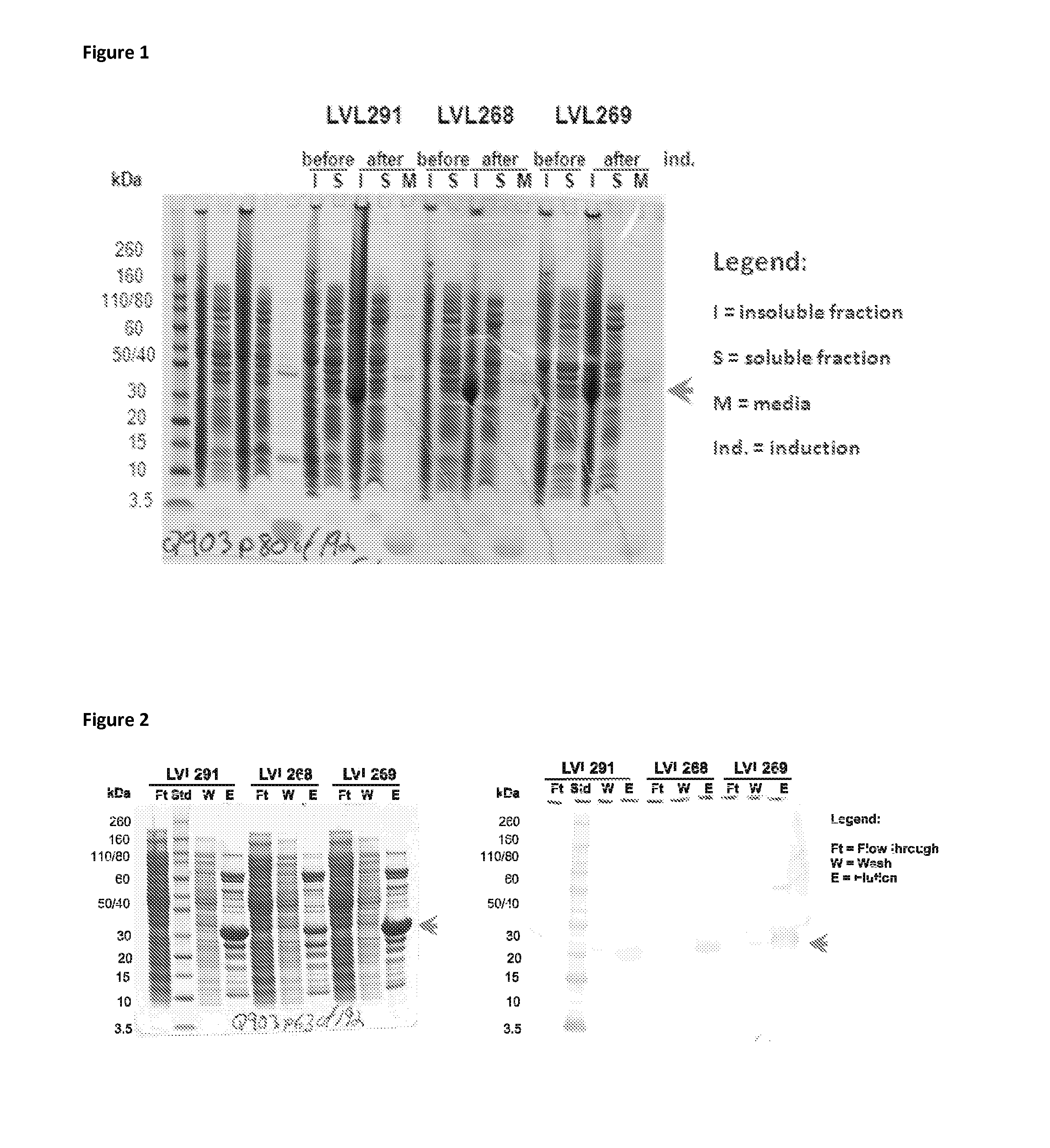
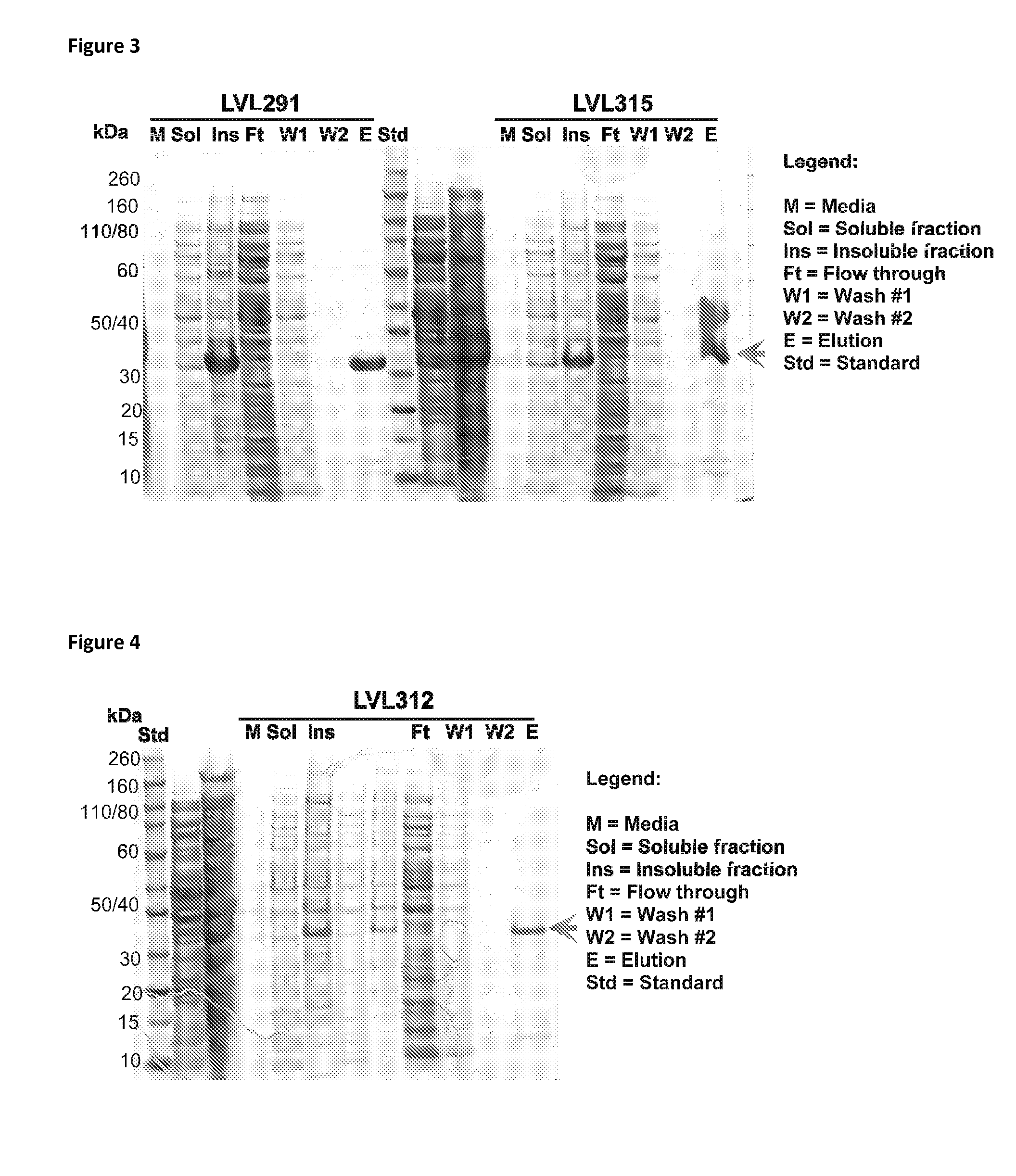
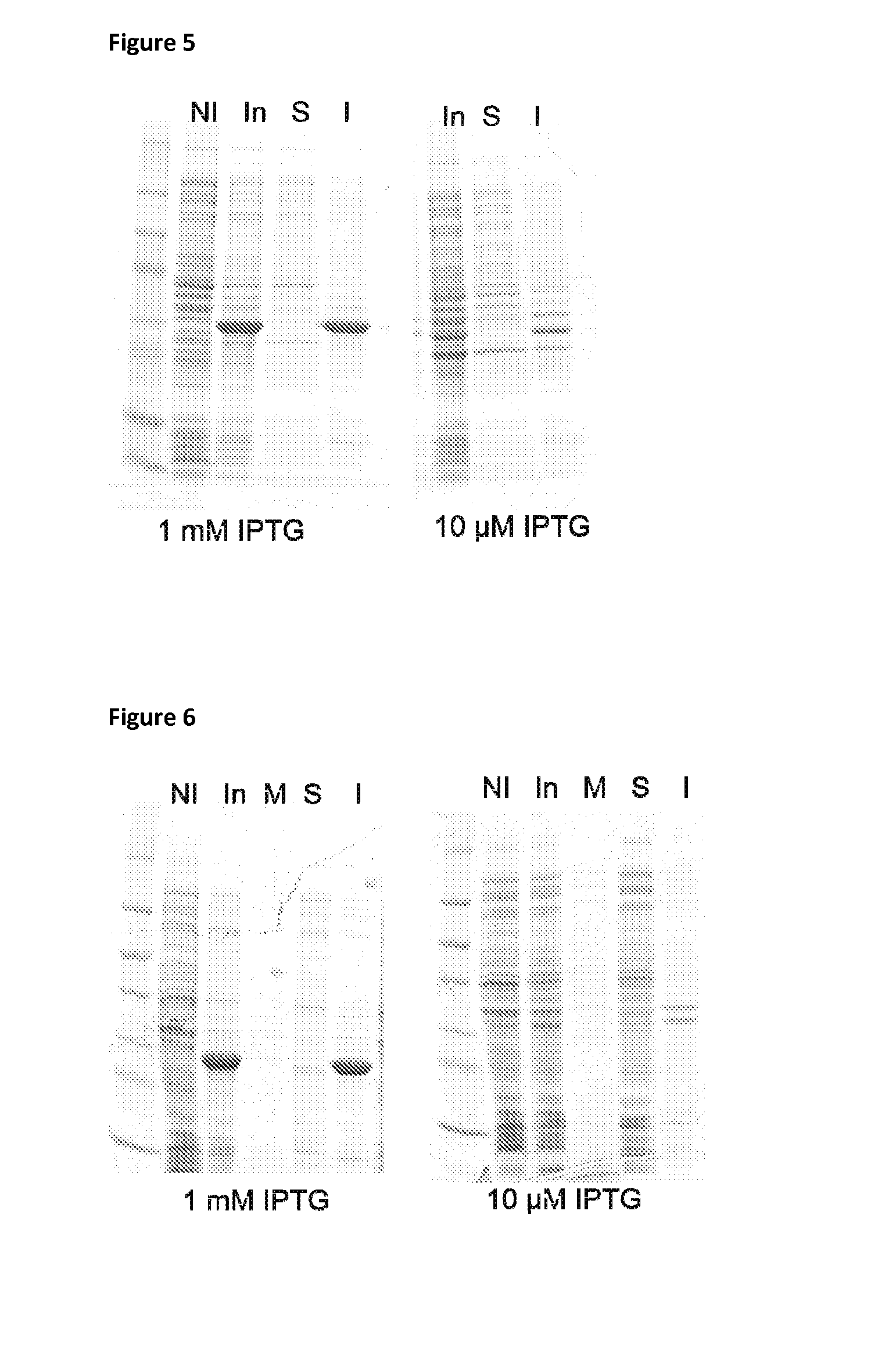
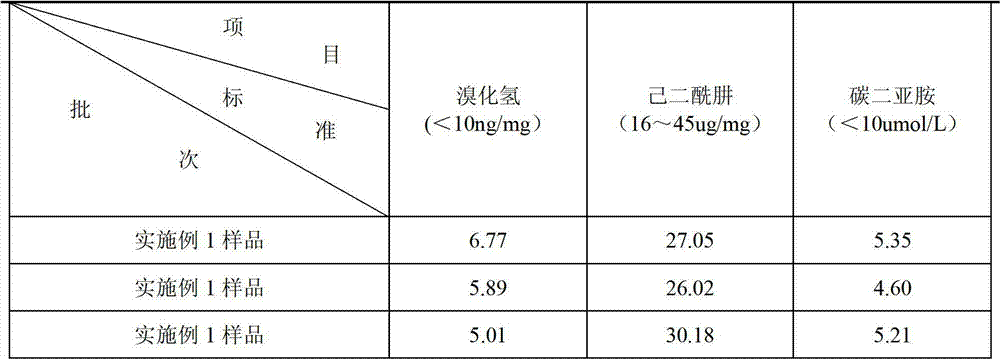
Patents
Literature
57 results about "Haemophilus influenzae type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Injectable vaccines against multiple meningococcal serogroups
ActiveUS20070082014A1Pharmaceutical delivery mechanismDepsipeptidesStreptococcus pneumoniaeSalmonella serotype typhi
An injectable immunogenic composition comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of Neisseria meningitidis, wherein said capsular saccharides are conjugated to carrier protein(s) and / or are oligosaccharides, and wherein (i) the composition comprises <50 mug meningococcal saccharide per dose, and / or (ii) the composition further comprises an antigen from one or more of: (a) serogroup B N. meningitidis; (b) Haemophilus influenzae type B; and / or (c) Streptococcus pneumoniae. Saccharide antigens in the compositions are generally conjugated to a carrier.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
Oligosaccharides derived from ribose-ribitol-phosphate, and vaccines containing them
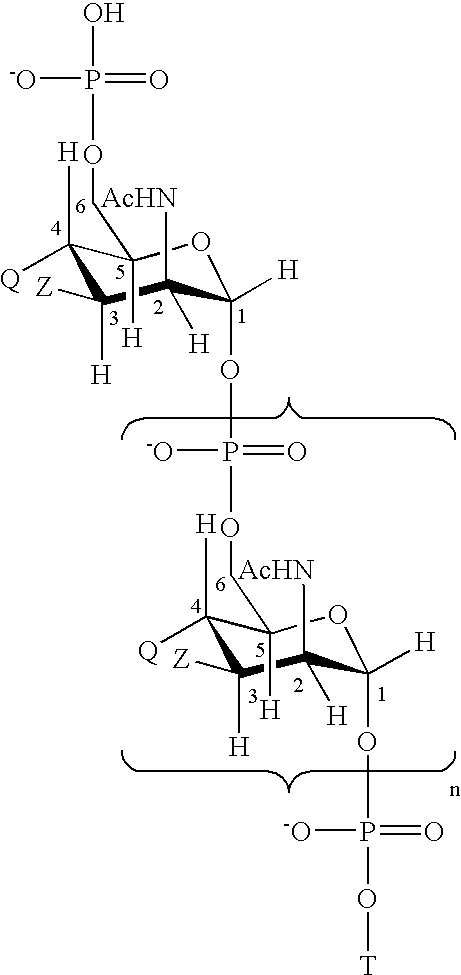
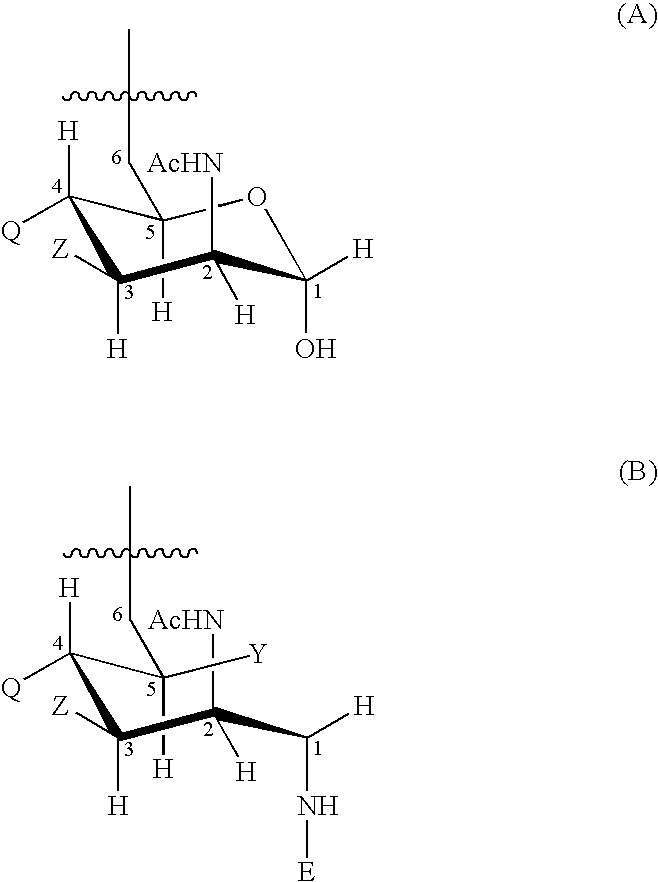
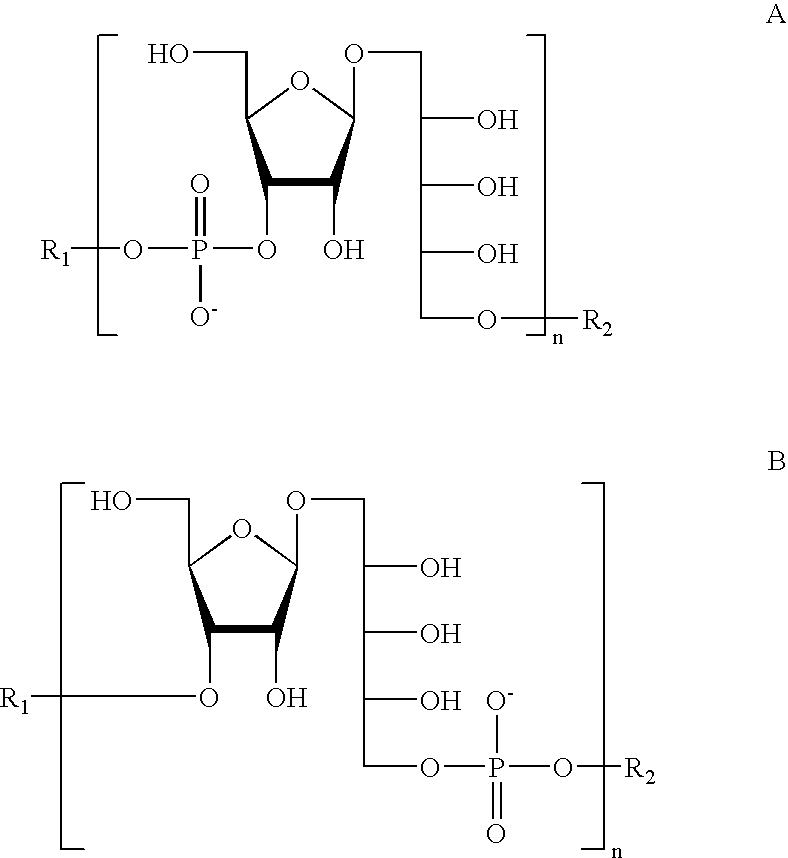
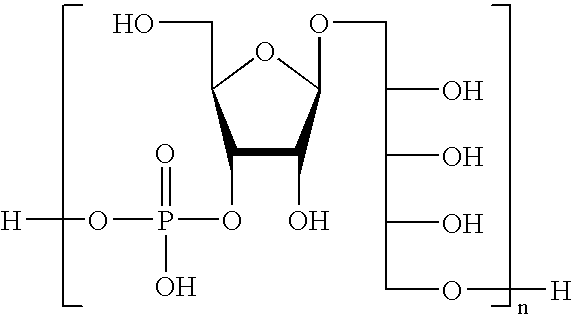
The present invention relates to the field of the Medicine, in particular with the chemical synthesis of oligosaccharide mixtures derived of ribose-ribitol-phosphate, which are used as active principle in vaccines for the prevention of infections caused by Haemophilus influenzae type b (Hib), as well as with the vaccines containing said oligosaccharide mixtures.The oligasaccharide mixtures obtained by chemical synthesis of the present invention, comprise repeating units of formulae (phosphate-ribosa-ribitol)n or (ribose-ribitol-phosphate)n of at least 5 compounds of structure A or B, which represent the repeating unit of the capsular polysaccharide of Haemophilus influenzae type b and differ only by n, being n a value contained between 4 and 25 (n>=4 y<=25), and wherein R1 or R2 is a spacer for conjugation to a carrier, with the condition of R1=spacer if R2=H, or R2=spacer if R1=H.The invention also is related with the immunogens containing such oligosaccharide mixtures, with the vaccines containing said immunogens and with the methods to prepare these oligosaccharides as mixtures. Furthermore, the invention includes the use of the vaccines, alone or combined with other vaccines, for the prevention of the infections caused by Haemophilus influenzae type b.
Owner:UNIV DE L HABANA +1
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
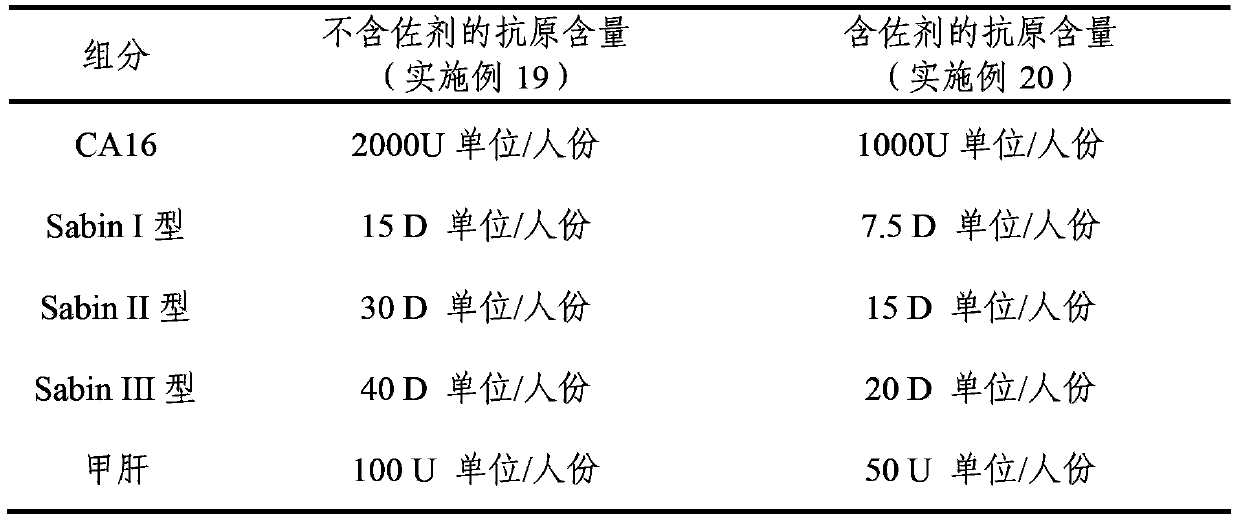
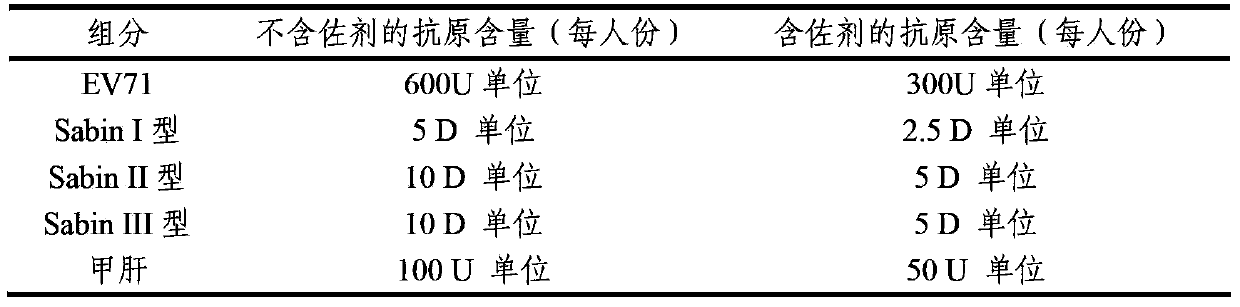
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Multivalent immunogenic composition containing enterovirus antigens
ActiveCN103386126AImproving immunogenicityImprove securityBacterial antigen ingredientsViral antigen ingredientsHepatitis A AntigensTetanus toxoids
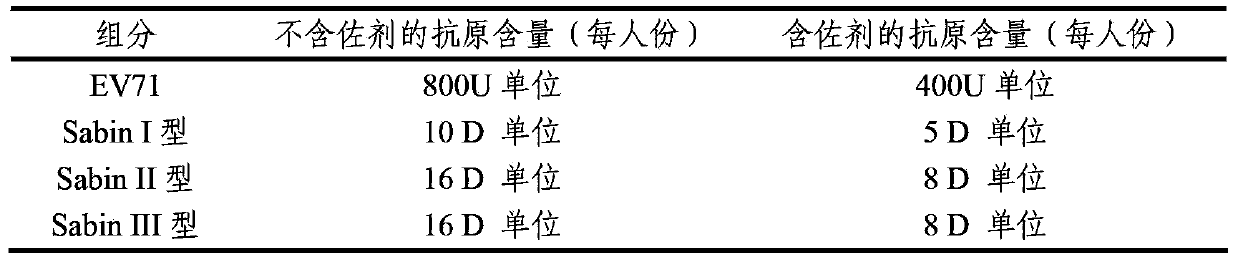
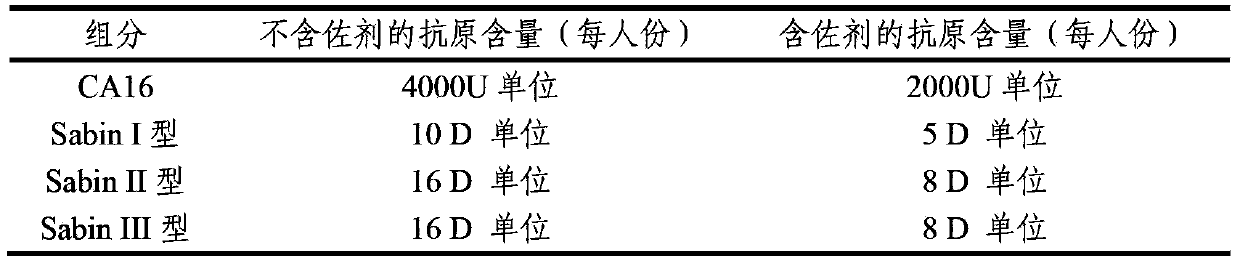
The invention provides a multivalent immunogenic composition containing enterovirus antigens. The composition comprises inactivated EV71 antigens and / or inactivated CA16 antigens, and inactivated polio antigens. The composition can further comprise antigens selected from hepatitis A antigens, hepatitis B antigens, acellular pertussis antigens, tetanus toxoid, diphtheria toxoid, Haemophilus influenzae type b capsular polysaccharide, and meningococcal polysaccharide antigens, as well as physiologically acceptable carriers combined with bacterial polysaccharide antigens. The invention also provides a preparation method of the composition. The composition can prevent invasion of a plurality of pathogens simultaneously without interference among the antigens, and the immunogenicity is no less than that of individually activated antigens. With the composition, vaccination processes are significantly simplified, and the vaccination efficiency is improved with reduced costs.
Owner:SINOVAC BIOTECH
Multivalent DTP-POLIO vaccines
InactiveCN101310769AEffective preventionSsRNA viruses negative-senseAntibacterial agentsTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
Diphtheria, tetanus and acellular pertussis/Haemophilus influenzae type b - group A and C meningococcus combined vaccine
ActiveCN102813917AGood freeze-drying effectRelieve painAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus
The invention provides a diphtheria, tetanus and acellular pertussis / Haemophilus influenzae type b - group A and C meningococcus combined vaccine, which has the characteristics of high safety, high efficacy, high controllability and prevention of multiple diseases through one injection, satisfies relevant requirements on absorbed diphtheria, tetanus and acellular pertussis combined vaccines, Haemophilus influenzae type b combined vaccines and group A and C meningococcus combined vaccines in the Third Part of Chinese Pharmacopoeia (2010) and proposed Rules on Production and Inspection of Diphtheria, Tetanus and acellular Pertussis / Haemophilus influenzae Type b - Group A and C Meningococcus Combined Vaccines, and achieves an effect of putting into practical application.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
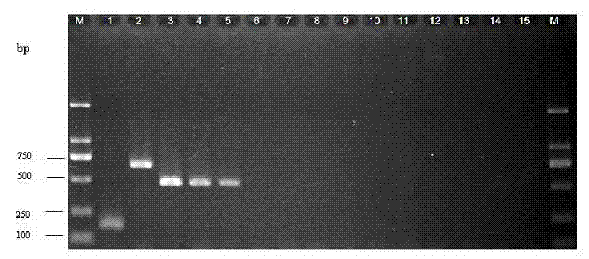
Multiple landing PCR(Polymerase Chain Reaction) detection kit and detection method for pathogenic bacteria of lower respiratory tract
InactiveCN102409103AHigh sensitivityMicrobiological testing/measurementMicroorganism based processesBovine serum albuminEpidemiologic survey
The invention designs specific primers for streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex, and establishes a multiple landing PCR(Polymerase Chain Reaction) detection kit and a detection method for simultaneously detecting the bacteria, adopting agarose gel electrophoresis for detecting PCR products. The kit comprises a 10*PCR buffer solution, MgCl2, dNTP, TaqDNA polymerase, BSA(Bovine Serum Albumin), positive control DNAs of streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex, a streptococcus pneumoniae primer, a haemophilus influenzae type b primer and a mycobacterium tuberculosis complex primer. The kit can simultaneously quickly detect streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex in a lower respiratory tract sample. The multiple landing PCR detection kit and detection method established by the invention are quick, simple, specific and sensitive, and can be used for rapid diagnosis and epidemiological investigation of infection of streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex.
Owner:JIANGSU UNIV
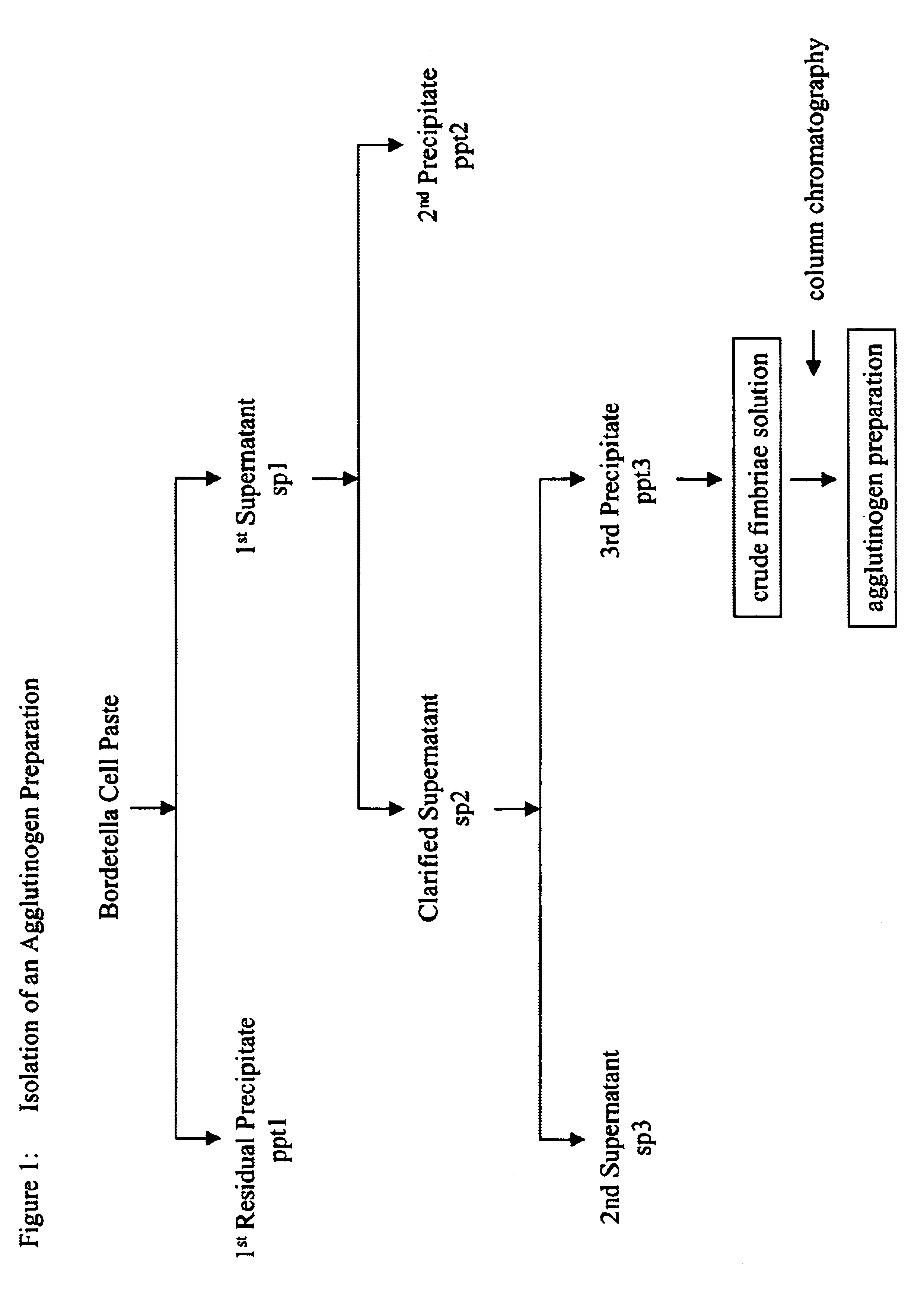
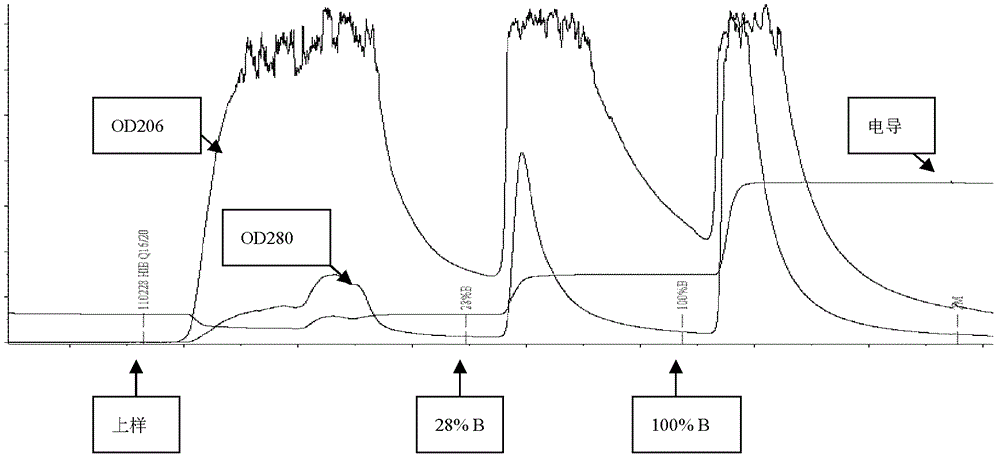
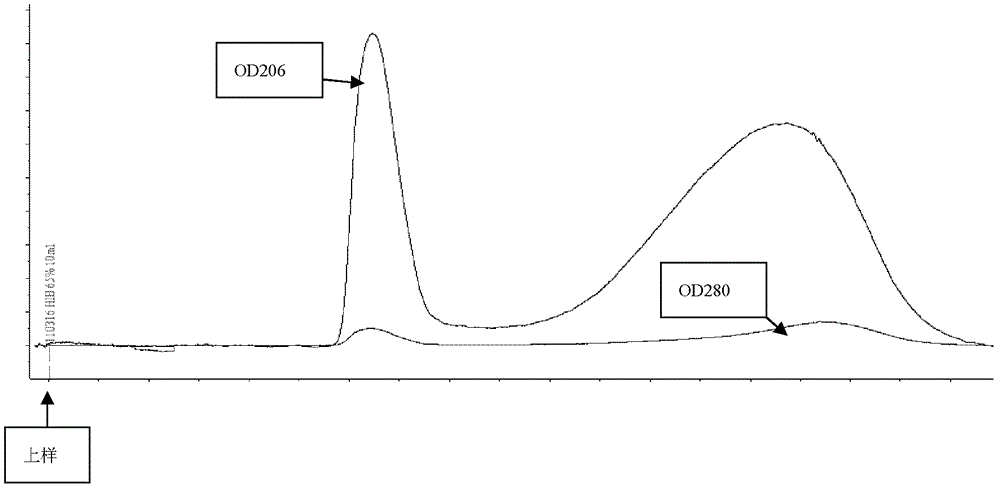
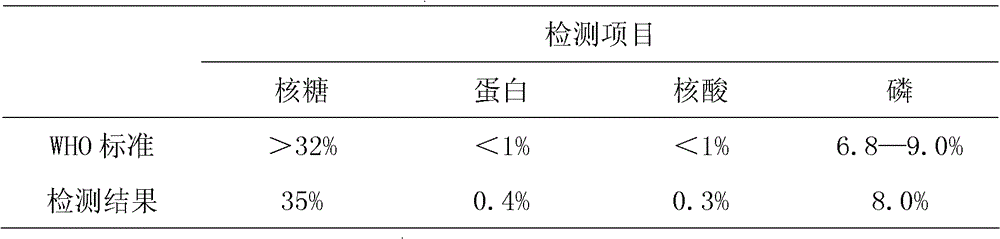
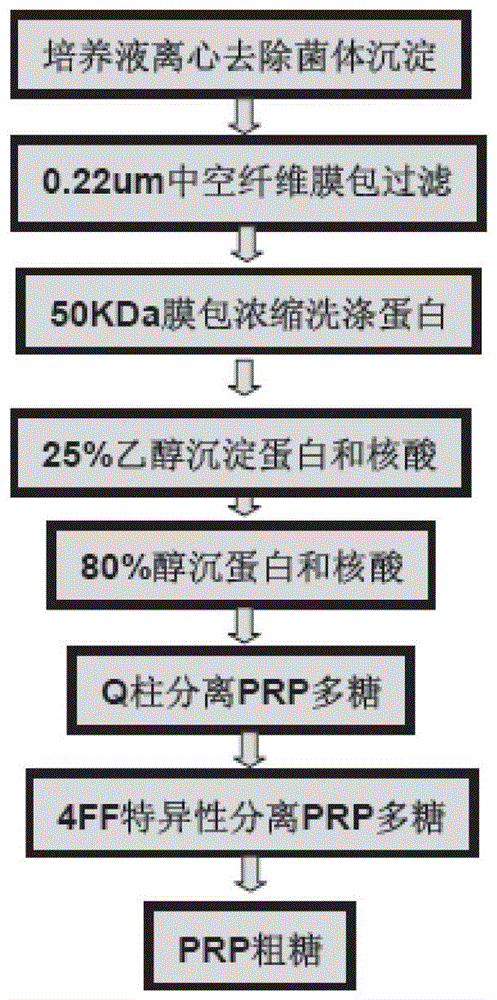
Method for rapid purification of bacterial capsular polysaccharide
The invention discloses a method for rapid purification of bacterial capsular polysaccharide. The method can fast remove pollutants comprising proteins and nucleic acid from a specific bacterial fermentation broth and retains purified capsular polysaccharide. The method comprises the following steps of fermentation of capsular polysaccharide-containing bacteria, acid adjustment of a pH value of a fermentation broth, precipitation of thalli and impurities of the fermentation broth, centrifugation, microfiltration, ultrafiltration condensation and filter wash so that a crude bacterial capsular polysaccharide solution is obtained. A basic principle of the method comprises that through acid adjustment, a pH value of a fermentation broth is in a range of 3 to 5 so that thalli having whole shapes and other impurities are removed and capsular polysaccharide is retained in the fermentation broth and thus purification of capsular polysaccharide is realized. The method can be used for purification of capsular polysaccharide of pneumococcocci, haemophilus influenzae type b, epidemic meningococcocci and typhoid bacillus.
Owner:KANVAX BIOPHARM
Immunogenic composition
ActiveUS20140105926A1Effective protectionBacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsStreptococcus pneumoniaeHaemophilus influenzae type
The present invention relates to immunogenic compositions comprising one or more Streptococcus pneumoniae capsular saccharide conjugates and a protein component comprising Protein E and / or PilA from Haemophilus influenzae.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Haemophilus influenzae type b (Hib) polysaccharide and refined tetanus toxoid coupling process
ActiveCN102861330AReduce dosageReduced Chances of ContaminationAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetanus toxoidsHaemophilus influenzae type
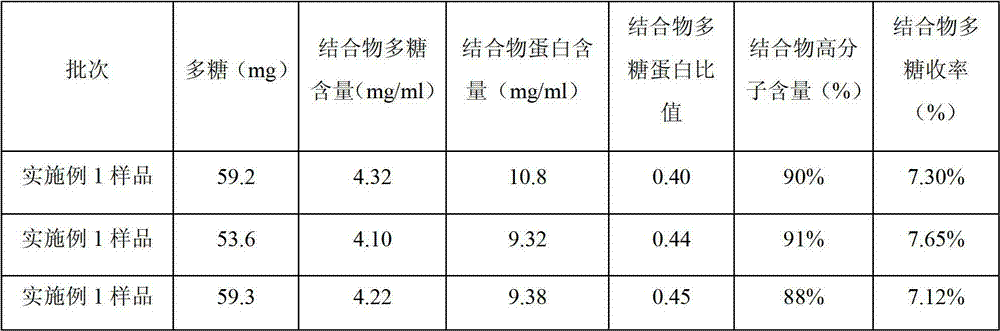
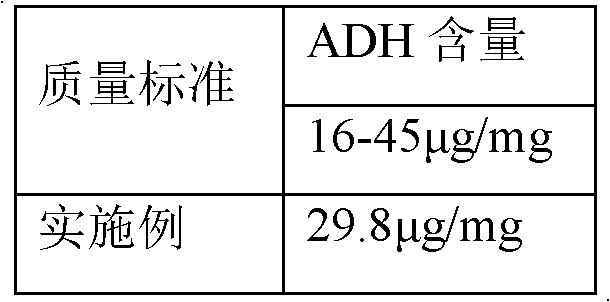
The invention discloses a haemophilus influenzae type b (Hib) polysaccharide and refined tetanus toxoid coupling process which includes steps of Hib polysaccharide-AH derivant generation and Hib polysaccharide-tetanus toxoid (TT) combination preparation. The Hib polysaccharide-AH derivant generation includes steps of A1, polysaccharide dissolving, A2, polysaccharide activation, A3, antidiuretic hormone addition for connection and A4, CNBr and antidiuretic hormone (ADH) removing. The Hib polysaccharide-TT combination preparation includes the following steps: B1, adding TT solution, B2, adding carbodiimide (EDAC), B3, removing the EDAC to obtain polysaccharide-TT carbodiimide; and B4, separating and pourifying the polysaccharide-TT carbodiimide, eluting, degerming and filtering to obtain the polysaccharide-TT combination. The Hib polysaccharide and refined tetanus toxoid coupling process has the advantages of improving working efficiency, saving time and labor and simultaneously saving using amount of NaCl solution and reduces the contaminated rate of samples compared with dialysis adopted by the traditional process.
Owner:CHENGDU OLYMVAX BIOPHARM
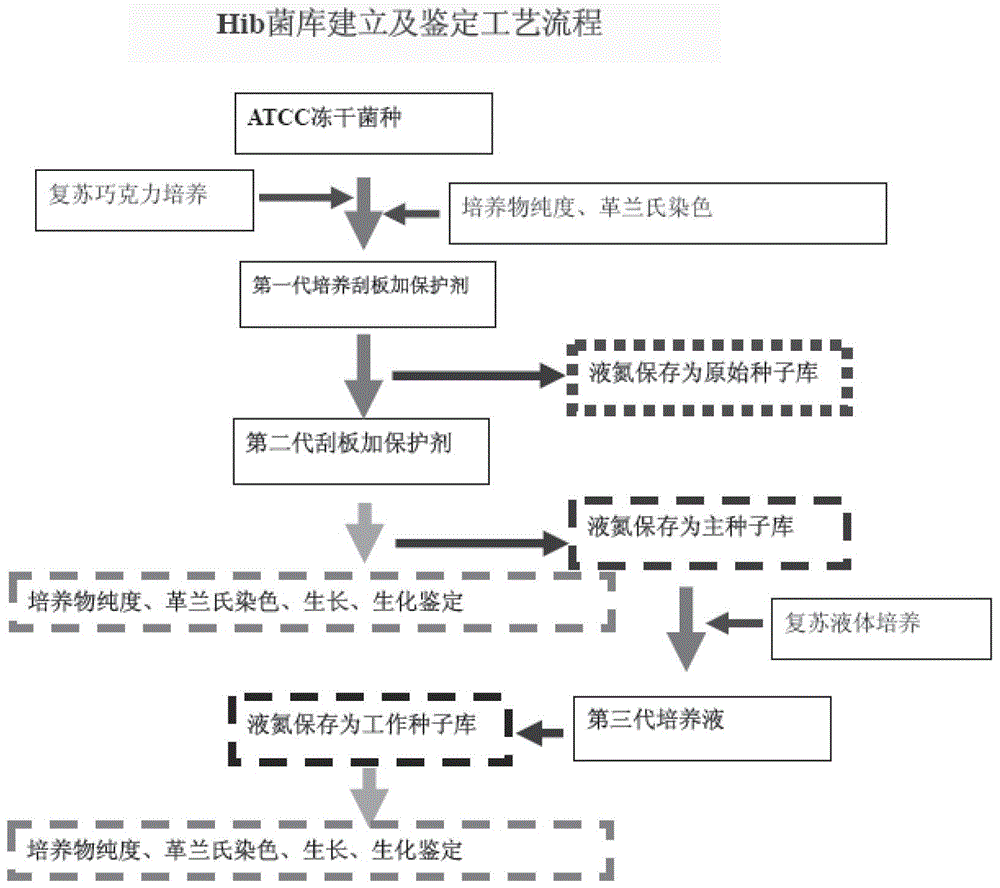
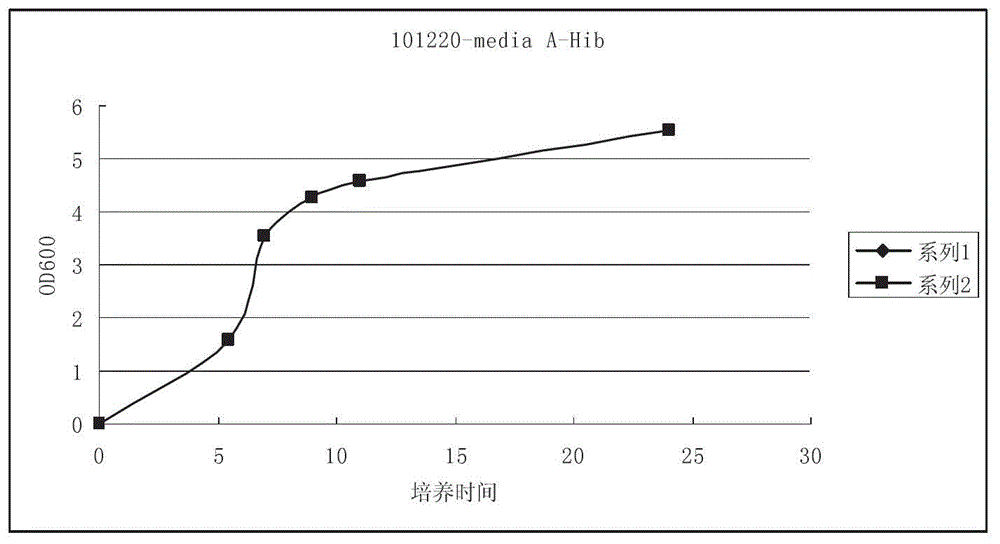
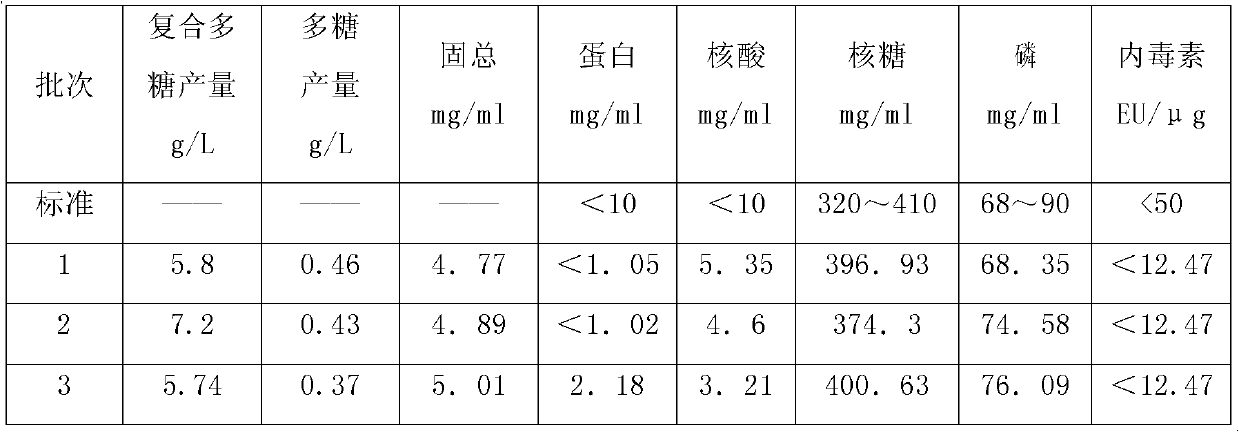
High-density culture and production method of bacterium capsular polysaccharide with haemophilus influenzae type b
ActiveCN102628068AIncrease productionReduce the number of extractions to remove impurity proteinsBacteriaMicroorganism based processesHigh concentrationBacteroides
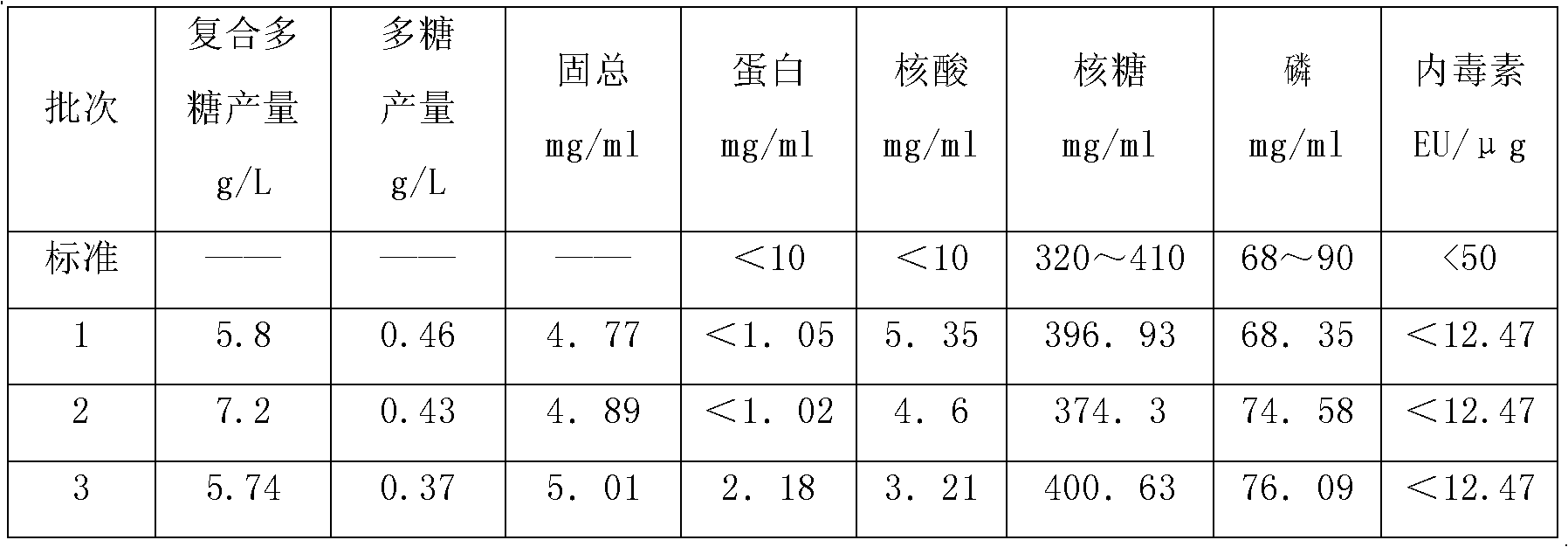
The invention discloses a high-density culture and production method of bacterium capsular polysaccharide with haemophilus influenzae type b. The method comprises following steps: subculturing Hib strains from a first generation to a fourth generation, carrying out culture in a seeding tank, carrying out fermentation and culture in a fermentation tank, killing bacteria, acquiring a supernatant, and purifying the supernatant. Beneficial effects of the invention are as follows: (1) by culturing Hib bacteria to a high concentration, output of Hib capsular polysaccharide is improved by 50% to 100% on the basis of the output of Hib capsular polysaccharide produced by the traditional way, and produced capsular polysaccharide meets the national standard; (2) by controlling nucleic acids, protein, and other impurities in a zymotic fluid to a low level, the number of times of phenol extracting for removing impurity protein is reduced for relatively low protein content, thereby reducing phenol amount used in subsequent purifying processes, reducing environmental pollution, and reducing production cost; and (3) output of polysaccharide is stable, and content of refined glycoprotein, endotoxin, and other impurities after the purifying is substantially lower than the national control standard.
Owner:CHENGDU OLYMVAX BIOPHARM
Process for activating Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine
ActiveCN102626515AAvoid harmImprove securityAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetrafluoroborateFreeze-drying
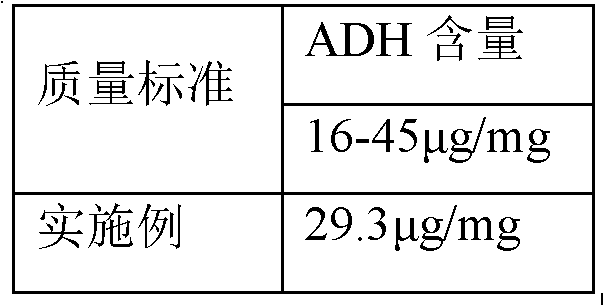
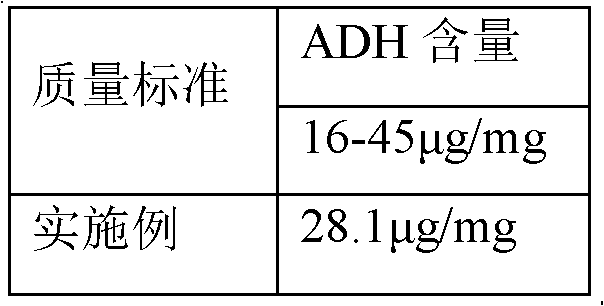
The invention discloses a process for activating a Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine, which comprises the following steps of: A, preparing Hib polysaccharide; B, dissolving 1-Cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) by using acetonitrile into a solution; C, preparing the Hib polysaccharide into a solution; D, adding the CDAP solution to the Hib polysaccharide solution, and stirring for 2-5 minutes at the room temperature; E, dissolving adipic dihydrazide (ADH) by using NaHCO3 into a solution, adding the ADH solution to a mixed solution, and stirring for 0.5-2 hours at the room temperature; and F, collecting eluent of the void volume from a loading solution after the reaction is ended at a SephadexG-25 gel chromatography column balanced in advance by water for injection, and performing freeze drying to obtain a Hib polysaccharide-ADH derivative. The process for activating the Hib polysaccharide conjugate vaccine has the beneficial effects that the quality index of the prepared Hib polysaccharide-ADH can reach the industrial standard, and moreover, the safe and nontoxic CDAP is adopted to serve as an activating agent instead of cyanogen bromide which is greatly harmful to human and environment, and therefore, the safety is enhanced, and the harm to the human and the environment are avoided.
Owner:CHENGDU OLYMVAX BIOPHARM
PRP ribose extraction method
The invention relates to a Haemophilus influenzae type B polyribosyl ribitol phosphate purification method, which comprises the following steps: 1) removing thallus in a Haemophilus influenzae type fermentation broth to obtain a fermented supernatant; and 2) removing nucleic acid and protein in the fermented supernatant to obtain PRP polysaccharide.
Owner:TIANJIN TASLY PHARMA CO LTD
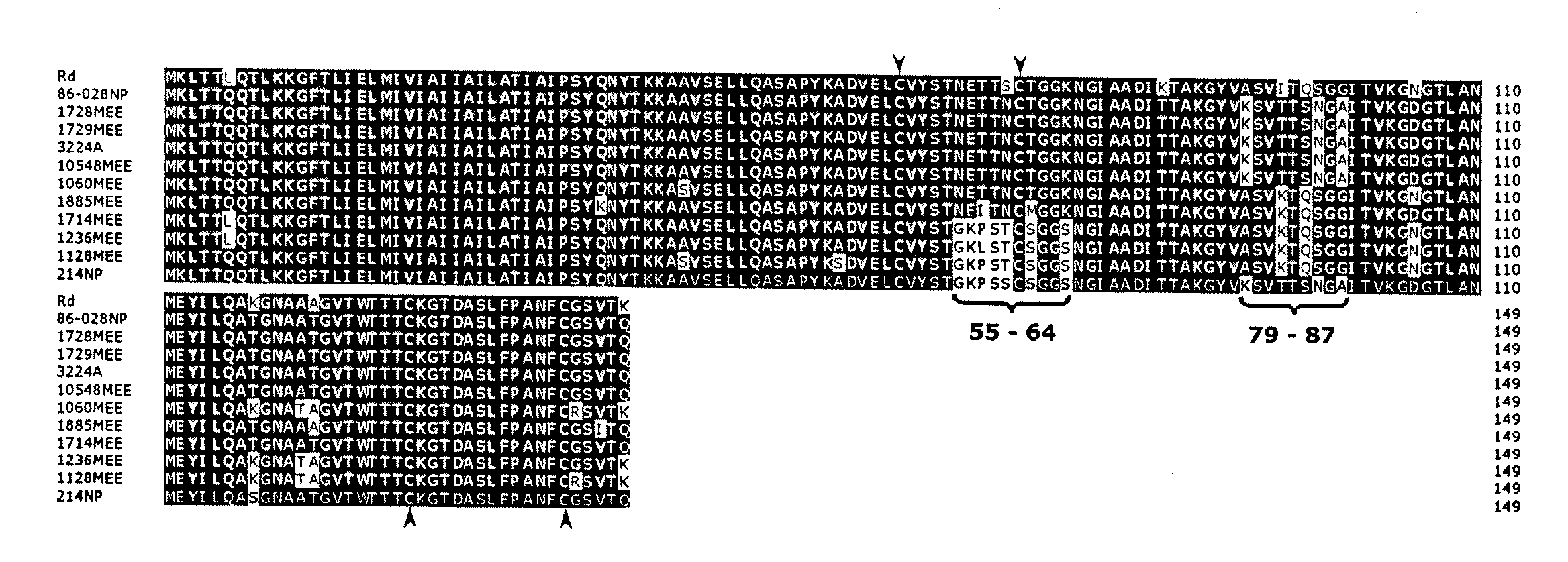
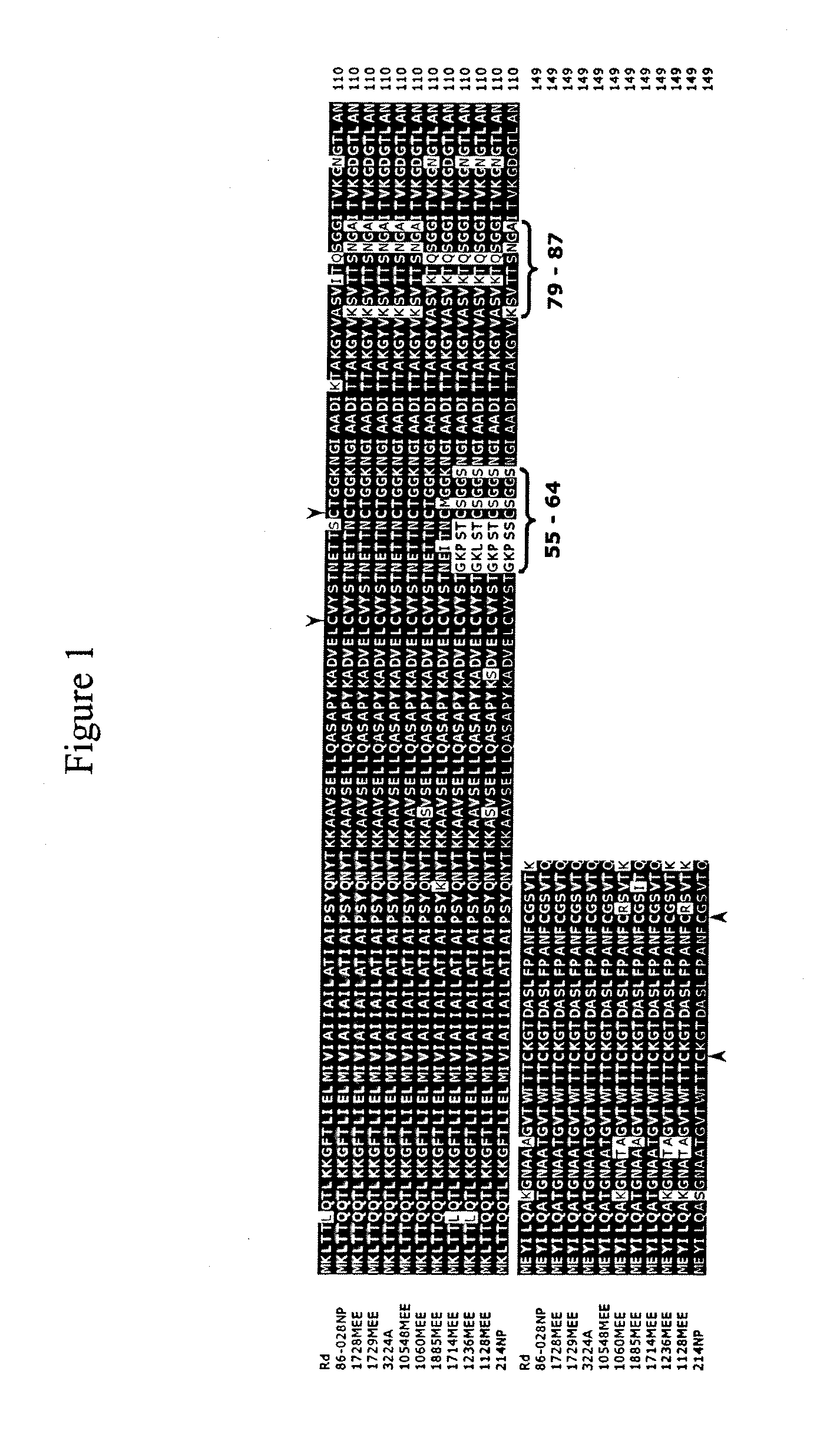
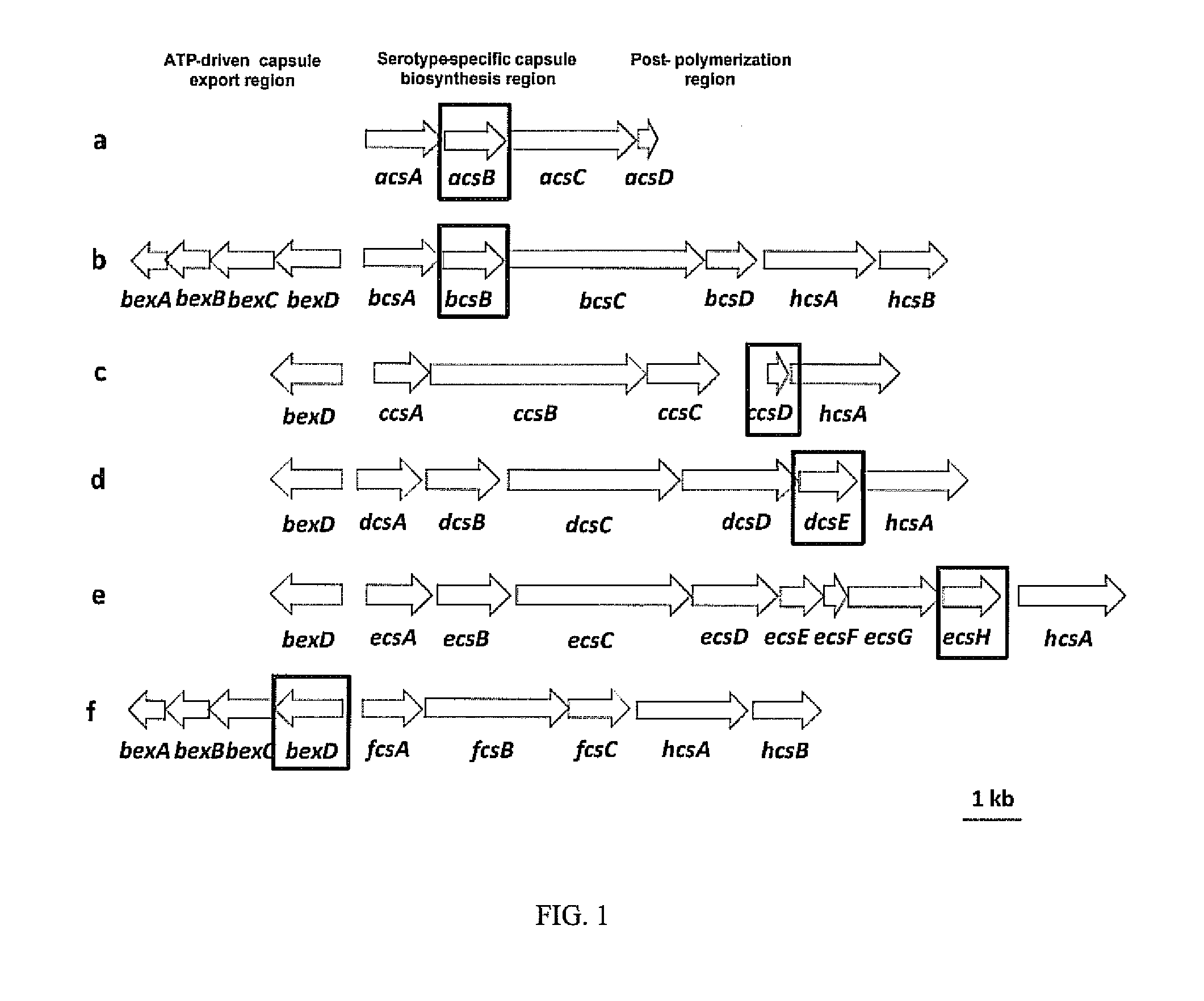
Haemophilus influenzae type IV pili
The invention described herein relates to a Haemophilus influenzae (H. influenzae) regulon encoding type IV pili. In particular, the invention relates to type IV pili from nontypeable H. influenzae (NTHi) and from H. influenzae strains a, b, c, e and f. The invention provides isolated H. influenzae pilus polynucleotides and polypeptides encoded by the polynucleotides as well as polynucleotides and polypeptides encoded by the polynucleotides involved in the assembly / disassembly of the structure. The invention also relates to uses of these polynucleotides and / or polypeptides including methods for eliciting an immune response to H. influenzae and methods of treating and preventing H. influenzae related pathological conditions.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Selective detection of Haemophilus influenzae
A process for detecting Haemophilus influenzae nucleic acid in a sample includes producing an amplification product by amplifying a Haemophilus influenzae nucleotide sequence and measuring the amplification product to detect Haemophilus influenzae in the sample. Some embodiments allow direct serotype determination in a single step assay. Also provided are reagents and methods for detecting and distinguishing Haemophilus influenzae from other infectious agents. A kit is provided for detecting and quantifying Haemophilus influenzae in a sample.
Owner:UNITED STATES OF AMERICA
Dyeing method for bacterial capsules in Haemophilus influenzae type b fermentation broth
InactiveCN102735517ALow operating skill requirementsEasy to operatePreparing sample for investigationBacteroidesMicroscopic exam
The invention discloses a dyeing method for bacterial capsules in Haemophilus influenzae type b fermentation broth. The method comprises the steps of pretreatment, smear preparation, smearing, drying, dyeing, secondary drying and microscopic examination The method employs the principle of negative dyeing, which guarantees that bacterial capsules are integral and thalluses occupy a great area and are easy to identify; a good dyeing effect is obtained, and color contrast between the capsules and a background is obvious, so the capsules can be easily observed; operation process is simple and does not have high requirements on operating skill of a tester, so the success rate of testing is high.
Owner:CHENGDU OLYMVAX BIOPHARM
Method detecting content of Hib (haemophilus influenzae type b) polysaccharide antibodies in serum through adopting ELISA method
InactiveCN102759618AEasy to getSolve the shortageMaterial analysisSerum igeHaemophilus influenzae type
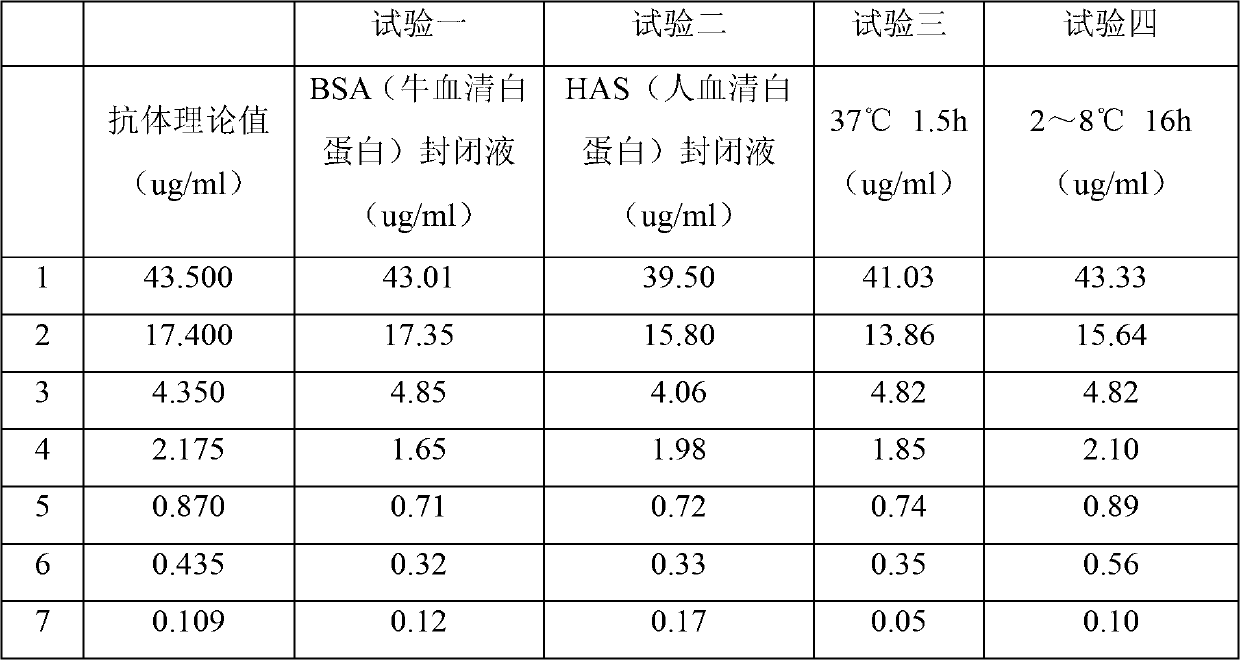
The invention discloses a method detecting content of Hib (haemophilus influenzae type b) polysaccharide antibodies in serum through adopting the ELISA method. The method provided by the invention mainly comprises the following steps: I, coating; II, applying sample; III, adding enzyme labeled antibodies; IV, substrate adding and coloration; V, reaction termination; and VI, color comparison. The method has the benefits that bovine serum albumin is adopted to substitute human serum albumin and serves as a sealing agent during the content detection of Hib polysaccharide antibodies in serum, so that the difficult problem that the sealing agent in an experiment needs a lot, yet the human serum albumin is short and high in price is solved, cost is greatly reduced, and the bovine serum albumin is easy to obtain, as a result, the popularization of the method can be facilitated; and the reaction time is changed into 16 hours at 2 to 8 DEG C, so that more serum can be detected at one time, the reaction time is sufficient, difference among plates is reduced, and the improvement on the detection accuracy is facilitated.
Owner:CHENGDU OLYMVAX BIOPHARM
Preparing method of group A and group C meningococcus and haemophilus influenzae type b combined vaccine
InactiveCN106075421AGood effectTraumaAntibacterial agentsPowder deliveryFreeze-dryingEconomic benefits
The invention discloses a preparing method of group A and group C meningococcus and haemophilus influenzae type b combined vaccine. The method includes the steps of preparing a monovalent vaccine stock solution, preparing the combined vaccine and freeze-drying the vaccine. The invention provides the preparing method of the group A and group C meningococcus and haemophilus influenzae type b combined vaccine, group A and group C meningococcus and haemophilus influenzae type b are combined together, and the prepared combined vaccine is remarkable in effect; as the vaccine is of combined type, the number of times of inoculation is reduced, infant trauma is reduced, the inoculation cost is reduced, and the combined vaccine has good social and economic benefits. The method is easy to operate and suitable for large-scale industrial production, and the combined vaccine is easy to prepare and low in cost.
Owner:CHENGDU OLYMVAX BIOPHARM
Haemophilus influenzae type IV pili
ActiveUS20090041774A1Improve efficiencyAntibacterial agentsOrganic active ingredientsHaemophilusPilus
The invention described herein relates to a Haemophilus influenzae (H. influenzae) regulon encoding type IV pili. In particular, the invention relates to type IV pili from nontypeable H. influenzae (NTHi) and from H. influenzae strains a, b, c, e and f. The invention provides isolated H. influenzae pilus polynucleotides and polypeptides encoded by the polynucleotides as well as polynucleotides and polypeptides encoded by the polynucleotides involved in the assembly / disassembly of the structure. The invention also relates to uses of these polynucleotides and / or polypeptides including methods for eliciting an immune response to H. influenzae and methods of treating and preventing H. influenzae related pathological conditions.
Owner:NATIONWIDE CHILDRENS HOSPITAL
A kind of preparation method of Haemophilus influenzae type b polysaccharide conjugate vaccine
ActiveCN103623404BLarge amount of processingSimple processAntibacterial agentsBacteriaConjugate vaccineHaemophilus
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Selective detection of haemophilus influenzae
Owner:UNITED STATES OF AMERICA
Multivalent DTP-POLID vaccines
InactiveCN100408095CEffective preventionAntibacterial agentsSsRNA viruses negative-senseTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
HIB (Haemophilus influenzae type B) synthetic medium, HIB conjugate vaccine and preparation method of HIB conjugate vaccine
ActiveCN105199998AStable passagePassage safe and reliableAntibacterial agentsBacteriaConjugate vaccineVaccine Production
The invention provides a HIB (Haemophilus influenzae type B) synthetic medium, a HIB conjugate vaccine and a preparation method of the HIB conjugate vaccine, relates to the field of a vaccine production preparation technology and solves the problems that the HIB conjugate vaccine contains haematogenous components, is likely to have an anaphylactic reaction, is unstable and high in cost and has high content of nucleic acid, egg white and endotoxin. The HIB synthetic medium comprises following components in terms of final concentration: 5-15 g / l of yeast extract powder, 5-15 g / l of glucose, 1-3 g / l of MgCl2*6H2O, 0.01-0.03 g / l of beta-coenzyme, 0.005-0.02 g / l of hemin, 22-32 g / l of Na2HPO4*12H2O and 2-5 g / l of NaH2PO4*2H2O. The medium does not contain the haematogenous components and macromolecular allergen components, so that strain passage is more stable, safer and more reliable, and the prepared HIB conjugate vaccine has low endotoxin content and good stability and is safe and effective.
Owner:ZHONGYI ANKE BIOTECH CO LTD +1
Vaccine for inducing an improved immune reaction
InactiveCN103384532AExcellent immune boosting effectImprove stabilityAntibacterial agentsSsRNA viruses negative-senseHepatitis B virusHuman immunodeficiency virus (HIV)
The present invention relates to a pharmaceutical vaccine composition comprising: (a) a pathogen-derived antigen selected from the group consisting of Mycobacterium tuberculosis antigen, Bacillus anthracis antigen, HAV (hepatitis A virus) antigen, HBV (hepatitis B virus) antigen, HCV (hepatitis C virus) antigen, HIV (human immunodeficiency virus) antigen, influenza virus antigen, HSV (herpes simplex virus) antigen, Hib (Haemophilus influenzae type b) antigen, Neisseria meningitidis antigen, Corynebacterium diphtheriae antigen, Bordetella pertussis antigen, Clostridium tetani antigen and Varicella virus antigen; (b) a deacylated non-toxic LOS (lipooligosaccharide); and (c) a pharmaceutically acceptable carrier.
Owner:EYEGENE INC
Polyvalent pneumococcus and B-type haemophilus influenzae combined vaccine
PendingCN106075420AInhibit side effectsPrevent oxidationAntibacterial agentsPowder deliverySerum igeMedicine
The invention provides a polyvalent pneumococcus and B-type haemophilus influenzae combined vaccine. Each dose of the combined vaccine contains 50-500 micrograms / ml and 10-60 micrograms / ml of B-type haemophilus influenzae capsule polysaccharide, and the serotype of pneumococcus is one or more of 1A, 3A, 4A, 5A, 6A, 6B, 7F, 8N, 9N, 9V, 12F, 14B, 15B, 18C, 19A, 19F, 22F and 23F. Compared with the prior art, the combined vaccine is safe, reliable and good in stability, and the application prospect of the combined vaccine is very wide.
Owner:BRAVOVAX
High-density culture and production method of bacterium capsular polysaccharide with haemophilus influenzae type b
ActiveCN102628068BIncrease productionReduce the number of extractions to remove impurity proteinsBacteriaMicroorganism based processesHigh concentrationHaemophilus influenzae type
The invention discloses a high-density culture and production method of bacterium capsular polysaccharide with haemophilus influenzae type b. The method comprises following steps: subculturing Hib strains from a first generation to a fourth generation, carrying out culture in a seeding tank, carrying out fermentation and culture in a fermentation tank, killing bacteria, acquiring a supernatant, and purifying the supernatant. Beneficial effects of the invention are as follows: (1) by culturing Hib bacteria to a high concentration, output of Hib capsular polysaccharide is improved by 50% to 100% on the basis of the output of Hib capsular polysaccharide produced by the traditional way, and produced capsular polysaccharide meets the national standard; (2) by controlling nucleic acids, protein, and other impurities in a zymotic fluid to a low level, the number of times of phenol extracting for removing impurity protein is reduced for relatively low protein content, thereby reducing phenol amount used in subsequent purifying processes, reducing environmental pollution, and reducing production cost; and (3) output of polysaccharide is stable, and content of refined glycoprotein, endotoxin, and other impurities after the purifying is substantially lower than the national control standard.
Owner:CHENGDU OLYMVAX BIOPHARM
Selective detection of haemophilus influenzae
ActiveUS20130295557A1Easy to understandMicrobiological testing/measurementHaemophilusHaemophilus influenzae type
A process for detecting Haemophilus influenzae nucleic acid in a sample includes producing an amplification product by amplifying a Haemophilus influenzae nucleotide sequence and measuring the amplification product to detect Haemophilus influenzae in the sample. Some embodiments allow direct serotype determination in a single step assay. Also provided are reagents and methods for detecting and distinguishing Haemophilus influenzae from other infectious agents. A kit is provided for detecting and quantifying Haemophilus influenzae in a sample.
Owner:UNITED STATES OF AMERICA
A kind of polymyxin b magnetic bead, preparation method and application thereof
ActiveCN109557303BImprove detection efficiencyHigh detection sensitivityMaterial analysisA lipoproteinMagnetic bead
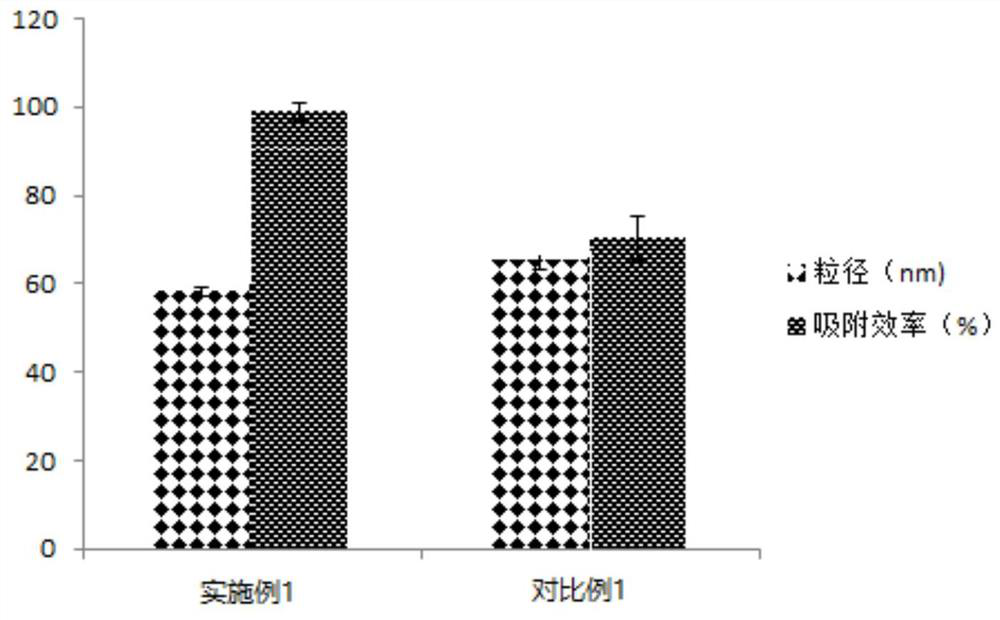
The invention discloses a polymyxin B magnetic bead, a preparation method and an application thereof. 3 o 4 Nano-magnetic beads and polymyxin B solution are mixed and then stirred at 40-60°C for 2-5 hours to obtain Fe coated with polymyxin B on the surface 3 o 4 Nano magnetic beads, that is, polymyxin B magnetic beads. The polymyxin B magnetic beads include Fe 3 o 4 Nano magnetic beads and coated in Fe 3 o 4 Polymyxin B on the surface of magnetic nanobeads. The polymyxin B magnetic beads prepared by the present invention are combined with the free negatively charged phosphate groups of lipoproteins on the cell membrane of Haemophilus influenzae type b through the specific polycationic ring of the polymyxin B molecule through electrostatic interaction, and have high accuracy. High and specific characteristics. It can quickly enrich trace amounts of Haemophilus influenzae type b from cerebral effusion and achieve good adsorption effect. At the same time, the detection efficiency and sensitivity of Haemophilus influenzae type b are improved.
Owner:SHAANXI NORMAL UNIV
A kind of preparation method of non-adjuvant Haemophilus influenzae type b conjugate vaccine freeze-dried agent
ActiveCN104491854BEliminate aggregationEliminate degradationAntibacterial agentsPowder deliveryConjugate vaccineAdjuvant
The invention discloses a preparation method of a non-adjuvant Haemophilus influenzae type b conjugate vaccine freeze-drying agent, which includes formulation, mixture preparation and freeze-drying process. In the process of vacuum freeze-sublimation drying in the freeze-drying process to remove free water, the present invention controls the temperature of the bottled material below the eutectic point of free water and bound water to eliminate the aggregation of CRM197 protein; removes free water by desorption and drying in vacuum During the process, the temperature of the bottled material is controlled below the degradation point to eliminate the degradation of the Hib capsule structure, so that the Haemophilus influenzae type b conjugate vaccine freeze-dried agent does not need to add an adjuvant technical solution, which overcomes the protein aggregation existing in the prior art , structural degradation, and the problems and shortcomings of adding adjuvants, the Haemophilus influenzae type b combined vaccine freeze-dried agent has achieved the purpose of eliminating protein aggregation and structural degradation without adding adjuvants.
Owner:艾美坚持生物制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com