Process for activating Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine
A Haemophilus influenzae and conjugate vaccine technology is applied in the field of Haemophilus influenzae type b polysaccharide conjugate vaccine activation technology, which can solve problems such as use, and achieve the effects of avoiding harm and improving safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
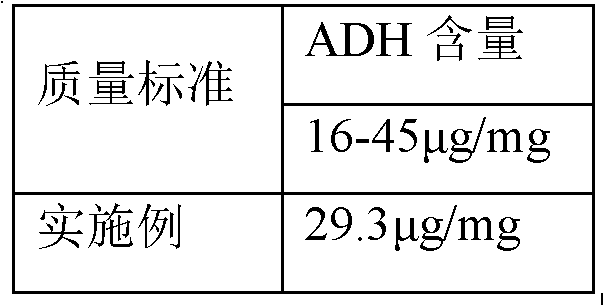
Embodiment 1
[0025] Embodiment 1: This embodiment is the best embodiment of the present invention.
[0026] Haemophilus influenzae type b polysaccharide conjugated vaccine activation process, it comprises the following steps:
[0027] A, prepare Hib polysaccharide, it comprises the following substeps:
[0028] A1. Centrifuge the sterilized culture solution at 10,000 rpm for 30 minutes to precipitate the bacteria, and collect the supernatant;
[0029] A2. Add CTAB to the supernatant, the weight-to-volume ratio (w / v) of CTAB to the supernatant is 1:1000, stir at room temperature for 1 h, and collect the composite of CTAB and Hib capsular polysaccharide after centrifuging the obtained mixture at 10,000 rpm for 30 min precipitation;
[0030] A3, the complex precipitate was dissolved with 1M sodium chloride solution, and stirred at room temperature for 2 hours to dissociate the Hib capsular polysaccharide from CTAB; add 4°C pre-cooled absolute ethanol until the final concentration of ethanol ...
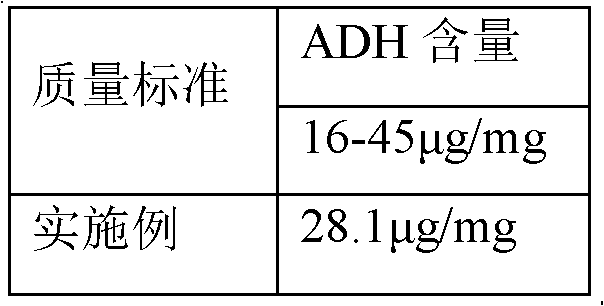
Embodiment 2
[0043] Haemophilus influenzae type b polysaccharide conjugated vaccine activation process, it comprises the following steps:
[0044] A, prepare Hib polysaccharide, it comprises the following substeps:
[0045] A1. Centrifuge the sterilized culture solution at 8000 rpm for 40 minutes to precipitate the bacteria, and collect the supernatant;
[0046] A2, CTAB is added in the supernatant, the weight-to-volume ratio (w / v) of CTAB and supernatant is 1: 800, stir at room temperature for 0.5h, and the obtained mixture is centrifuged at 8000rpm for 40min to collect the mixture of CTAB and Hib capsular polysaccharide complex precipitation;
[0047] A3. Dissolve the complex precipitate with 0.5M sodium chloride solution, stir at room temperature for 1 hour to dissociate Hib capsular polysaccharide from CTAB; add 3°C pre-cooled absolute ethanol until the final concentration of ethanol is 20% (v / v) , stirred overnight at 3°C;
[0048] A4. Centrifuge at 800rpm for 40min, collect the su...
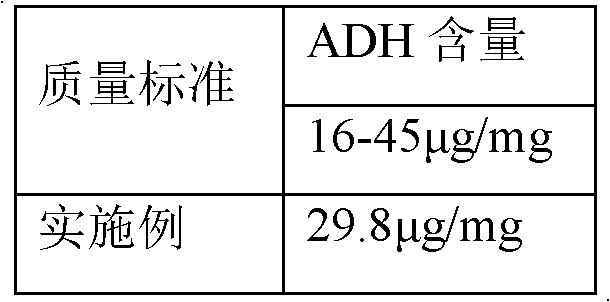
Embodiment 3
[0060] Haemophilus influenzae type b polysaccharide conjugated vaccine activation process, it comprises the following steps:
[0061] A, prepare Hib polysaccharide, it comprises the following substeps:
[0062] A1. Centrifuge the sterilized culture solution at 12000 rpm for 20 minutes to precipitate the bacteria, and collect the supernatant;
[0063] A2. Add CTAB to the supernatant, the weight-to-volume ratio (w / v) of CTAB to the supernatant is 1:1200, stir at room temperature for 1.5h, and collect the mixture of CTAB and Hib capsular polysaccharide after centrifuging at 12000rpm for 20min. complex precipitation;
[0064] A3. Dissolve the complex precipitate with 1.5M sodium chloride solution, stir at room temperature for 3 hours to dissociate Hib capsular polysaccharide from CTAB; add 5°C pre-cooled absolute ethanol until the final concentration of ethanol is 30% (v / v) , stirred overnight at 5°C;
[0065] A4. Centrifuge at 12000rpm for 20min, collect the supernatant, conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com