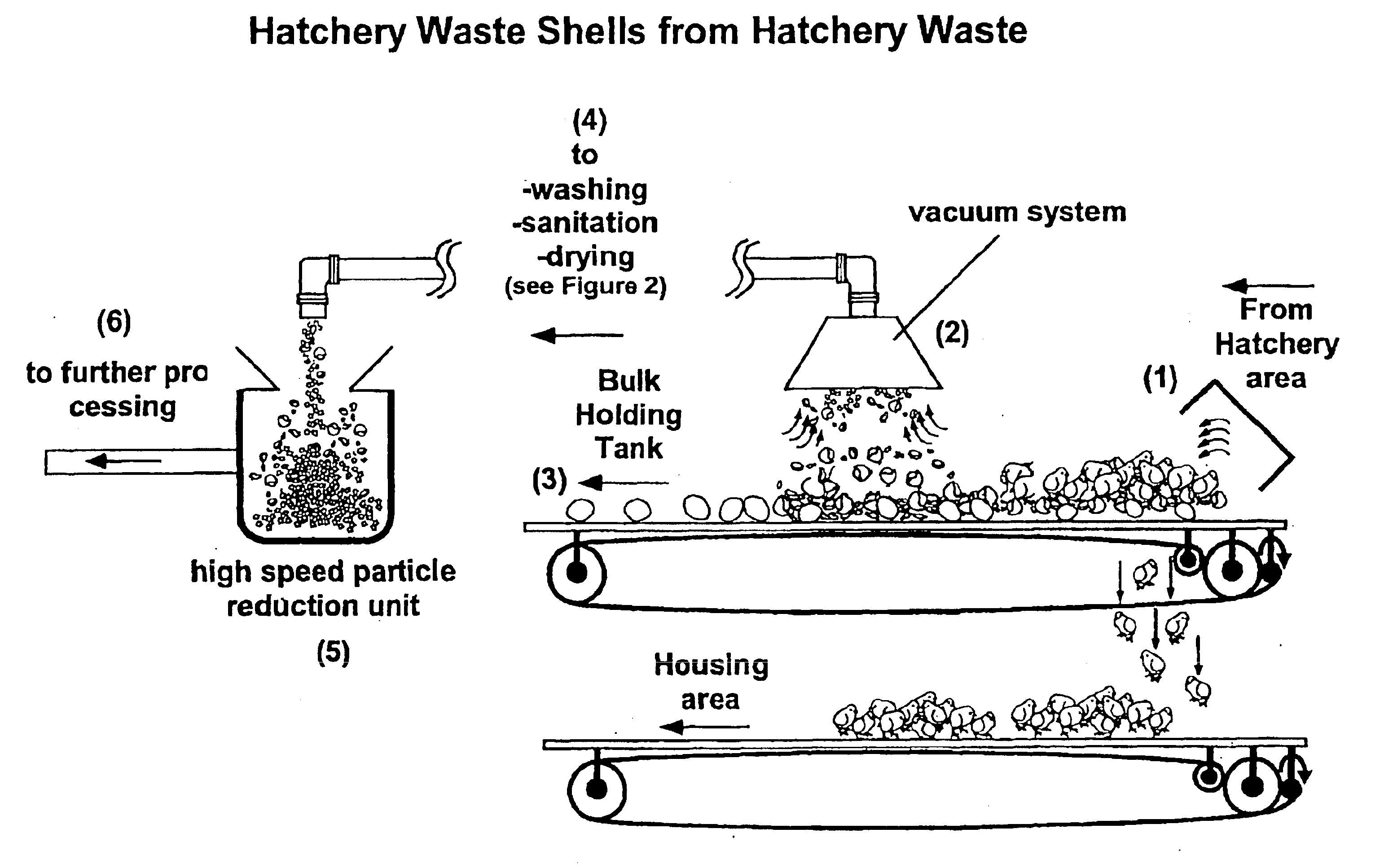
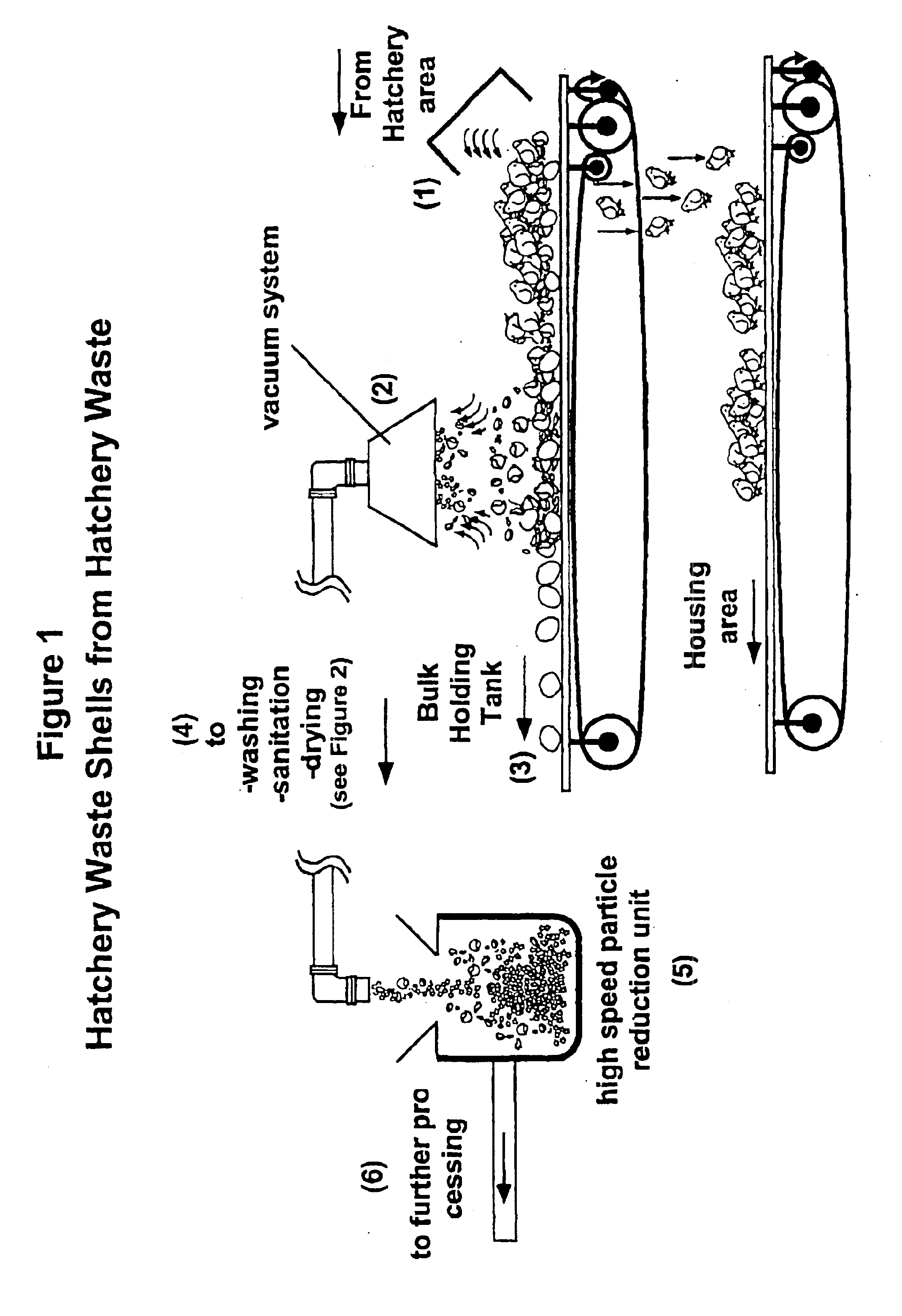
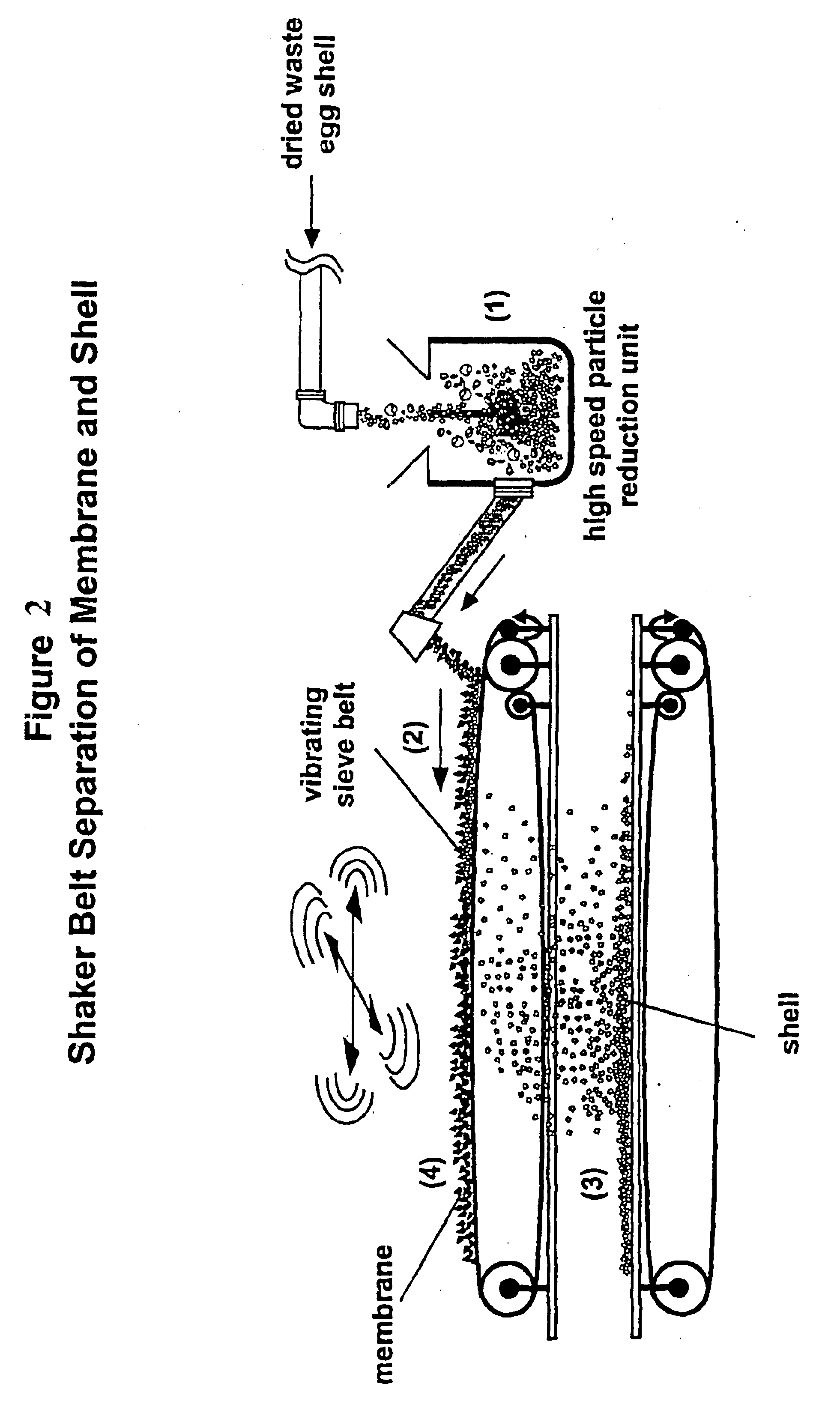
Hatchery eggshell waste processing method and device
InactiveUS6899294B2Reduce environmental impactEconomic benefitVitamin food ingredientsPeptide/protein ingredientsWaste processingEggshell
A method and apparatus for separating the organic membrane portion of waste egg shells from the hard inorganic mineral portion thereof, so that each can be used or further processed as desired, thereby addressing the environmental and economic issues associated with disposing of waste egg shells. The membrane and shell are separated by first processing waste eggshells so as to yield small waste eggshell particles. During this processing, the waste eggshell particles are at least slightly abraded, whereby the linking structure between the membrane and shell is at least partially disrupted. Thereafter, the waste eggshell particles are conveyed to a device, which isolates the two components based on their size and weight differences. The methods listed above can provide products from the eggshell waste including precipitated calcium carbonate, membrane, protein, amino acids, collagen and other important components.
Owner:PENN STATE RES FOUND
Nutrient enrichment selenium-enriched rice and cultivation method thereof
ActiveCN103120107AGood selenium enrichment effectHigh nutritional valueBiocidePlant growth regulatorsNutrient solutionSeedling
The invention relates to nutrient enrichment selenium-enriched rice and a cultivation method of the nutrient enrichment selenium-enriched rice. A nutrient solution which is adopted comprises a selenium material, protein, amino acid, microelement, an energy accelerator, an enhancer, a reaction accelerator, active water, a penetrating agent, a protective agent, a buffer agent, emulgator and the like, during a rice seedling period, a heading to flowering period and a grouting fructicative period, the diluted selenium nutrient solution is evenly sprinkled on leaf surfaces of paddies. After the process, the selenium content of the nutrient enrichment selenium-enriched rice can be 189 micrograms per kilogram, and the yield per mu can be improved by about 5% to 10%.
Owner:谢超
Anti-aging skincare mask containing crithmum maritimum
InactiveCN107334725ALow freezing pointEasy to synthesizeCosmetic preparationsToilet preparationsFennel extractCentella asiatica extract
The invention discloses an anti-aging skincare mask containing crithmum maritimum. The anti-aging skincare mask is prepared from a skin conditioner component, an auxiliary component and water, wherein the skin conditioner component is prepared from 2%-6% by mass of butanediol, 2%-5% by mass of glycerin, 0.1%-0.5% by mass of a crithmum maritimum extract, 1%-5% by mass of a pseudoalteromonas fermentation product extract, 1%-5% by mass of acetyl hexapeptide-8, 0.5%-3% by mass of a Centella asiatica extract, 0.5%-2% by mass of D-panthenol, 0.03%-0.1% by mass of sodium hyaluronate, 1%-5% by mass of Chamomile German water, 0.5%-3% by mass of corn protein amino acids, 1%-3% by mass of a lotus extract, 0.5%-2% by mass of bio-saccharide gum-1 and 0.1%-0.3% by mass of allantoin. The anti-aging skincare mask containing crithmum maritimum can inhibit lipid peroxidation and inflammatory reaction, rebuild water balance and brighten complexion and has effects of preserving moisture, resisting aging, brightening the complexion and realizing anti-allergic repair.
Owner:广州蜜妆生物科技有限公司
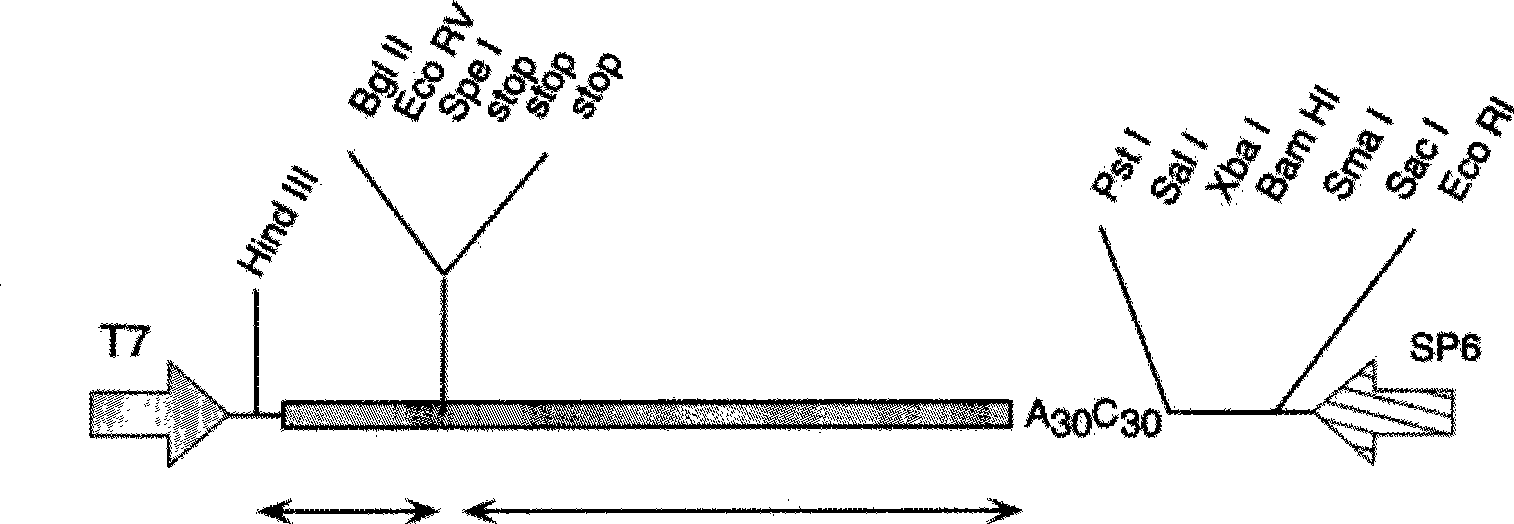
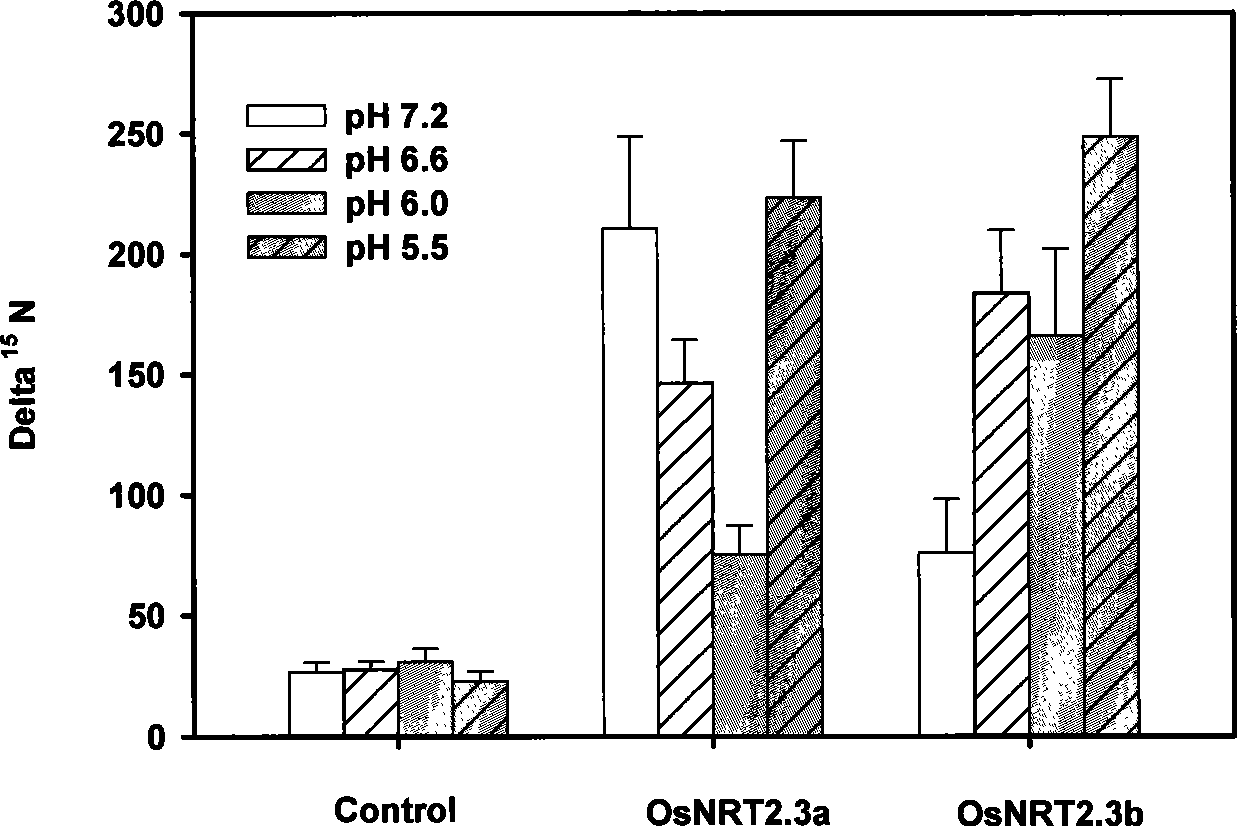
Genetic engineering application of nitrate transport protein genom OsNRT2.3 in Rice
ActiveCN101392257AImprove adaptabilityVerification functionPlant peptidesFermentationBiotechnologyHeterologous
The invention discloses a genetic engineering application of nitrate transport protein genome OsNRT2.3 of paddy rice, pertaining to the genetic engineering field. The nucleotide sequence of the nitrate transport protein genome OsNRT2.3 of paddy rice is SEQ ID NO.1, and OsNRT2.3a protein amino acid sequence expressed thereby is SEQ ID NO.2 and OsNRT2.3b protein amino acid sequence expressed thereby is SEQ ID NO.3. The gene of the invention is a first report on paddy rice. By participation in the transport of nitrate of paddy rice and especially under the condition short of nitrogen and poor pHenvironment, the analysis of mRNA expression shows that OsNRT2.3a is induced by low nitrate nitrogen so that the expression only occurs in lateral roots; and OsNRT2.3b has expressions in root system and parts over the ground and is responsible for the transport of nitrate. The OsNRT2.3a and OsNRT2.3b proteins are expressed in a frogspawn heterogenous system and determined as high compatible nitrate transport protein. A pH regulation and control site is found to exist in both proteins which are regulated and controlled by pH. Under low nitrogen condition, OsNRT2.3 transgenic plant shows higherefficiency in transport of nitrate, thus improving the utilization efficiency of nitrogen and the ultimate output.
Owner:NANJING AGRICULTURAL UNIVERSITY
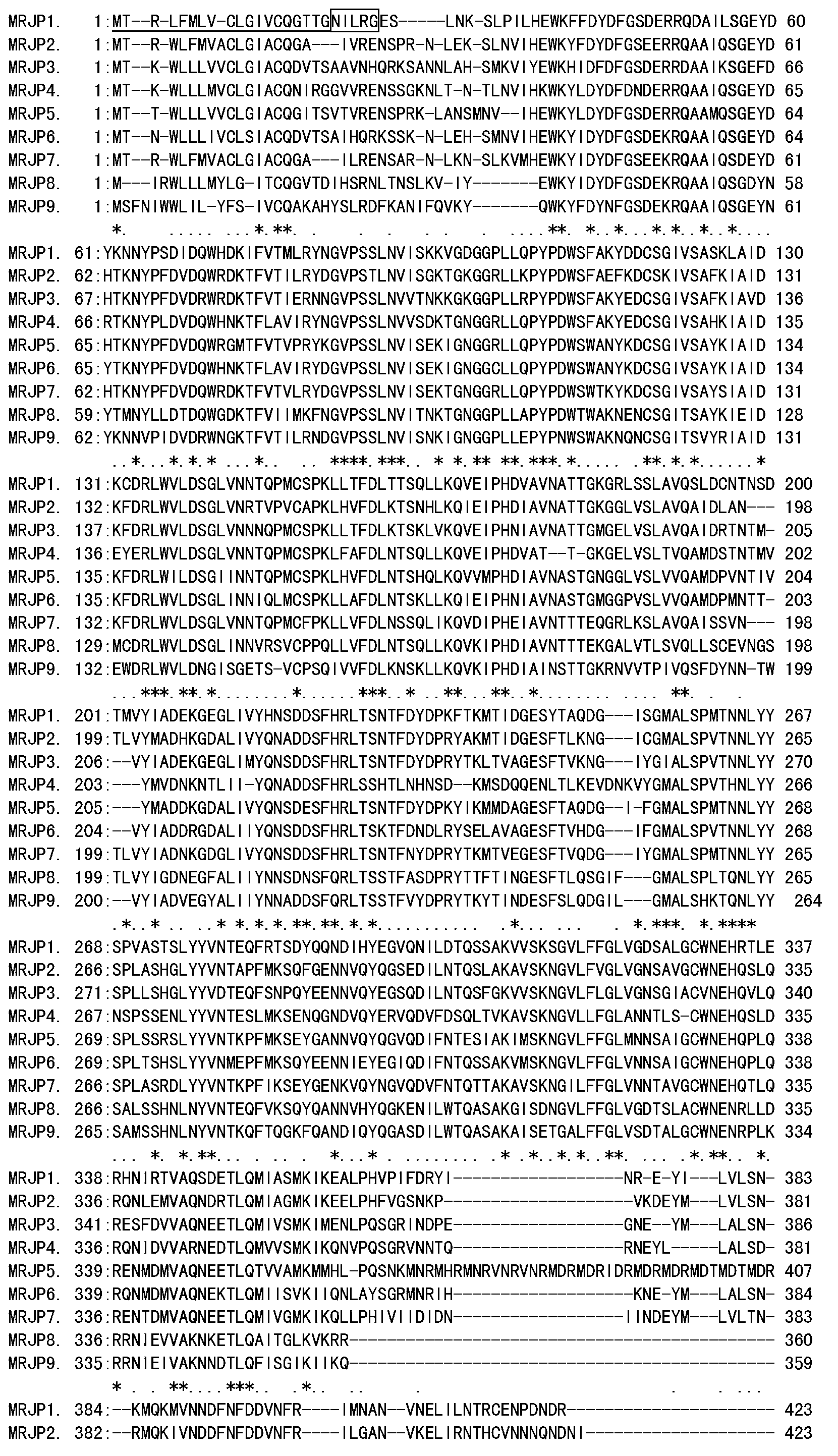
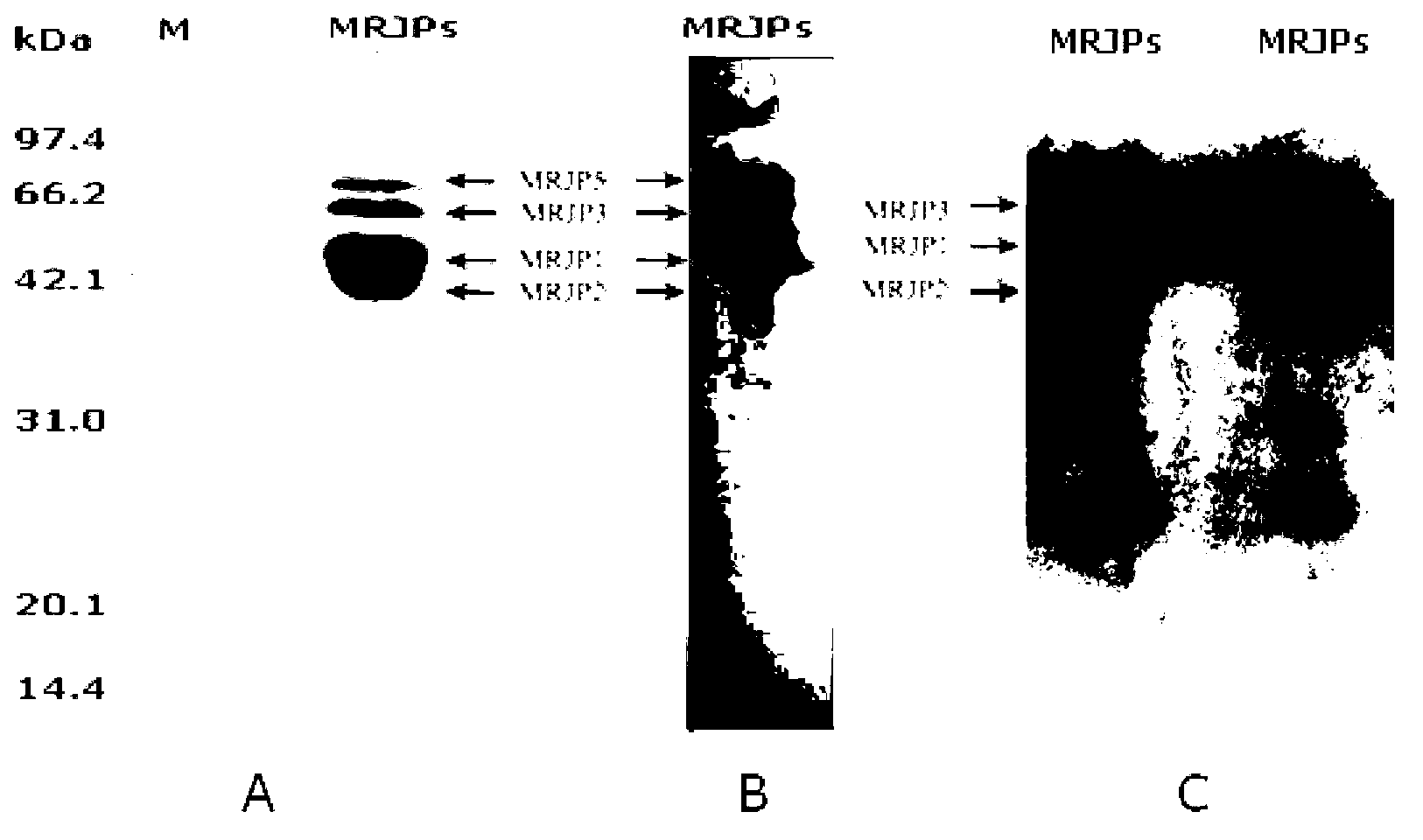
A specific antibody of major royal jelly protein MRJP1 and a preparation method thereof and Elisa quantitative detection thereof
ActiveCN103059135AStrong specificityIndividual bigSerum immunoglobulinsImmunoglobulins against animals/humansNew Zealand white rabbitSpecific antibody
The invention discloses a specific antibody of major royal jelly protein MRJP1 and a preparation method thereof and Elisa quantitative detection thereof. First, homology analysis is performed to amino acid sequences of proteins of all members of the Apismellifera major royal jelly protein MRJPs family (MRJP1-MRJP9) to select a specific polypeptide amino acid sequence unlike other MRJPs family members in MRJP1. The related specific polypeptide is synthesized by using a chemical method, and is used as an antigen to immunize New Zealand white rabbits; taking serum, and performing Elisa assay obtaining polyclonal antibody R2 with a relatively high titer, then purifying the antibody by using an affinity column prepared from the synthesized MRJP1 polypeptide. The titer of antibody R2 is detected via Elisa assay by using MRJP1 as the antigen, and the titer of the antibody is greater than 1:20000. The present invention provides a very reliable new rapid detection method for the qualitative and quantitative detection of MRJP1 in royal jelly, and also provides a very reliable technical means for quality control, freshness detection, and identification of genuine products of royal jelly and honey products for bee product quality supervision departments and processing and trading enterprises.
Owner:ZHEJIANG UNIV
Intra-intestinal nutrient emulsion for tumor patients
InactiveCN103609933ANutritional balanceRecovery functionMetabolism disorderEmulsion deliveryNutritionTryptophan
The invention belongs to the medicine field, and specifically relates to an intra-intestinal nutrient emulsion for tumor patients. The emulsion is characterized by comprises the following effective components: protein, fat, carbohydrate, total dietary fiber, green tea theine, taurine, L-carnitine, composite vitamins and mineral substances, and water. The protein is provided in an amino acid powder form, the formula of the amino acid powder is adjusted on the basis of 20 kinds of protein amino acids, wherein methionine is replaced by homocysteine, glutamine and glutamic acid are not added, the tryptophan content is low, and the content of branched chain amino acid reaches 35% or more; fat is provided in a saturated aliphatic acid or unsaturated fatty acid form, wherein in the unsaturated aliphatic acid the value of omega-6 / omega-3 is equal to 2-6 / 1; and the carbohydrate is provided in a form of glycerin, glucose, fructo-oligosaccharide or lentinan. Besides one prominent characteristic of the emulsion is that the emulsion is a high-fat and low-sugar type, and the ratio of sugar to fat is 1:1. The emulsion formula is adjusted according to the nutrient and metabolism requirements of tumor patients so as to develop an individualized intra-intestinal nutrient preparation for tumor patients, the preparation can provide balanced nutrients for tumor patients, effectively cures the malnutrition symptom, improves the life quality of patients, and can also be used to assist the tumor treatment.
Owner:湖北一半天制药有限公司
Method for preparing active algae extract foliar fertilizer
Owner:ZHEJIANG OCEAN UNIV
Mutation type and mutation frequency of hereditary hearing loss gene SLC26A4 in Chinese crowd and usage of mutation type
InactiveCN101445799AImprove diagnostic efficiencyMicrobiological testing/measurementFermentationMutation frequencyCrowds
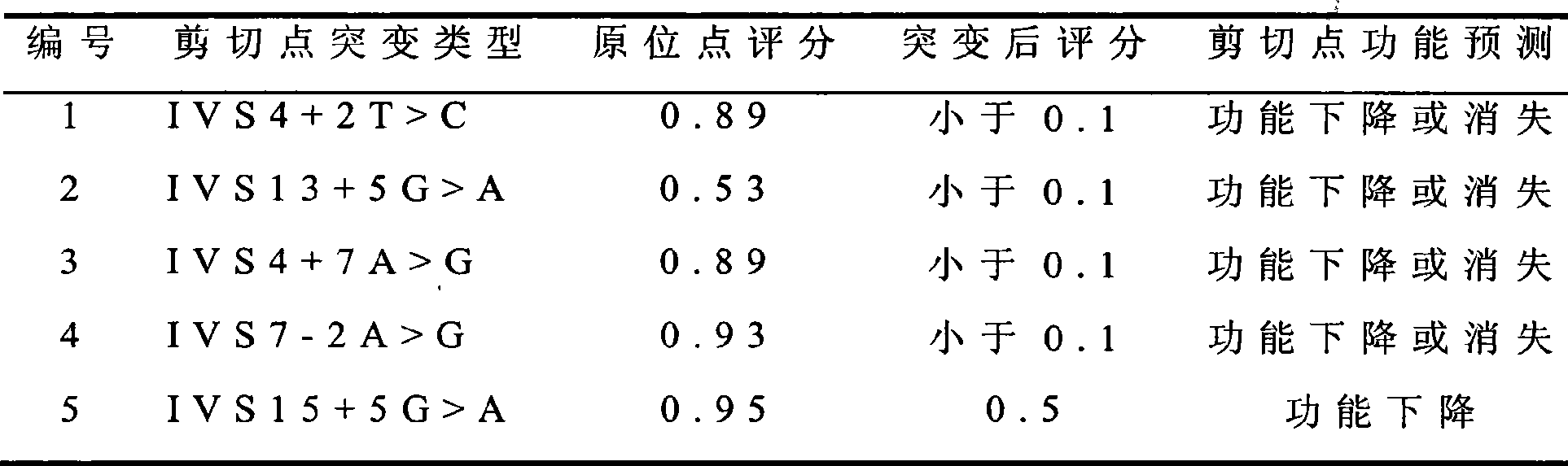
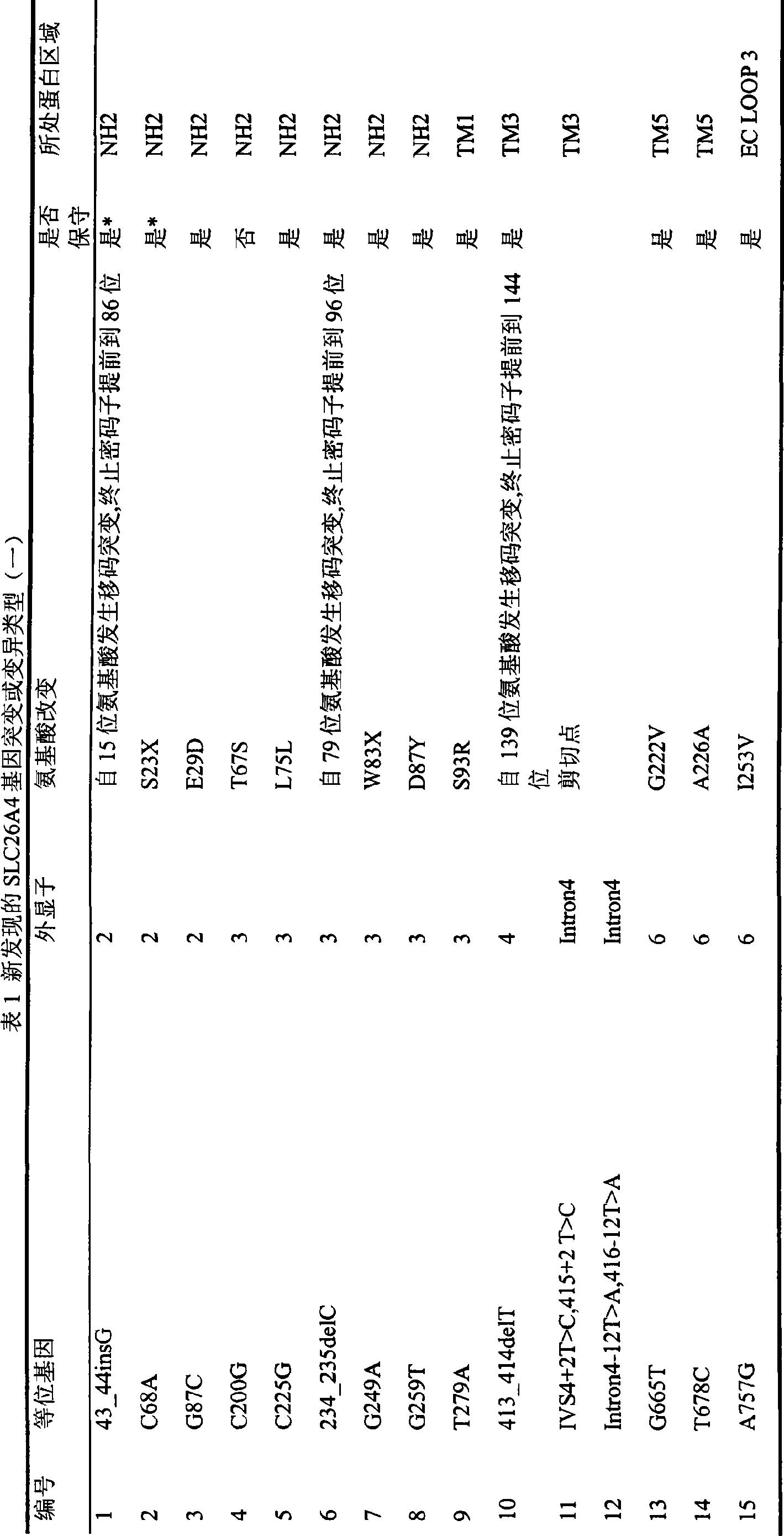
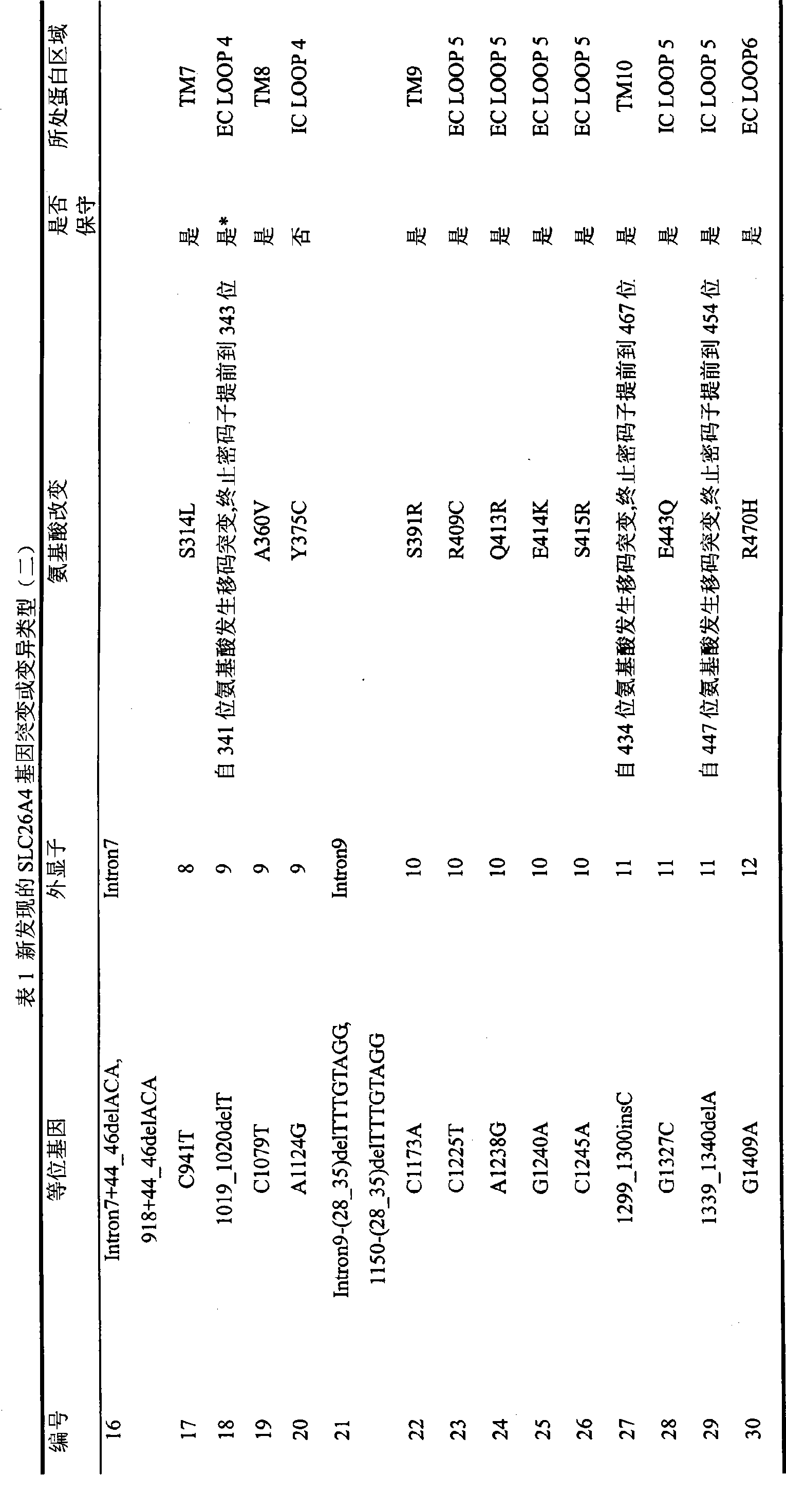
The invention relates to 86 types of particular mutation type atlases of SLC26A4 gene related to hearing loss in Chinese crowd, 27 types of relative hot-spot mutation type atlases and frequency information thereof, 13 types of hotspot mutation type atlases and frequency information thereof, and 2 types of hottest-spot mutation type atlases and frequency information thereof. 59 SLC26A4 gene mutation types are newly discovered in the Chinese crowd, wherein, 47 mutation types lead to the change of encoded protein amino acid of the SLC26A4 gene or influence genetic transcription and translation, 6 mutation types lead to base change rather than the change of amino acid, and 6 types are intron mutation types of the SLC26A4 gene. The discovery has a vital practical significance in developing an SLC26A4 gene diagnosing chip and a kit, which conform to hereditary features of the Chinese crowd suffering hearing loss.
Owner:韩东一 +1
Duck feed containing palm kernel meal and preparation method thereof
ActiveCN102038113ASave scarce raw materialsIncrease profitAnimal feeding stuffAccessory food factorsNutritionManihot esculenta
The invention discloses a duck feed containing palm kernel meal and a preparation method thereof. Raw materials comprising corn, cassava slice, corn protein feed, palm kernel meal and the like are adopted, smashed and mixed to be uniform, nutrition preparations comprising lysine hydrochloride, methionine, multiple vitamins and complex enzymes are added, steam blending is carried out, so that raw powder is cured to certain extent, and then granulation is carried out, thus obtaining the finished product, wherein the palm kernel meal is obtained by stewing, peeling, squeezing and separating fresh palm berry fruit strings. In the invention, by products after palm berry fruit is squeezed for oil is used fully, the palm kernel meal is used in meat duck feed, corn and soybean meal raw materials in short supply can be saved, thus reducing production cost; other raw materials are combined, thus product is full of nutrients, has balance of protein and amino acid, is rich in vitamins microelements, has good palatability and high digestibility and can be applicable to different meat ducks, especially cherry valley meat ducks.
Owner:JIEYANG TONGWEI FEED
Protein for stimulating hypersensitive response of plants and encoding genes of protein
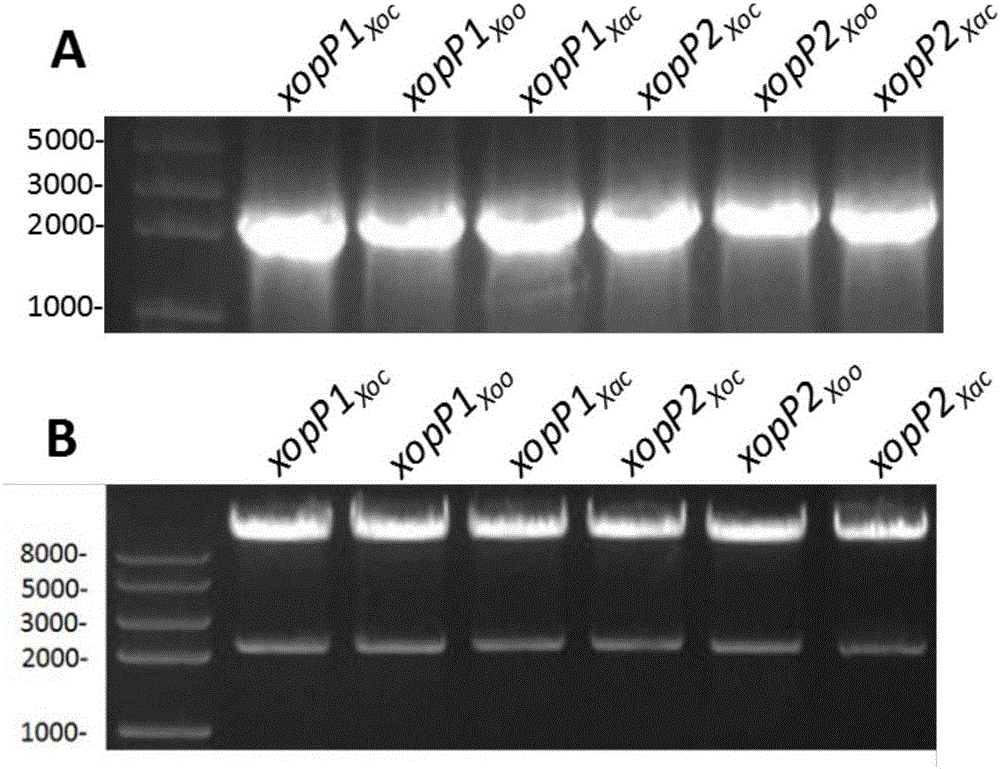
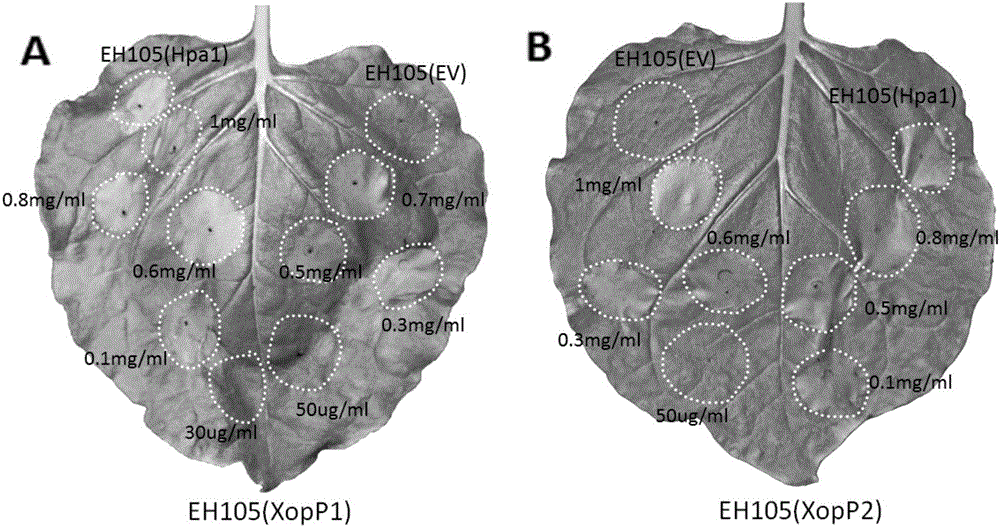
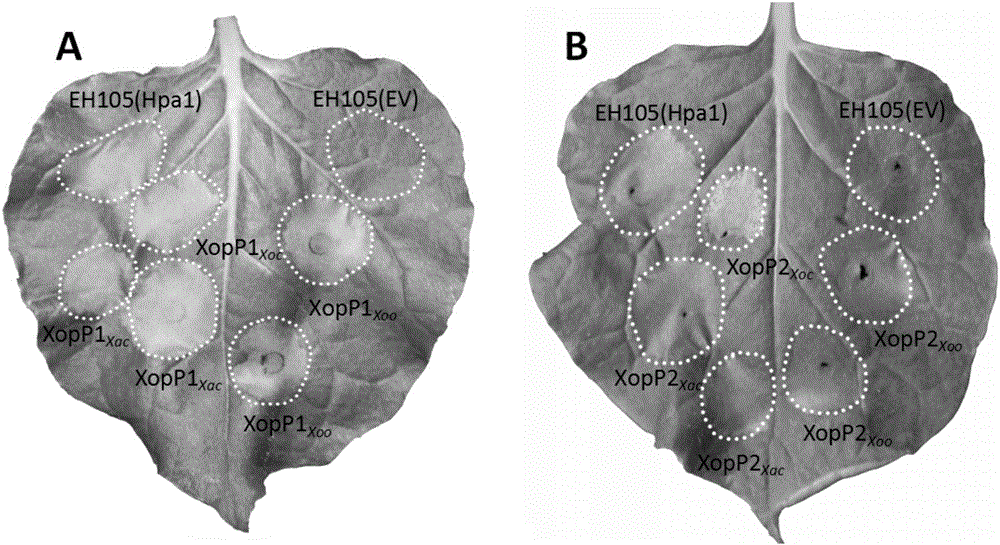
The invention relates to a protein for stimulating hypersensitive response of plants and encoding genes of the protein, and belongs to the field of biotechnologies. XopP1 amino acid sequences of the protein for stimulating the hypersensitive response of the plants are shown as SEQ ID NO.2, and xopP1 nucleotide sequences of the encoding genes of the protein are shown as SEQ ID NO.1; XopP2 amino acid sequences of the protein are shown as SEQ ID NO.4, and xopP2 nucleotide sequences of the encoding genes of the protein are shown as SEQ ID NO.3. The content of cysteine in amino acid sequences of the two encoding genes is lower than 1%, the isoelectric point of the protein is 8.8, the protein is weakly alkaline and contains abundant aliphatic amino acid such as alanine and leucine and abundant alkaline amino acid such as serine and arginine, the molecular weight of the protein is 7.9 kD approximately, and the total content of the alanine and the leucine can reach 25% at least. Hypersensitive response can be carried out by tobaccos. Compared with the prior art, the protein and the encoding genes have the advantages that xopP genes can be used as gene resources for plant disease resistance breeding, and XopP proteins can be used as protein medicines for stimulating the disease resistance of the plants.
Owner:SHANGHAI JIAO TONG UNIV
Gene engineering application of rice auxin transport protein gene OsPIN2
InactiveCN101736014AEnhanced tillering abilityImprove utilization efficiencyFermentationHorticulture methodsBiotechnologyNucleotide
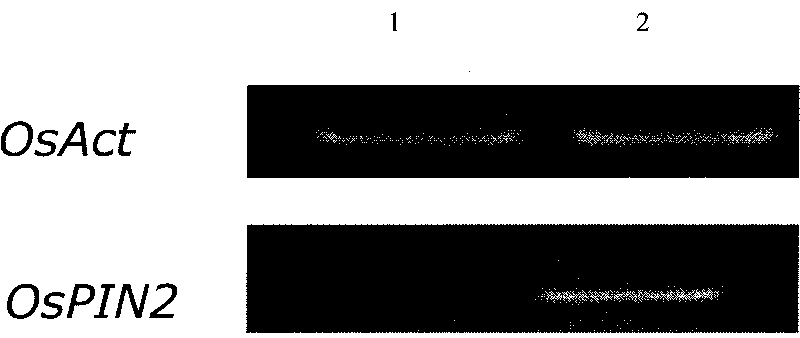
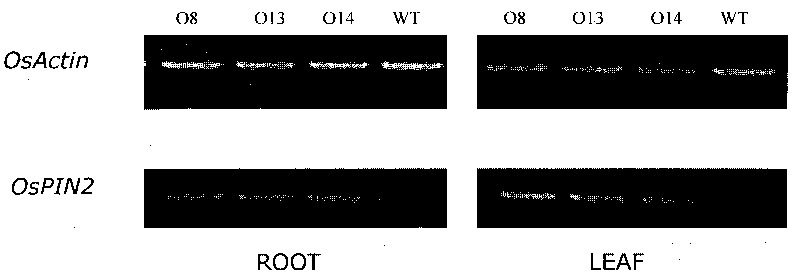
The invention discloses gene engineering application of rice auxin transport protein gene OsPIN2, which belongs to the field of gene engineering. The accession numbers of the nucleotide sequence of the rice auxin transport protein gene OsPIN2 and the expression OsPIN2 protein amino acid sequence thereof are AK101191 in an NCBI website (www. ncbi. nlm. nih. Gov / ). The engineering application of the gene is firstly reported in the rice, and participates in the transport of rice auxin so as to increase the rice roots, tiller number, tiller angle, nitrogen utilization efficiency and finial output.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method of preparing functional monascus mycelia and fermentation liquor by liquid fermentation, and products of the monascus mycelia and the fermentation liquor
ActiveCN103602590ANo pollution in the processAdvanced production technologyFungiNervous disorderBiotechnologyMonascus anka
The invention discloses a monascus induced strain CGMCC8167, and a method of preparing functional monascus mycelia and monascus fermentation liquor by liquid fermentation by utilization of the strain. The method is characterized by comprising steps of (1) preparing a fermentation culture medium; (2) preparing the strain; (3) preparing culture media for producing the strain; (4) fermenting; (5) performing treatment after fermentation; (6) collecting the mycelia; and (7) collecting the monascus fermentation liquor. The method adopts liquid fermentation and has an advanced pollution-free technology in place of traditional manual operation modes. The method is simple in operation step, small in labor intensity, short in period and high in quality stability, and is suitable for large-scale production. The biomass can be detected quantitatively and can reach the international health standards. The invention also provides products of the monascus mycelia and the monascus fermentation liquor. The products are rich in a plurality of functional active compounds, such as proteins, amino acids, beta-glucan, ergosterol, Monacolin-K, gamma-aminobutyric acid, and the like.
Owner:广东省真红生物科技有限公司
Herbal American ginseng skin beautifying improvement drug and cosmetic application and preparation
ActiveCN106727147ASolve the problem of easy exceeding the standardMoisturizing skin careCosmetic preparationsToilet preparationsAdditive ingredientActive ingredient
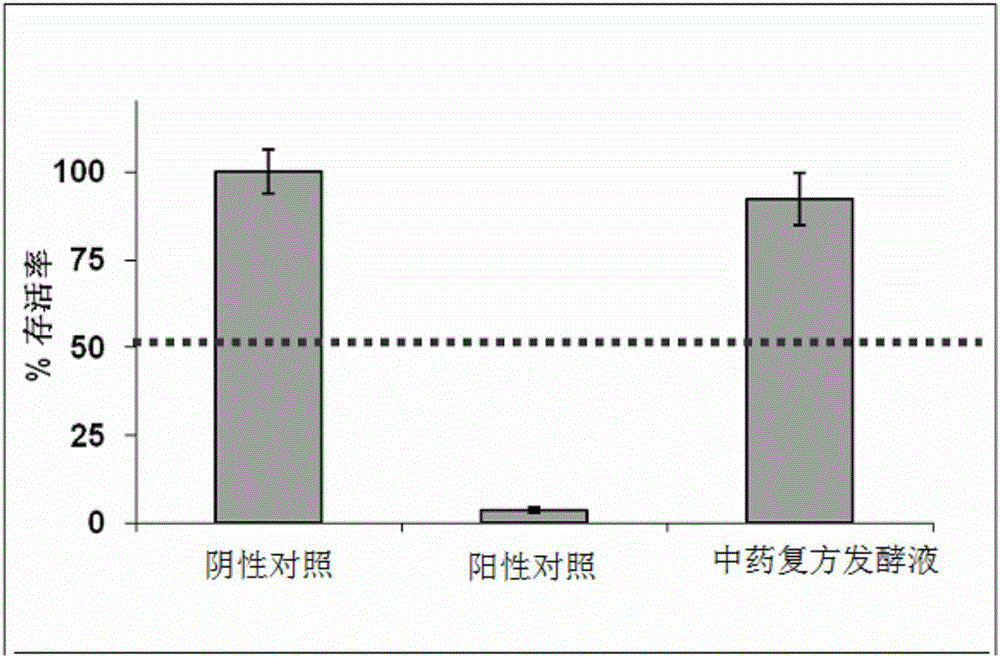
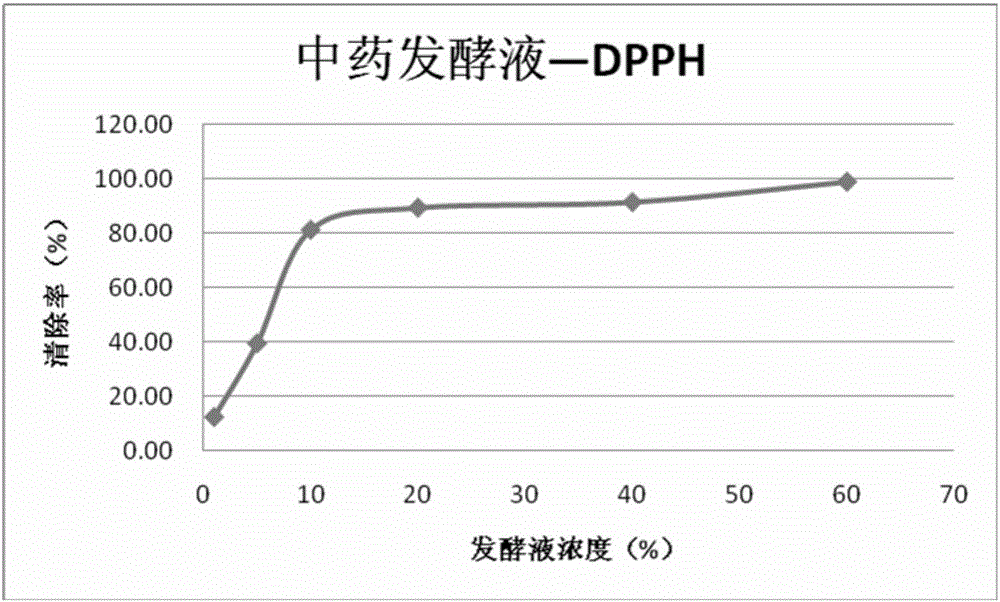
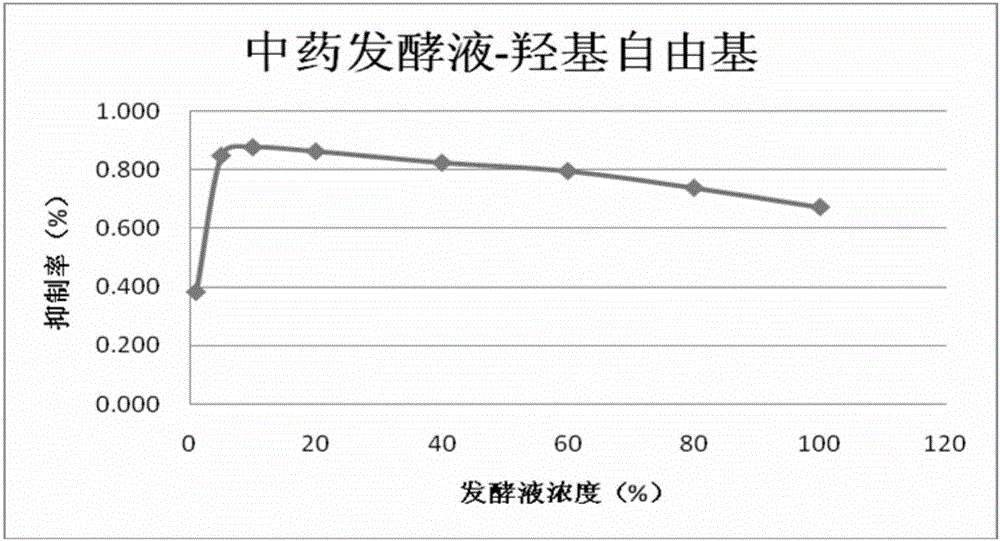
The present invention relates to a traditional Chinese medicine composition, and especially relates to a herbal American ginseng skin beautifying improvement drug and cosmetic application and preparation. A herbal American ginseng skin beautifying improvement composition comprises American ginseng, motherwort, pseudo-ginseng, white Poria and aloe. The herbal American ginseng skin beautifying improvement drug comprises the herbal American ginseng skin beautifying improvement composition as an active ingredient. A herbal American ginseng skin beautifying improvement reagent box comprises traditional Chinese medicine preparation reagent units, and each traditional Chinese medicine preparation reagent unit comprises the composition with the effective dosage of 0.03g-1g. The invention also provides the application of the traditional Chinese medicine preparation or the reagent box or a compound traditional Chinese medicine fermentation liquid in the skin beautifying improvement cosmetic field. The active components of traditional Chinese medicines are maximally protected by traditional Chinese medicine fermentation. The active components such as protein, amino acids, vitamins and trace elements and the like in the traditional Chinese medicines are not destroyed and can be fully utilized.
Owner:BEIJING CHINESE MEDICINE HOSPITAL AFFILIATED CAPITAL MEDICAL UNIV
A transcription factor PwNAC2 related to plant stress tolerance, a coding gene thereof and applications of the transcription factor
ActiveCN107827964AImprove germination rateImprove drought tolerancePlant peptidesFermentationWAS PROTEINAgricultural science
A transcription factor PwNAC2 related to plant stress tolerance, a coding gene thereof and applications of the transcription factor are disclosed. Protein provided by the invention is a), b), c) or d), wherein the a) is protein the amino acid sequence of which is shown as a sequence 2; the b) is fusion protein obtained by connecting a label to the N end and / or C end of the protein shown as the sequence 2; the c) is protein having same functions and obtained by substitution and / or deletion and / or addition of one or a plurality of amino acid residues to the amino acid sequence shown as the sequence 2; and the d) is protein which has functions same to functions of the amino acid sequence shown as the sequence 2, and which has 75% or 75% or above of homology with the amino acid sequence shownas the sequence 2. The new gene PwNAC2 is found and is introduced into arabidopsis thaliana to obtain a PwNAC2-transgenicarabidopsis thaliana plant. Experiments prove that drought tolerance and salt tolerance of the PwNAC2-transgenicarabidopsis thaliana plant are significantly improved, thus proving that the PwNAC2 or the protein coded by the PwNAC2 has stress tolerance.
Owner:BEIJING FORESTRY UNIVERSITY
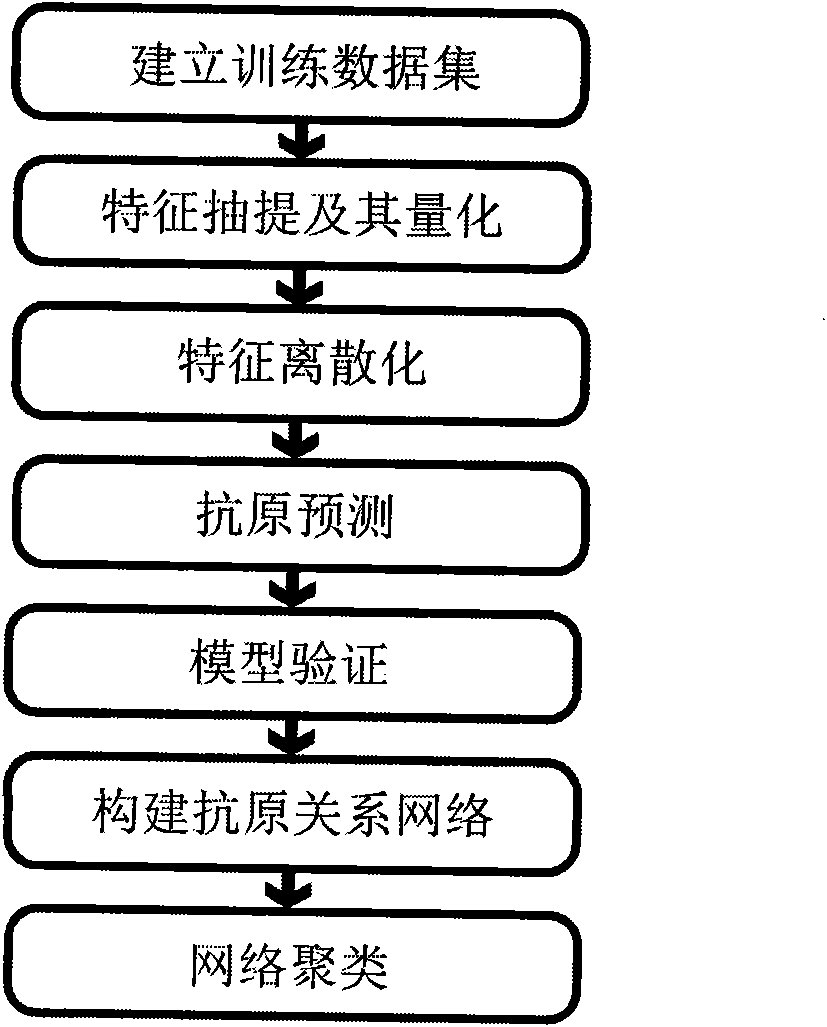
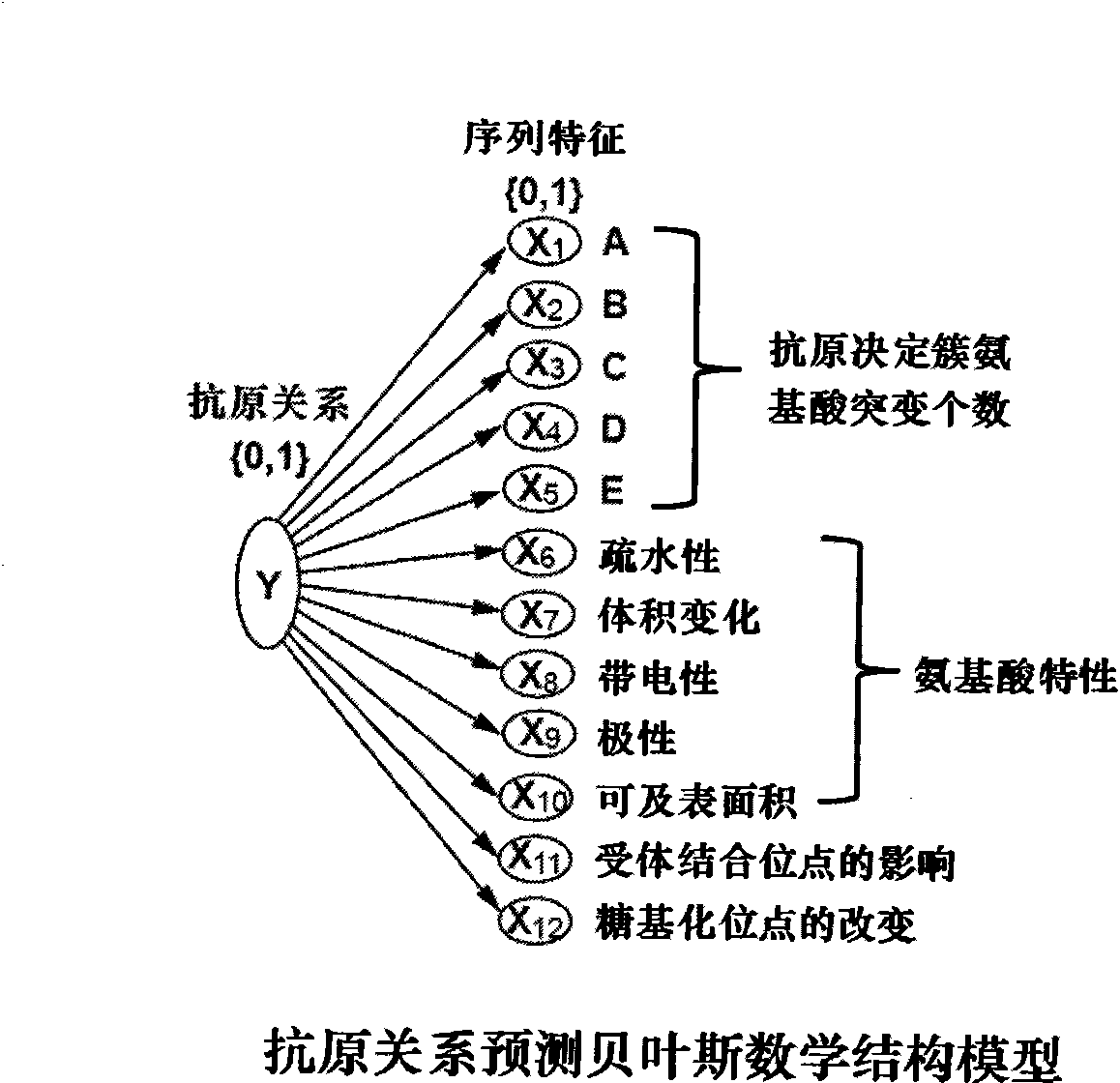
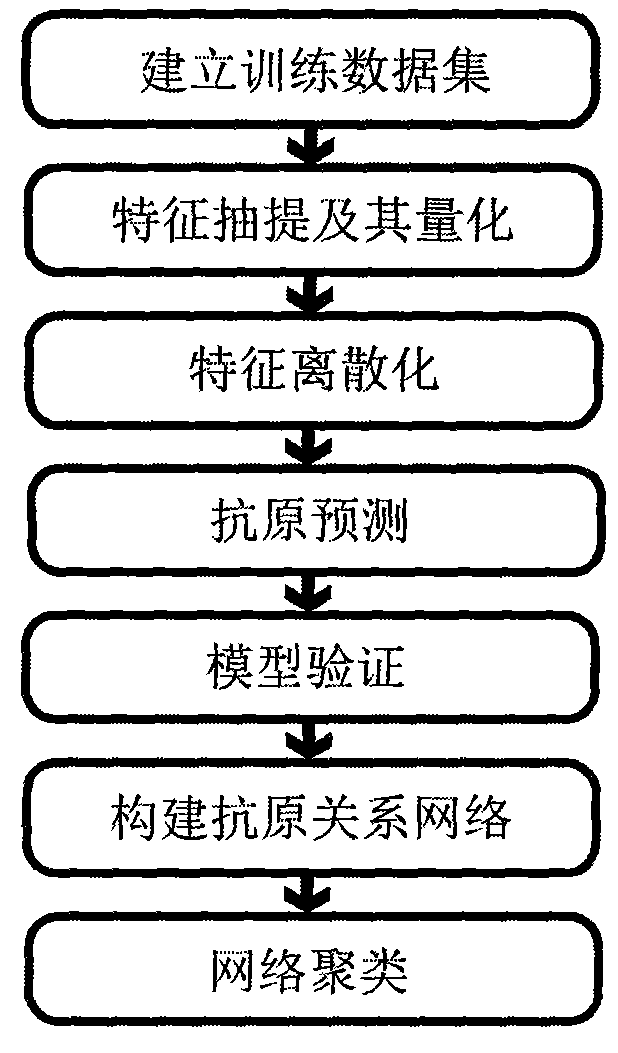
Method for predicting flu antigen through model and application thereof
ActiveCN101847179AAntigen relationship is simpleAntigen relationship is convenientSpecial data processing applicationsReceptorProteinogenic amino acid
The invention discloses a method for predicting flu antigen through a model and an application thereof. The method comprises the following steps: extracting 12 features affecting the flu antigen, wherein the 12 features include mutation numbers of five antigenic determinant amino acid, five physical and chemical properties of HA protein amino acid, factor affecting receptor, and number of glycosylated site, the five physical and chemical properties of amino acid include hydrophobicity, volume change, electriferous property, polarity and integrable surface area; and making statistics on the 12 features of 3681 virus pairs similar to known antigen and 1720 virus pairs variant from antigen, thereby establishing a prediction model on antigen relation. On the basis of sequence, the method can present the antigen relation among viruses, and thus having the advantages of simpleness, convenience and high sensitivity. The process of antigen evolution can be visually displayed through the network. The invention is used for revealing flu spreading rules, screening vaccine candidate strains and the like.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Protein powder produced by fluidized bed spraying lecithinum granulation technique and preparing method thereof
InactiveCN100998368ANeed to meetFull and even coatingVegetable proteins working-upPhosphatide foodstuff compositionsSolubilityWhey protein
A protein powder features its ideal protein-amino acid structure mode and contains soybean protein, whey protein, stabilizer, lecithin and 9 amino acids necessary to human body. Its preparing process features use of boiling bed to spray lecithin and granulating.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Germinated oat rice and preparation method thereof
InactiveCN101507504AIncrease incomeImprove the level of technologyAgriculture gas emission reductionCultivating equipmentsActive enzymeAdditive ingredient
The invention relates to a method for preparing germinated oats. The method comprises the steps of selecting raw materials, performing wet-type careful selection, soaking, accelerating the germination of oats, drying germinated oats and packaging the dried germinated oats. Scientific tests show that the nutrient components of the germinated oats prepared by the method have the following changes as compared with oats: 1, a change in protein and amino acid: antihypertensive functional nonprotein amino acid gamma-aminobutyric acid increases by 10 to 40 times; 2, a change in carbohydrate: the rough mouthfeel of oats is obviously improved, so that people is easy to accept the oats; 3, a change in mineral elements: the content of the mineral elements is increased, and the absorption rate of the mineral elements in human bodies is raised; 4. a change in vitamins: the vitamin content of the oats after germination obviously increases; 5, a change in functional factors: as the content of the functional factors of the oats greatly increases, and as a plurality of active enzymes of the oats are fully activated and released, the oats are good in the function of resisting oxidation and favorable for eliminating free radicals in human bodies; and 6, a change in dietary fiber: the function of the oats in treating various chronic diseases is strengthened. The method has the advantages of scientific process flow, extensive raw materials, eating convenience and easy popularization.
Owner:田向东
Environment-friendly type textile sizing agent
Owner:SUZHOU RUNHONG TRADING
High performance liquid chromatography separating column suitable for amino acid chiral resolution
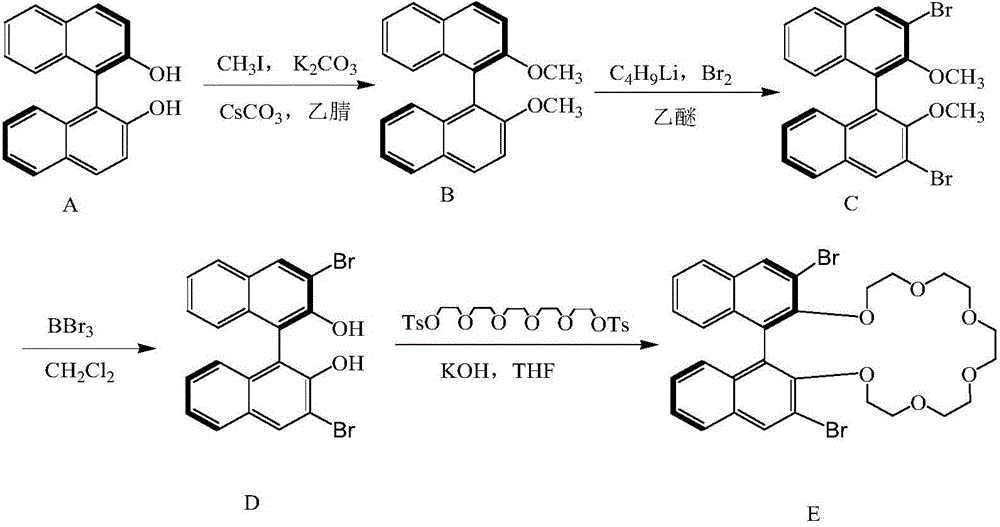
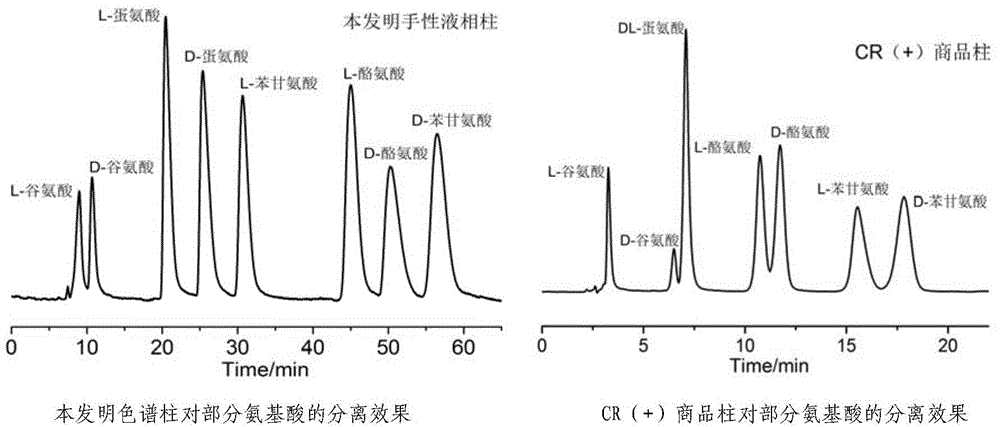
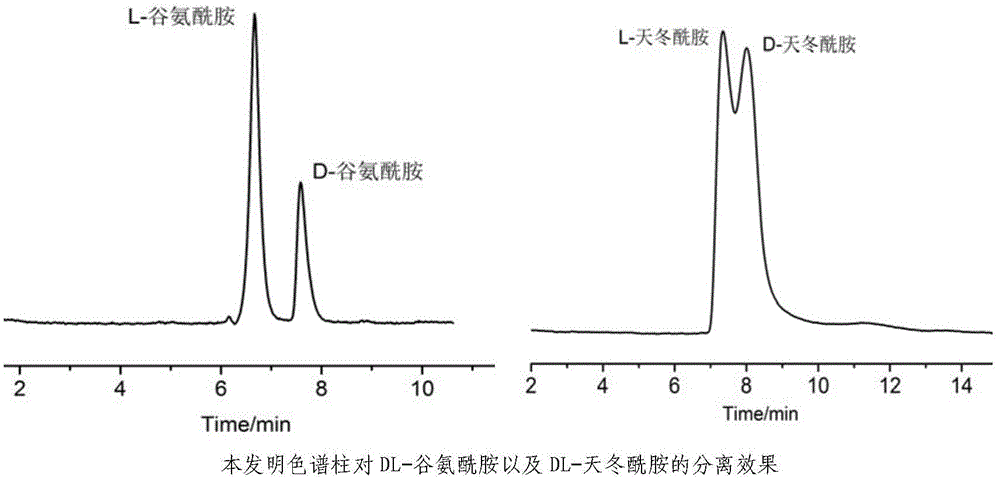
InactiveCN104475066AEfficient separationHigh resolutionOther chemical processesOrganic compound preparationEvaporationSlurry
The invention discloses a high performance liquid chromatography separating column suitable for amino acid chiral resolution. The separating column is prepared by the following steps: dissolving R(or S)-(3,3'-bromo-1,1'-dinaphthyl)-20-crown-6 in dichloromethane, uniformly dispersing the crown ether solution to C18 silica gel, carrying out rotary evaporation to remove the solvent to prepare a chiral stationary phase; and mixing the chiral stationary phase in a methanol water solution, stirring to obtain a homogenate solution, and filling the chromatographic column by a wet process by using the methanol water solution as a displacement fluid. The separating column can effectively separate all the 19 protein amino acids with chiral site at normal temperature, and can effectively separate chiral drugs with primary amine on the chiral site. The separating column has the obvious characteristics of high resolving power, high separating rate, high reproducibility, lower preparation cost and the like, and can be used repeatedly. The separating column has obviously better separating effect for amino acids than the like products at home and abroad.
Owner:YUNNAN NORMAL UNIV
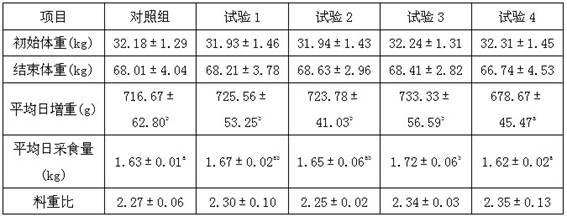
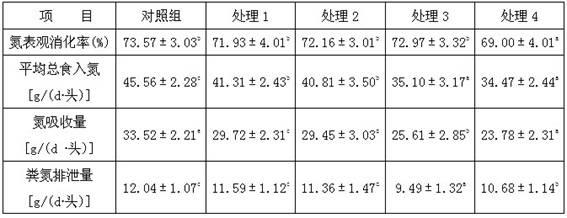
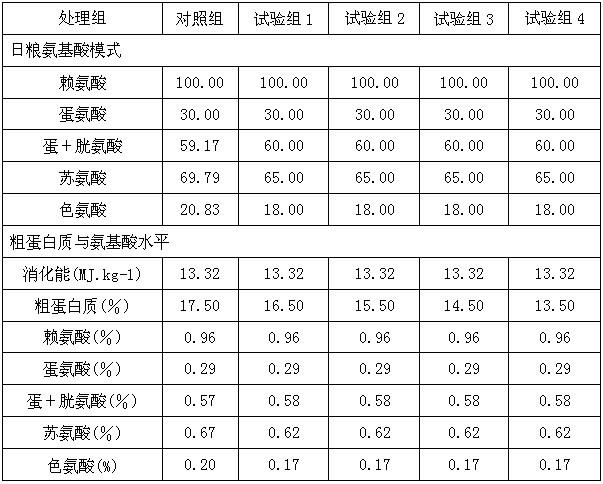
Low-protein daily ration of three-way cross growing pig
The invention discloses a low-protein daily ration of a three-way cross growing pig and belongs to the technical field of a pig industry of animal husbandry. The formula of the daily ration comprises 66-68 parts of corns, 4-4..6 parts of wheat bran, 15.4-24 parts of soybean meal, 0.88-0.92 part of stone powder, 0.89-1.07 parts of calcium hydrophosphate, 0.4 part of edible salt, 0.168-0.44 part of L-lysine hydrochloride. 0.040-0.081 part of DL-methionine, 0-0.12 part of L-threonine, 0-0.03 part of L-tryptophan, 0.208-0.582 part of zeolite powder and 1.00 part of premixed powder. According to the technology disclosed by the invention, according to an ideal protein amino acid model of the three-way cross growing pig, the rough protein level of the daily ration of the three-way cross growing pig are reduced by three percent, so that the aims of keeping the growing performance, reducing the cost of a feed formula and reducing nitrogen content in excrements are achieved.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Cordyceps taishanensis ferment product and production method thereof
ActiveCN104068377AImprove health benefitsConvenience dietFungiMicroorganism based processesSuperoxide dismutaseIngested food
The invention discloses a cordyceps taishanensis ferment product and a production method thereof. A main method comprises the following steps: selecting moringa tree saw dust powder, millets and rice as main materials; adding a suitable amount of glucose and peptones as cordyceps taishanensis culture base materials; mashing a great amount of cordyceps taishanensis mycelia generated in the base materials, and mixing the mashed cordyceps taishanensis mycelia with rock candies, honey, aspergillus and the like; bottling and sealing; brewing to generate a moringa cordyceps taishanensis mycelium edible ferment raw solution; or mixing the edible ferment raw solution and dextrin according to a suitable ratio; and drying in air to prepare a ferment buccal tablet food. The innovation points are that the health-care plant moringa trees and five cereals including the millets and the rice which have abundant nutrition are developed and used as the raw materials; the cordyceps taishanensis mycelia are cultured to prepare the ferment product; the ferment product is abundant in nutrition, convenient to eat and good for body health. The product contains proteins, amino acids, vitamins, microelements and the like needed by a human body; functional active components including cordycepin, cordycepic acid, cordyceps polysaccharides, SOD (Superoxide Dismutase) and the like are effectively accumulated; various health-care effects of the ferment are effectively improved.
Owner:LIAONING SHENGQI HEAVEN BIO MEDICAL SCI TECH CO LTD
Mink growth hormone releasing hormone cDNA and application thereof
The invention discloses a full-length nucleotide sequence and a mature peptide nucleotide sequence of a mink growth hormone releasing hormone cDNA gene, and encoded protein amino acid sequences thereof. The mink growth hormone releasing hormone cDNA sequence is a complete open reading frame with 321 nucleotide sequences, and 106 amino acids are encoded. The mature peptide is encoded by 132 nucleotide sequences, and 44 amino acid residues are encoded. The mink growth hormone releasing hormone cDNA can be used for promoting the growths of economic animals such as minks.
Owner:CHINA AGRI UNIV
Fast-growing aquatic plant nitrate transport protein GeNRT2.1 and coding gene and application thereof
ActiveCN105481955AImprove nitrogen use efficiencyGrow fastMicroorganism based processesPlant peptidesNitrate transportProtein C
The invention discloses a fast-growing aquatic plant nitrate transport protein GeNRT2.1 and a coding gene and application thereof. The protein is the protein a or the protein b or the protein c, wherein the protein a is provided with an amino acid sequence shown in the sequence 5 in a sequence table; the protein b is a fusion protein obtained by connecting labels to the end N and / or the end C of the protein shown in the sequence 5 in the sequence table; the protein c has the same function and is the protein obtained through substitute and / or deletion and / or addition of one or more amino acid residues for the amino acid sequence shown in the sequence 5 in the sequence table. A test proves that the protein GeNRT2.1 has the function of a nitrate transport protein and can improve the plant nitrogen utilization efficiency, so that the plant grows rapidly.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Method for brewing black millet iron-rich nutritional soy
InactiveCN103564406AIncrease added valueEfficient use ofFood ingredient functionsFood preparationBiotechnologyNutrition
The invention relates to a method for brewing black millet iron-rich nutritional soy, which mainly overcomes the shortage of low organic iron content in general soy taking soybeans and wheat as raw materials and the shortcoming of addition of an iron additive into finished soy, and can be used for increasing iron-containing microelements. The method comprises the following step: with black crops with high organic iron content as raw materials, carrying out biochemical reaction on various enzyme systems under specific environmental conditions through a combined constant-temperature fermentation process of a low-salt solid-state fermentation process and a high-salt diluted-state fermentation process of various strains to enable iron microelements and other nutritional ingredients such as proteins, amino acids and vitamins in the raw materials to be fully decomposed and effectively enriched in soy so as to generate soy with unique color, aroma, taste and shape and high content of various nutritional ingredients such as organic iron.
Owner:山西省农业科学院农业科技信息研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com