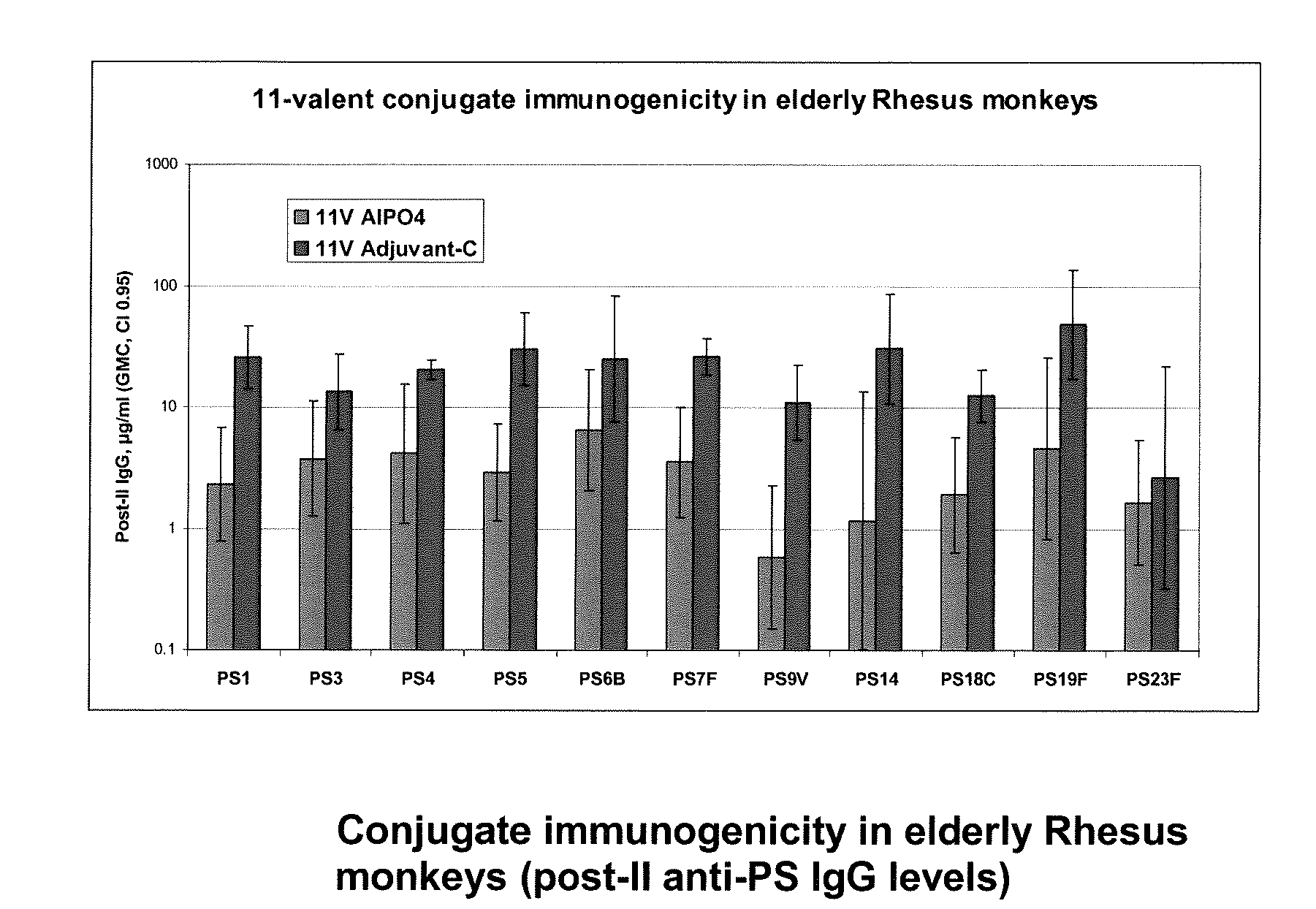
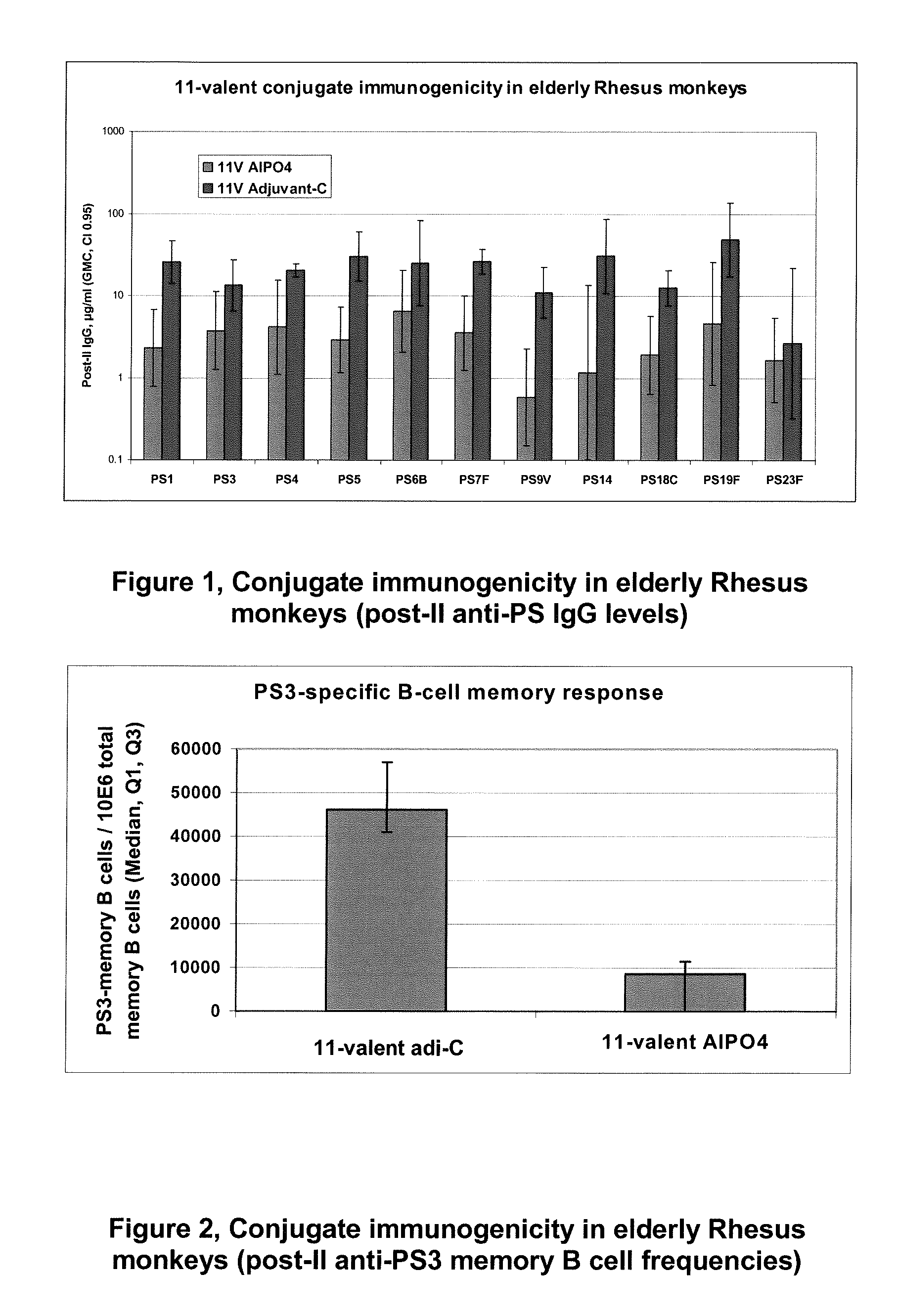
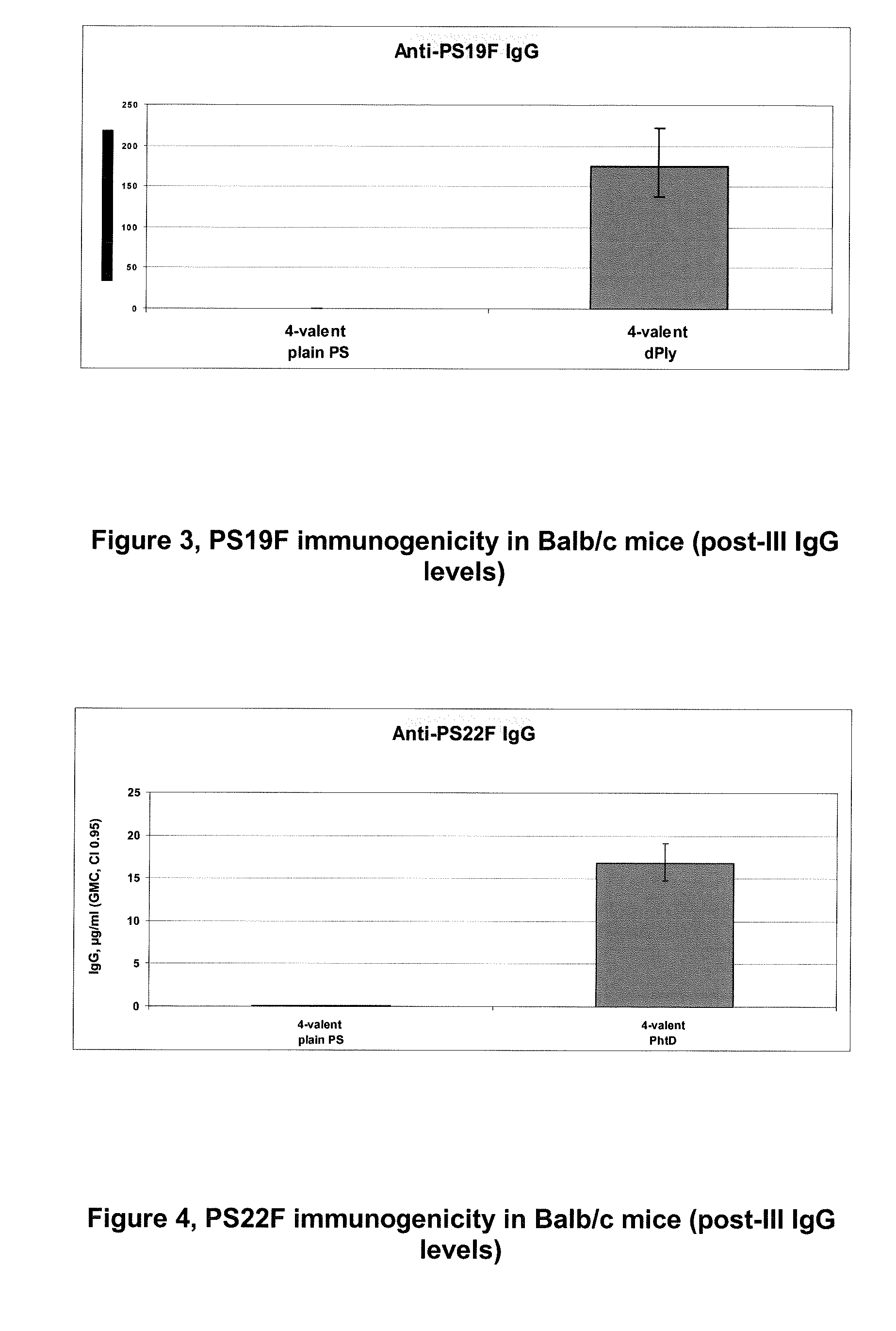
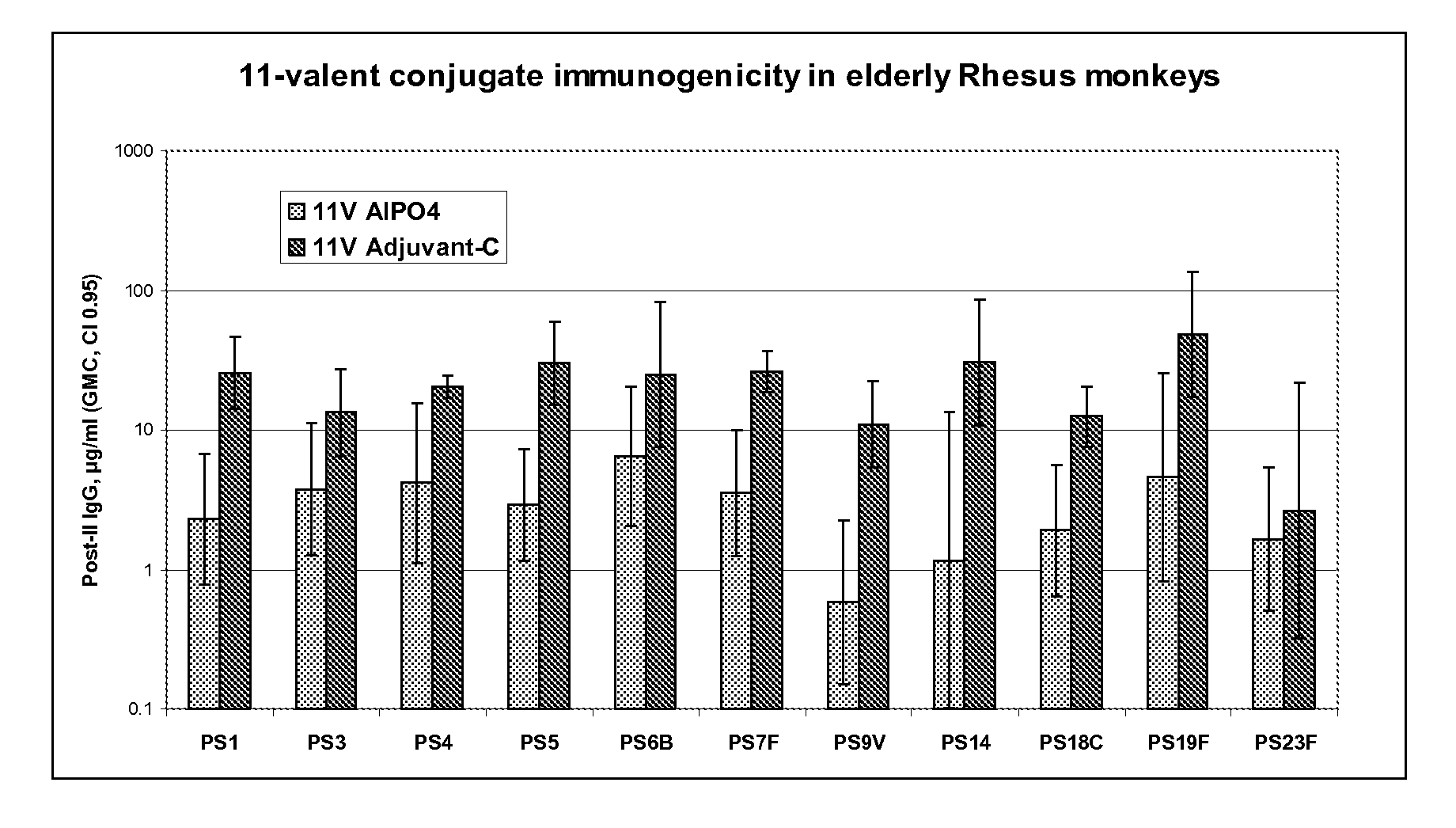
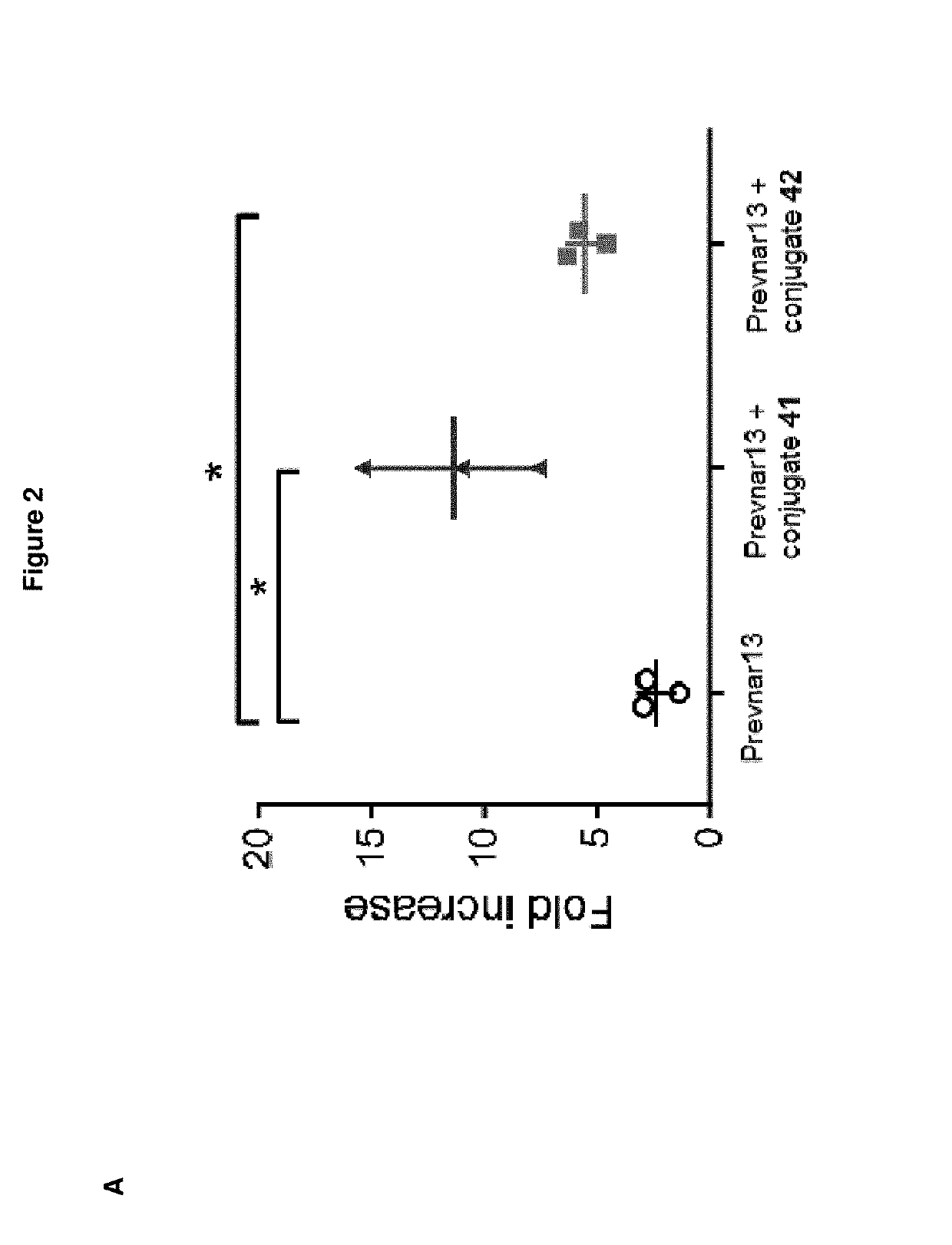
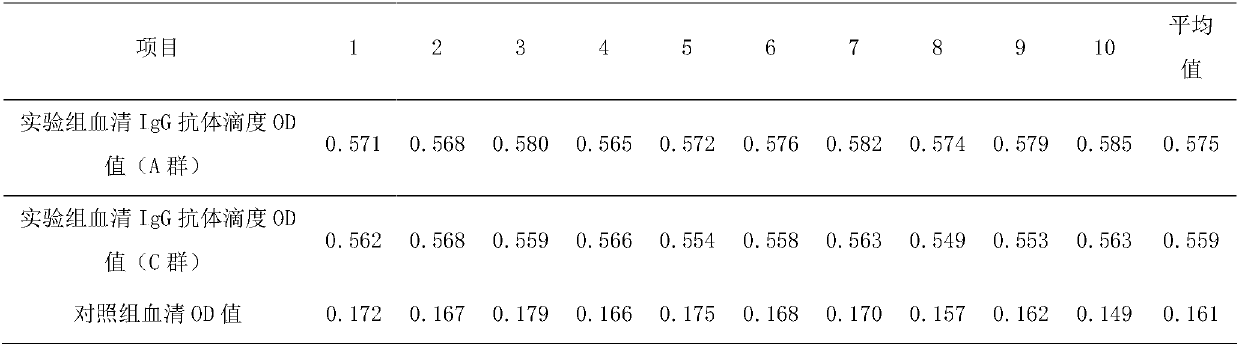
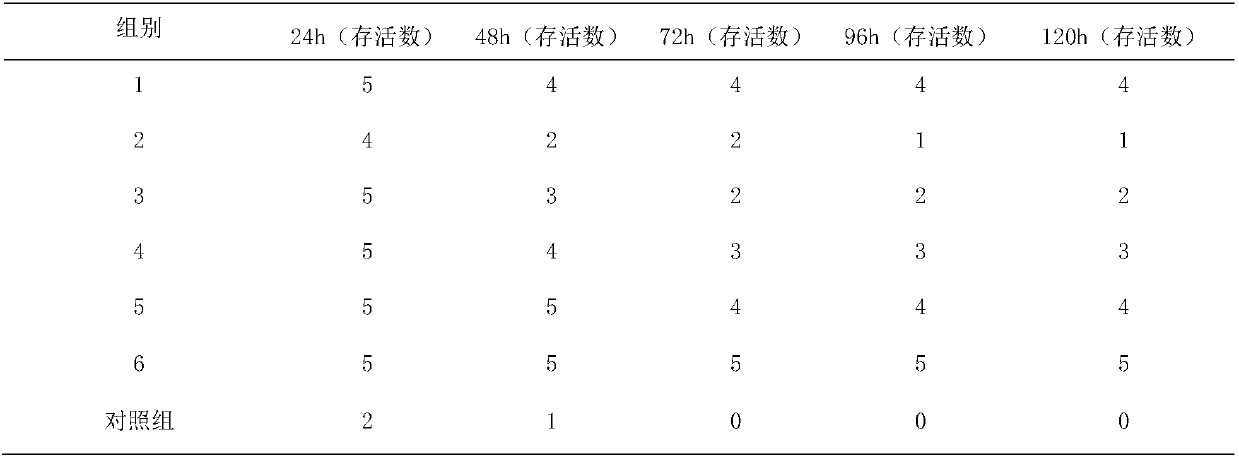
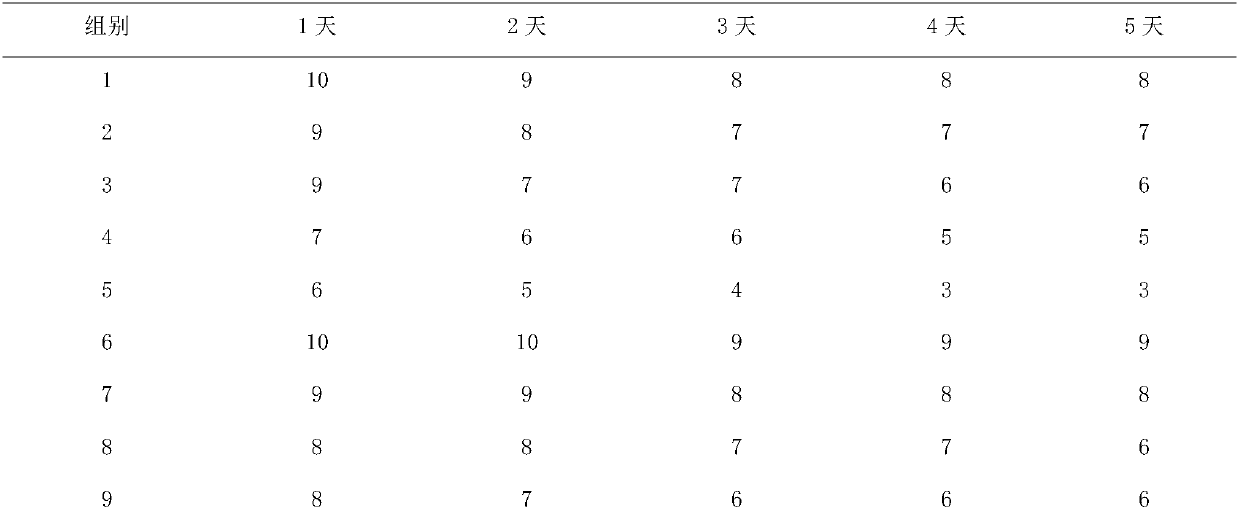
Patents
Literature
53 results about "Diptheria toxoid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The tetanus and diphtheria toxoids vaccine (also called Td) is used to help prevent these diseases in adults and children who are at least 7 years old. This vaccine works by exposing you to a small dose of the bacteria or a protein from the bacteria, which causes the body to develop immunity to the disease.
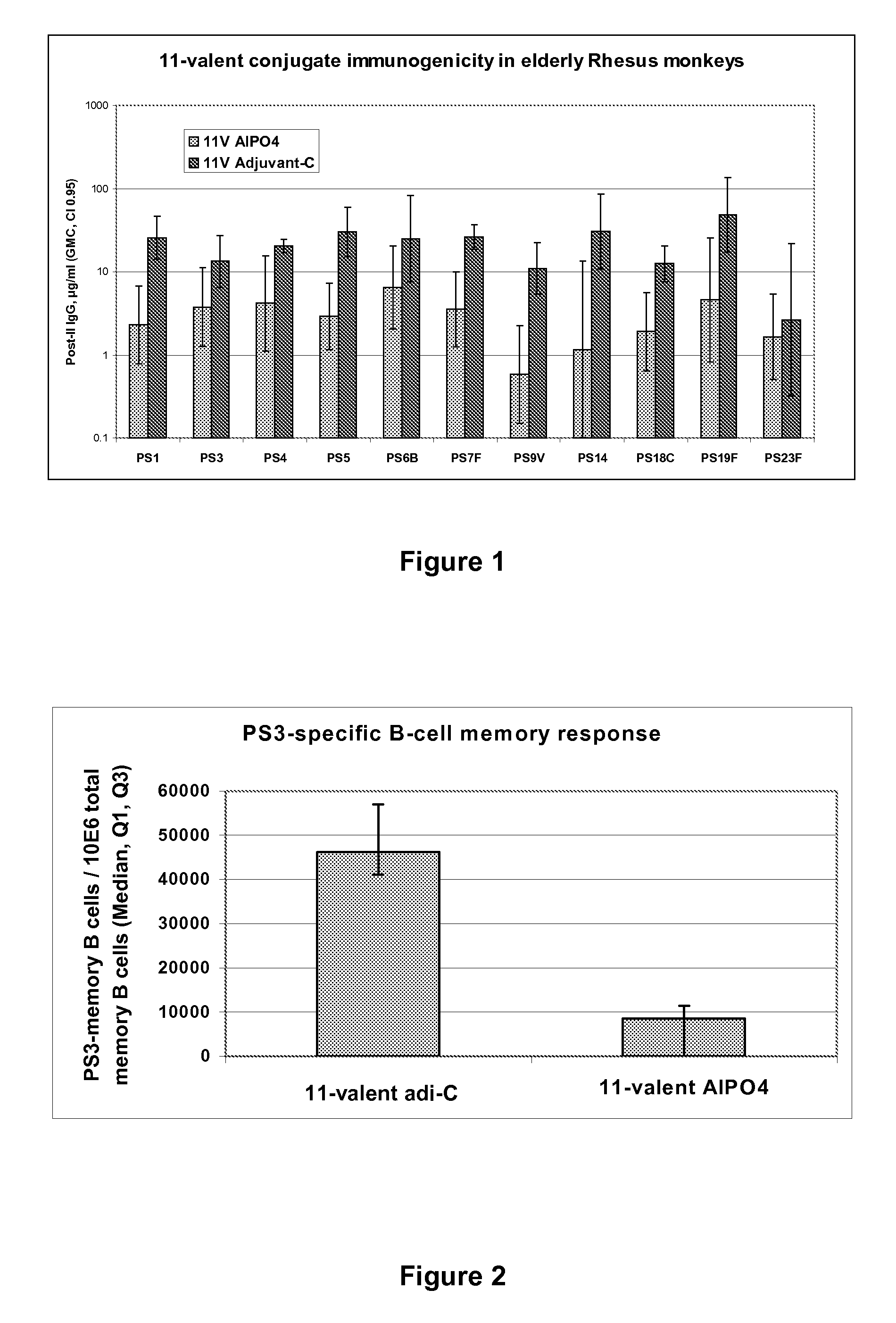
Pneumococcal Polysaccharide Conjugate Vaccine
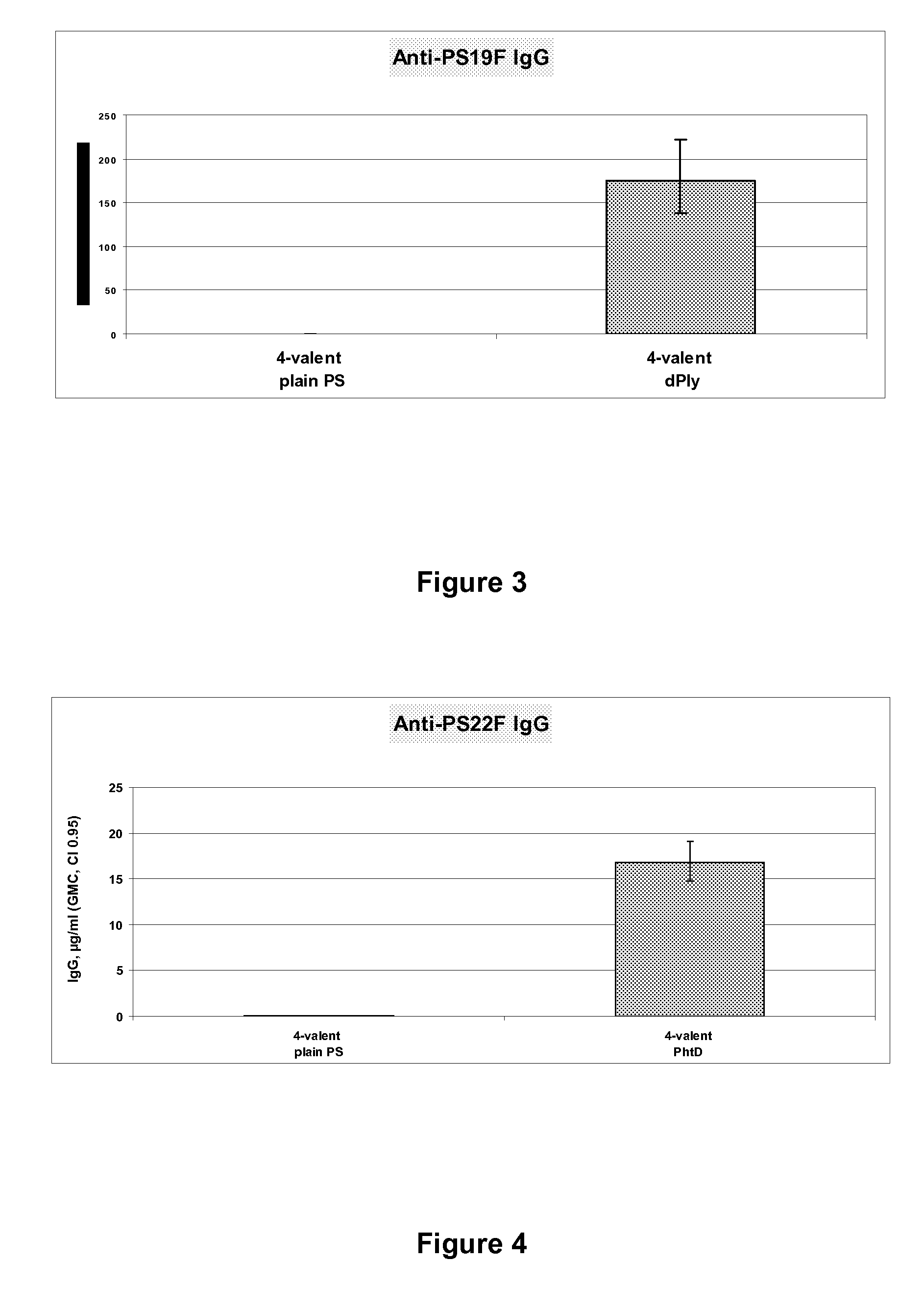
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid (DT) or CRM197, optionally wherein 19F is the only saccharide in the composition conjugated to diphtheria toxoid (DT) or CRM197.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100209450A1Antibacterial agentsSenses disorderConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
Separated and purified acellular pertussis-diphtheria-tetanus, b-type haemophilus influenzae and A-group and C-group meningococcus combined vaccine and preparation method thereof
InactiveCN104689309ARelieve painReduce the burden onAntibacterial agentsBacterial antigen ingredientsHemagglutininAluminium hydroxide
The invention discloses a combined vaccine and a preparation method thereof. The combined vaccine is formed by a component A and a component B, wherein the component A is a liquid preparation and composed of pertussis toxin, filamentous hemagglutinin, pertussis adhesion, diphtheria toxoid, tetanus toxoid, aluminium hydroxide and sodium chloride; the component B is a freeze-drying preparation and composed of A-group meningitis polysaccharide conjugate, C-group meningitis polysaccharide conjugate, b-type haemophilus influenzae polysaccharide conjugate and lactose. The preparation method of the vaccine includes the steps of preparing of acellular pertussis-diphtheria-tetanus vaccine semi-finished products, A-group and C-group meningococcocci and b-type haemophilus influenzae combined vaccine semi-finished products, split charging and packaging. The vaccine has the characteristics of being safe, effective, controllable and capable of preventing diseases through an injection, the preparation method is easy to operate, preparation is facilitated, cost is low, and the combined vaccine is suitable for industrialized mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
Pneumococcus polysaccharide protein coupling vaccine and its preparing method
The present invention is pneumococus polysaccharide protein coupling vaccine comprising covalently connected pneumococus capsule polysaccharide and recombinant pneumolysin without hemolytic activity modification and its preparation process. The vaccine has pneumolysin without hemolytic activity as protein carrier, no need of eliminating hemolysis toxicity of pneumolysin with formalin and ensured safety, and may be used for infant below 2 yeas old to prevent tympanitis. Owing to the pneumolysin as the self protein, the vaccine has no probable immune interference reaction and strengthened immune protecting effect. The vaccine has cross immunizing protection effect on various kinds of serum type pneumococus and raised immune memory response to pneumococus infection.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Multivalent immunogenic composition containing enterovirus antigens
ActiveCN103386126AImproving immunogenicityImprove securityBacterial antigen ingredientsViral antigen ingredientsHepatitis A AntigensTetanus toxoids
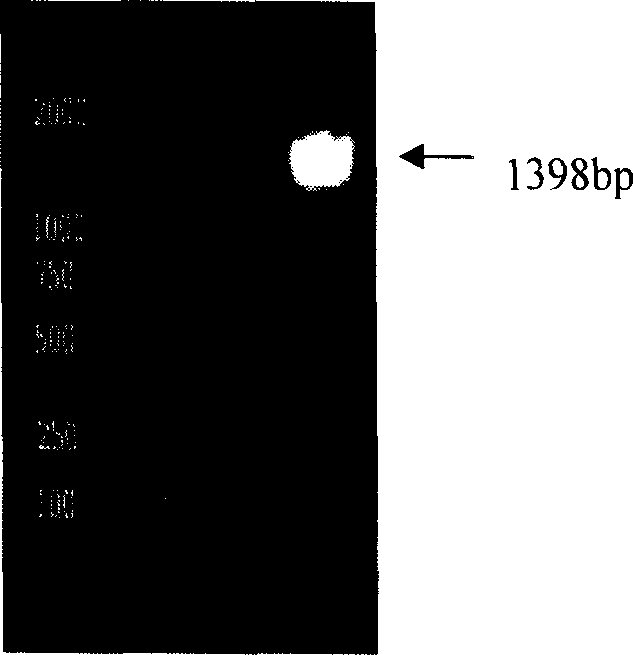
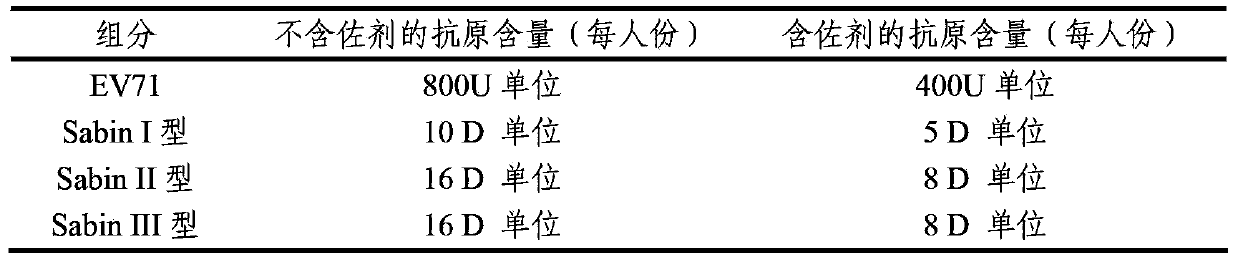
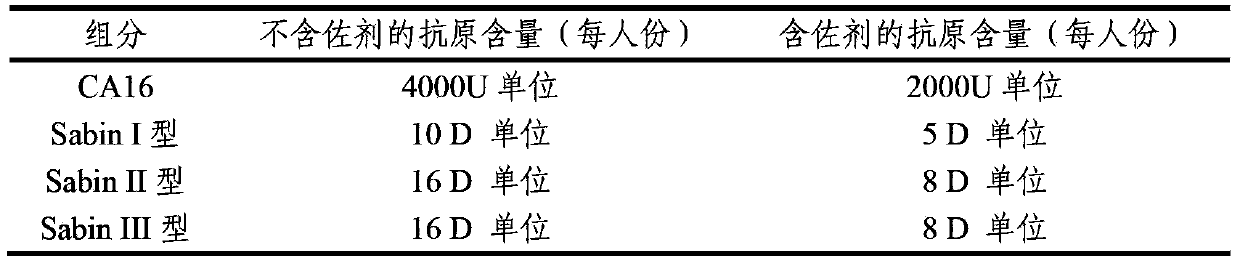
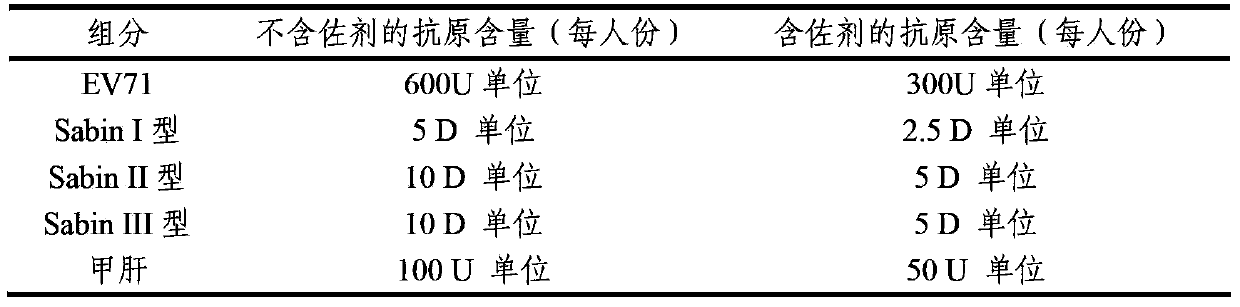
The invention provides a multivalent immunogenic composition containing enterovirus antigens. The composition comprises inactivated EV71 antigens and / or inactivated CA16 antigens, and inactivated polio antigens. The composition can further comprise antigens selected from hepatitis A antigens, hepatitis B antigens, acellular pertussis antigens, tetanus toxoid, diphtheria toxoid, Haemophilus influenzae type b capsular polysaccharide, and meningococcal polysaccharide antigens, as well as physiologically acceptable carriers combined with bacterial polysaccharide antigens. The invention also provides a preparation method of the composition. The composition can prevent invasion of a plurality of pathogens simultaneously without interference among the antigens, and the immunogenicity is no less than that of individually activated antigens. With the composition, vaccination processes are significantly simplified, and the vaccination efficiency is improved with reduced costs.
Owner:SINOVAC BIOTECH
Fermentation media free of animal-derived components for production of diphtheria toxoids suitable for human vaccine use
ActiveUS20130122040A1Minimising and avoiding cross-linkingLower potentialAntibacterial agentsBacterial antigen ingredientsDetoxification ProcessDiptheria toxoid
The present invention relates to a fermentation medium for cultivating Corynebacterium diphtheriae. The present invention also relates to the use of the fermentation medium in processes for obtaining diphtheria toxin from the Corynebacterium diphtheriae bacteria being cultivated and the preparation of vaccines using the diphtheria toxin obtained in the processes. The present invention further relates to a purification and detoxification processes specifically adapted for preparing a diphtheria toxoid for inclusion into a vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent DTP-POLIO vaccines
InactiveCN101310769AEffective preventionSsRNA viruses negative-senseAntibacterial agentsTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
Integration of meningococcal conjugate vaccination
ActiveUS20090060945A1Easy to useAvoid carrier suppressionAntibacterial agentsCarrier-bound antigen/hapten ingredientsDiphtheria vaccinationCarrier protein
Conjugated meningococcal capsular saccharides will be introduced into immunisation schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunising a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
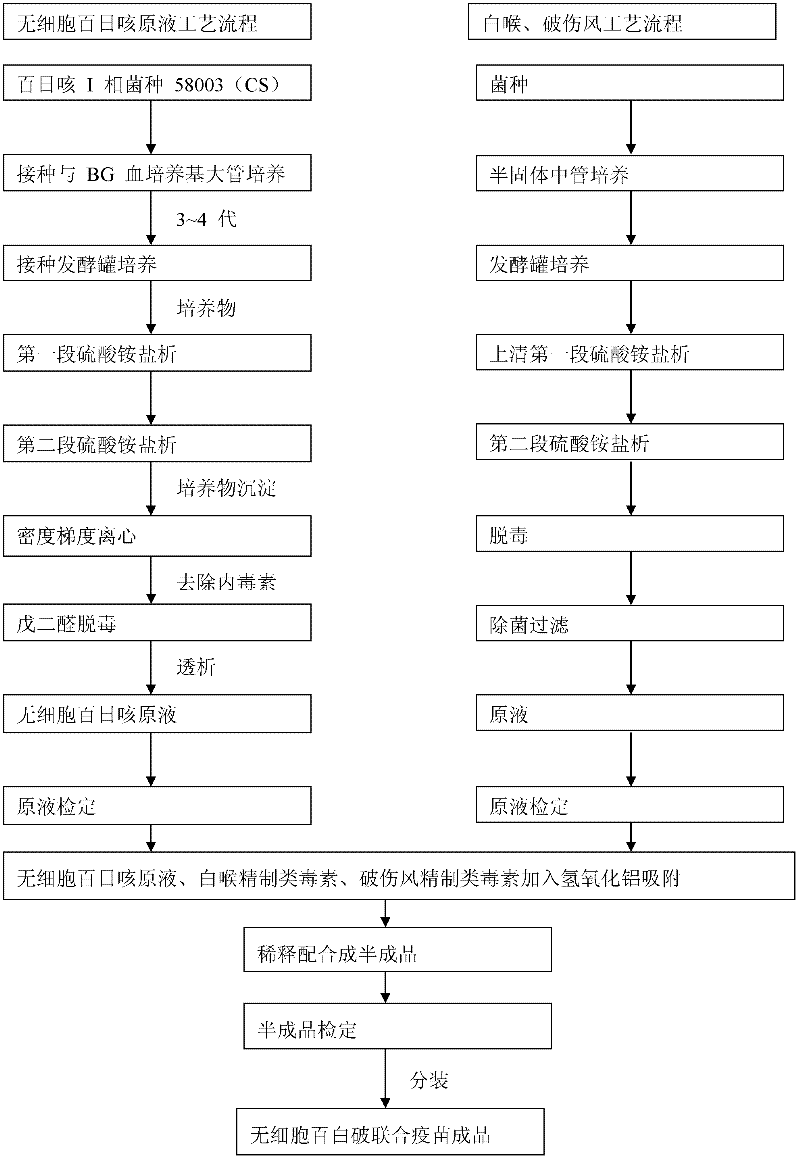
Technique for improving purity of diphtheria toxoid
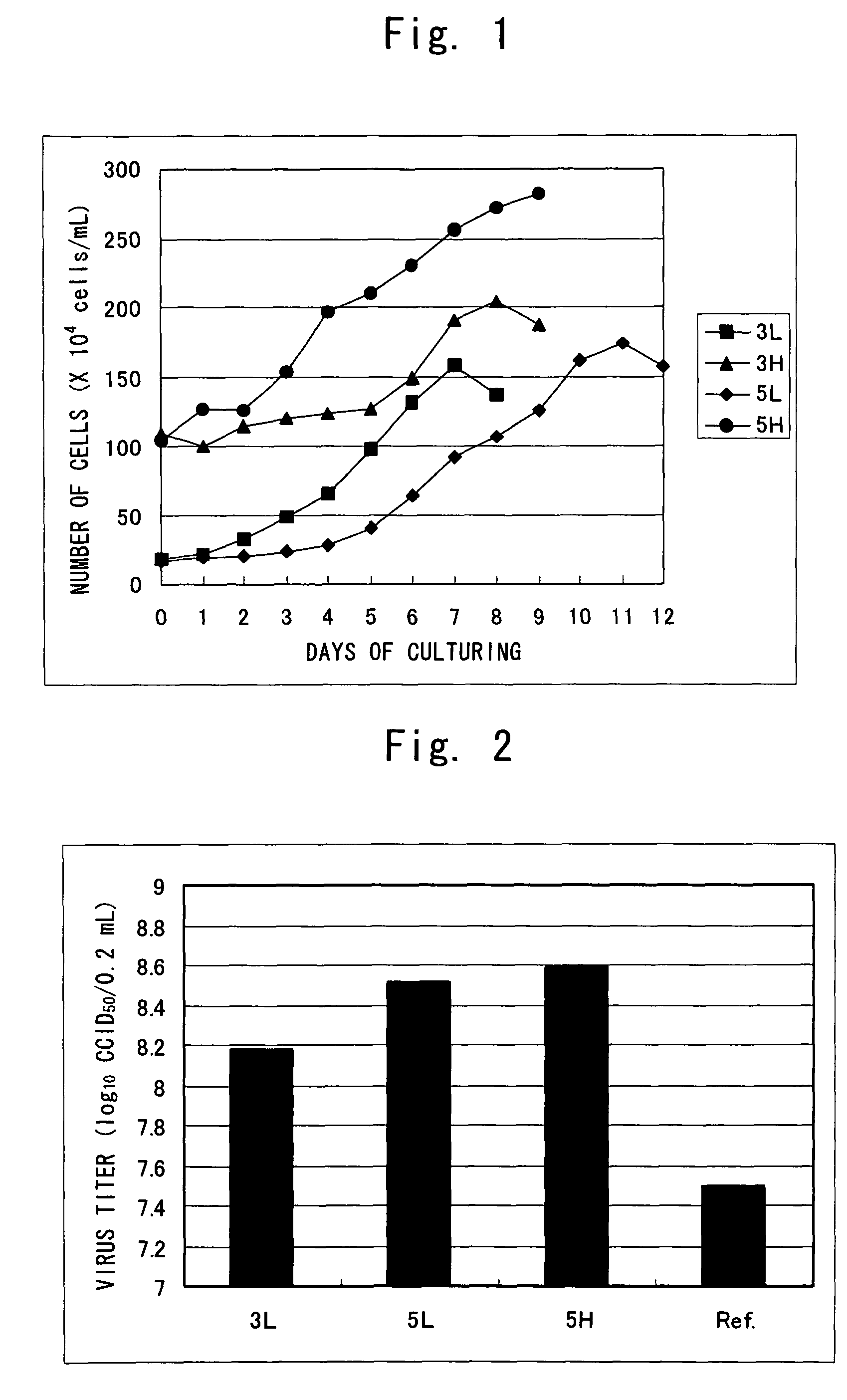
ActiveCN101503725AHigh purityReasonable process routeAntibacterial agentsDepsipeptidesLiquid productUltrafiltration
The invention relates to a process for improving the purity of diphtheria toxin. The manufacture process comprises the following steps of solid culture medium transfer, culture in a liquid culture medium fermentor, removal of thalli by centrifugation, primary salting-out, secondary salting-out, ultrafiltration desalting, gel filtration, aseptic filtration, removal of toxin, detection and storage stock solution, preparation of semi-finished products, finished products, wherein the liquid culture medium in the liquid culture medium fermentor is treated to remove proteins with the molecular weight of more than 50,000, and the culture products are almost diphtheria toxin with the molecular weight of more than 50,000; and by salting out and gel filtration, the proteins and other impurity proteins of which the molecular weight is less than 50,000 in the liquid product are further removed, so that the diphtheria toxin with high purity is obtained.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
IPV-DPT vaccine
InactiveUS8753646B2Efficient productionAntibacterial agentsBacterial antigen ingredientsProtective antigenTetanus toxoids
The invention provides a process for producing a combined vaccine containing an inactivated Sabin strain of poliovirus, a Bordetella pertussis protective antigen, a diphtheria toxoid and a tetanus toxoid, the process including a step of producing a high-titer Sabin strain poliovirus. The inventive process for producing a combined vaccine, including a step of culturing, in the presence of from about 4 g / L to about 6 g / L of a microcarrier, Vero cells to be inoculated with a Sabin strain of poliovirus, is useful as a process for efficiently producing a combined vaccine containing an inactivated Sabin strain of poliovirus.
Owner:TAKEDA PHARMA CO LTD +1
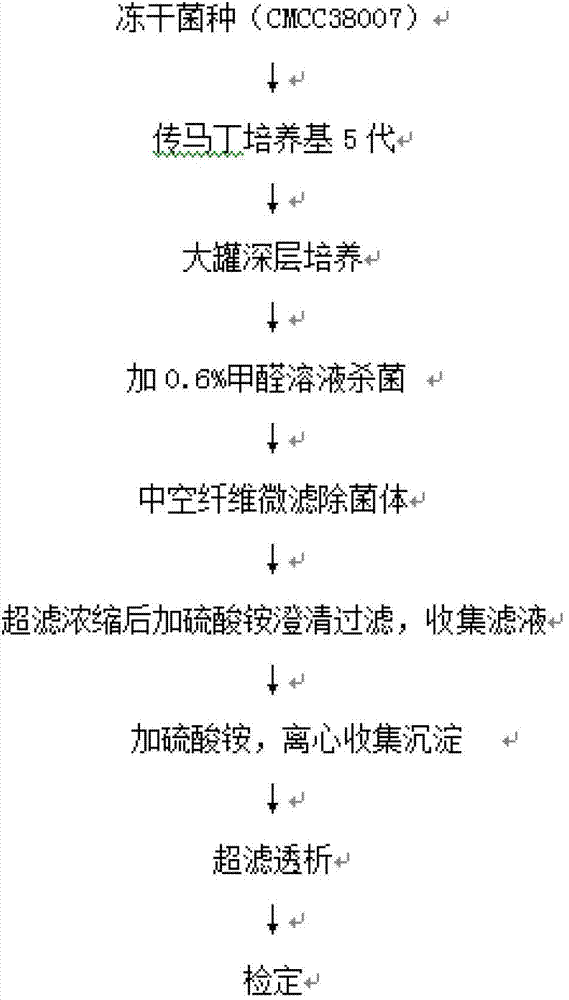
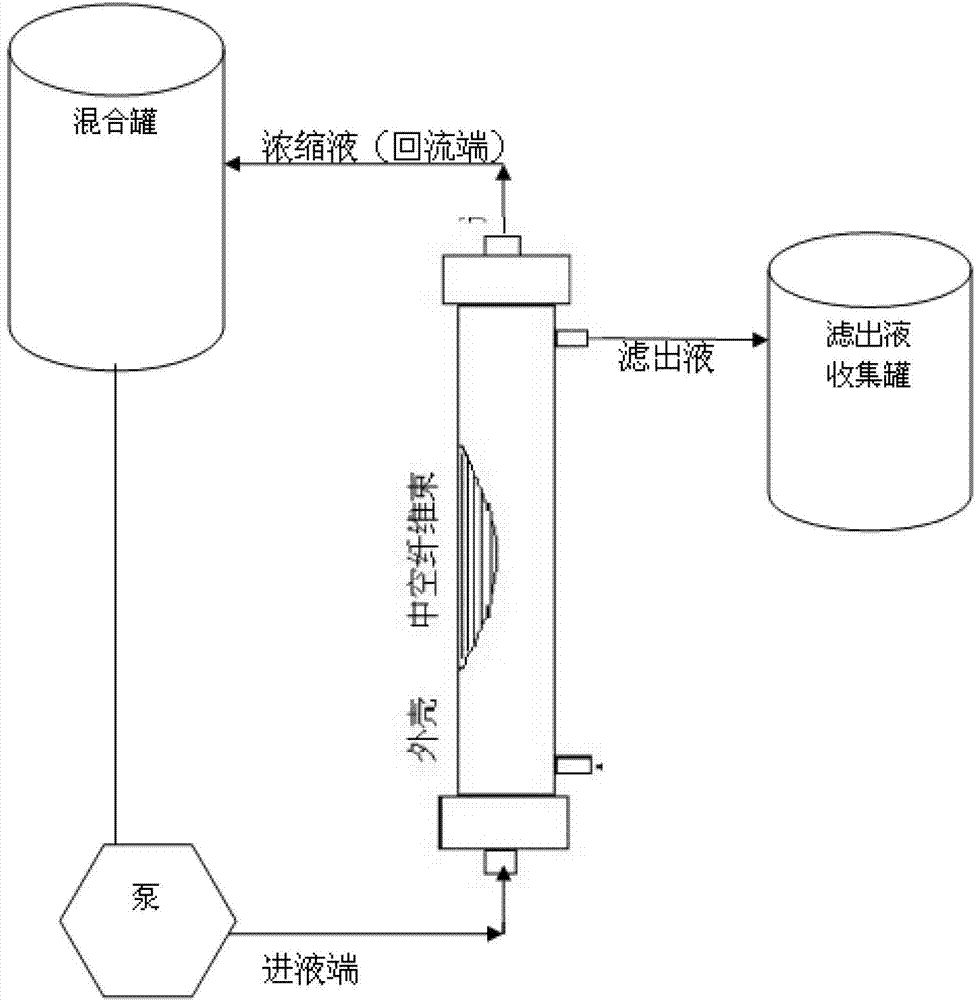
Preparation method of diphtheria toxoid vaccine
ActiveCN102961740AAvoid damageEfficient removalAntibacterial agentsBacterial antigen ingredientsFiberUltrafiltration
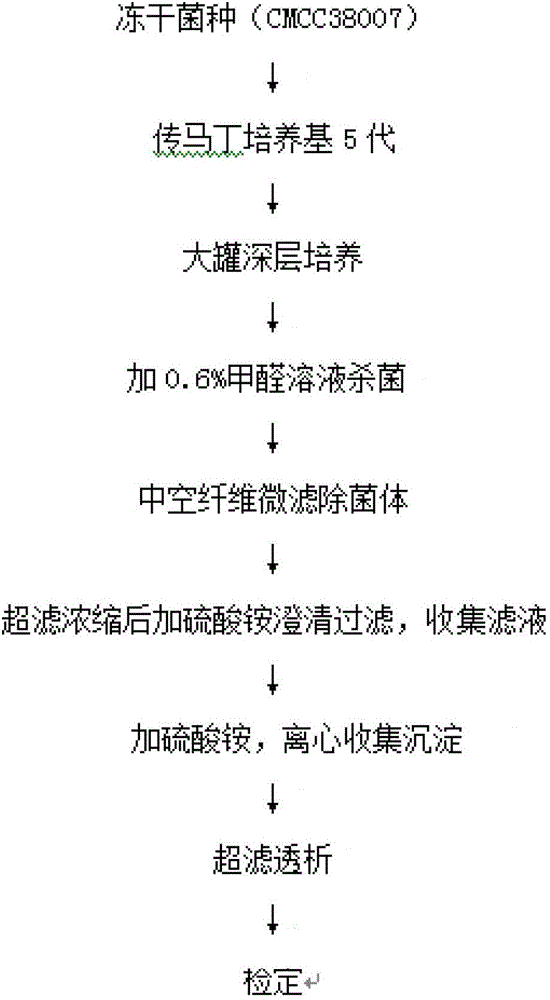
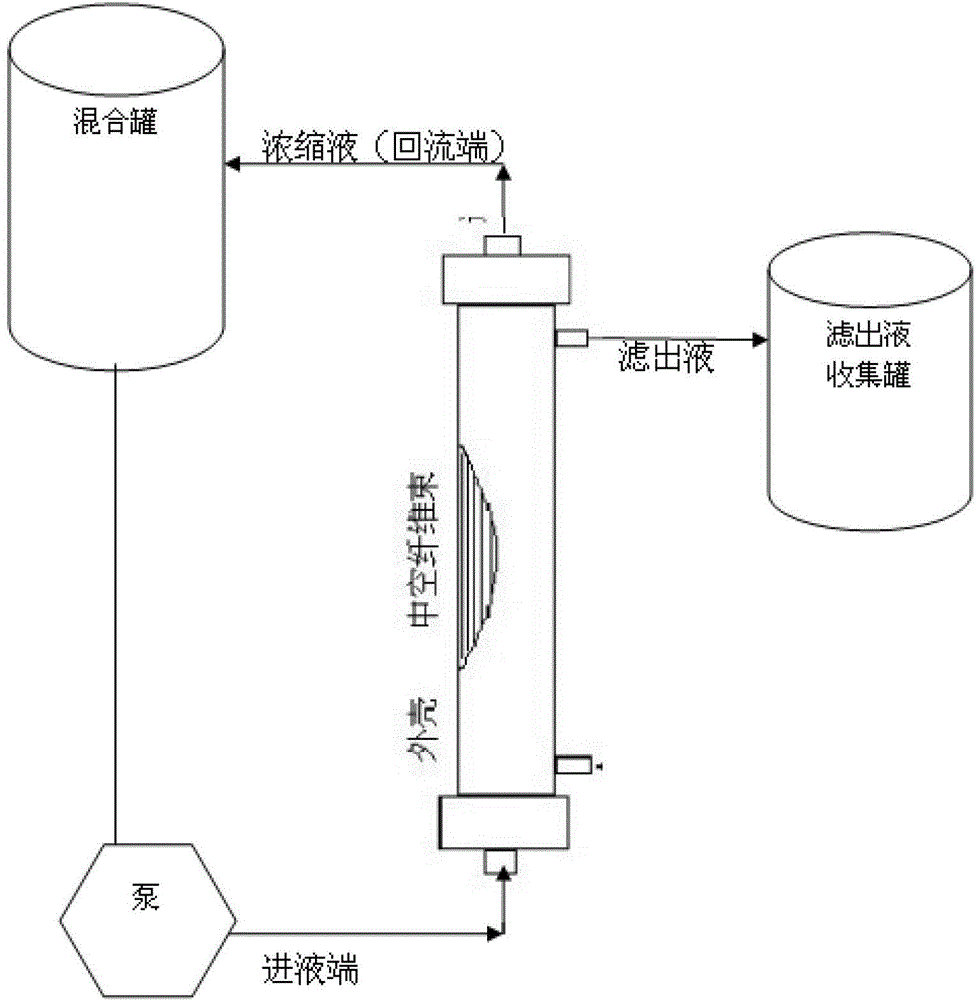
The invention discloses a preparation method of a diphtheria toxoid vaccine. The diphtheria toxoid vaccine is prepared by taking a diphtheria bacillus strain as a raw material and then carrying out the steps of diphtheria toxoid culture, bacteria-liquid separation, ultrafiltration and concentration, two-time salting out, ultrafiltration and desalination and the like. According to the preparation method, the thalli of the culture solution is removed by using a hollow fiber filter membrane and then refining is carried, so that the pore blockage caused by the accumulation of the thalli and other impurity fragments in the subsequent filter process is prevented; through an improved salting out method, the toxin proteins and other allergens in the culture solution are effectively removed; and a tangential flow ultrafiltration method is used for both the concentration of the culture solution and the desalting method after the salting out, so that the antigen damage caused by the shearing to the toxin proteins is decreased and the precipitation of the proteins is avoided. According to the preparation method, the preparation time of the diphtheria toxoid vaccine is shortened and the production efficiency is improved.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Immunogenic compositions
InactiveUS20150283232A1Improve protectionImprove purification effectAntibacterial agentsBacterial antigen ingredientsAcyl carrier proteinSerotype
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Pastevula mulfocida capsular polysaccharide-protein conjugate vaccine and preparation method thereof
ActiveCN108721616AIncrease virulencePlay the role of cross protectionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineHeterologous
Belonging to the technical field of biology, the invention in particular relates to a pastevula mulfocida capsular polysaccharide-protein conjugate vaccine and a preparation method thereof. The pastevula mulfocida capsular polysaccharide-protein conjugate vaccine provided by the invention is formed by connection of pastevula mulfocida capsular polysaccharide and diphtheria toxoid carrier protein through nano-microspheres. The combination of pastevula mulfocida capsular polysaccharide with the carrier protein diphtheria toxoid enhances the immune efficacy of the vaccine, and also reaches a cross protection effect on the infection of homologous strains and heterologous strains. The preparation method of the pastevula mulfocida capsular polysaccharide-protein conjugate vaccine provided by theinvention has the characteristics of simple operation and cost saving, and has good application prospect.
Owner:广州渔跃生物技术有限公司
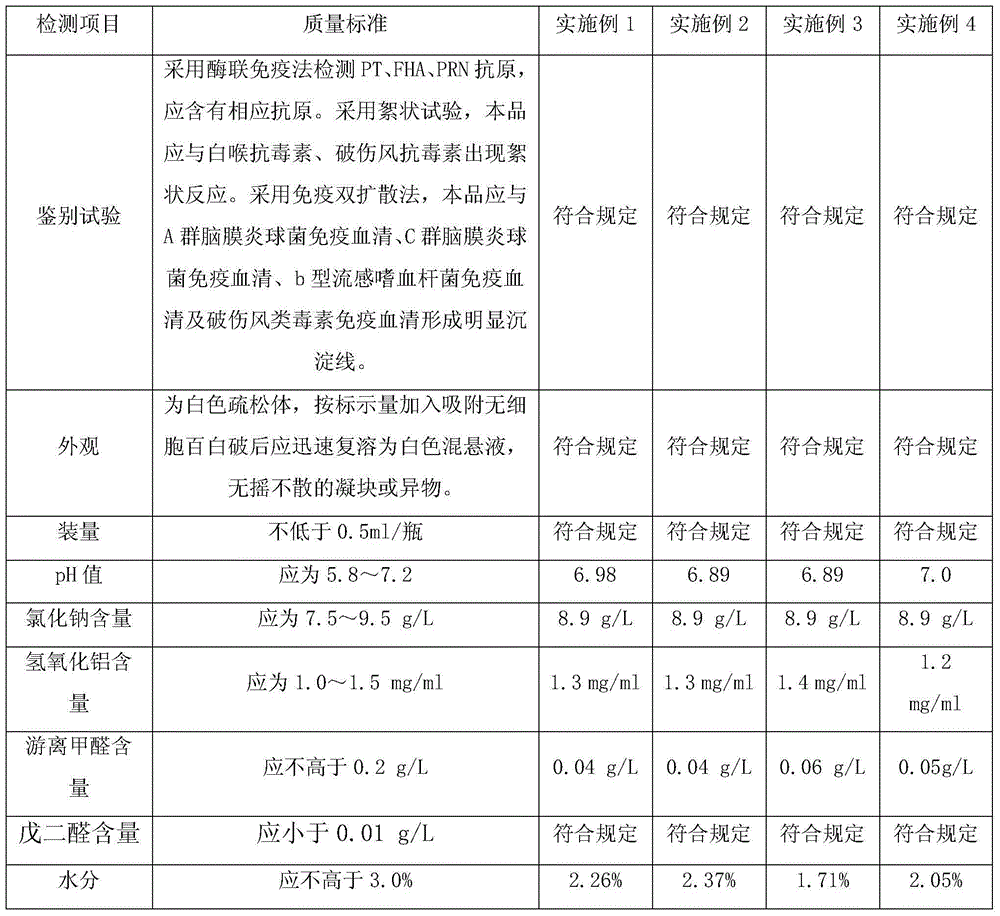
Preparation method for absorbed acellular diphtheria, tetanus and pertussis combined vaccine
InactiveCN102526717AAntibacterial agentsBacterial antigen ingredientsTetanus toxoidsDiptheria toxoid
The invention relates to a method using stain cultivation toxoid to prepare diphtheria, tetanus and pertussis vaccine, which uses modes of ammonium sulfate salting out, density gradient centrifugation and glutaraldehyde detoxification to prepare acellular pertussis raw liquid containing effective antigen of PT and FHA. acellular diphtheria, tetanus and pertussis combined vaccine for preventing diphtheria, tetanus and pertussis can be finally prepared by preparing the pertussis raw liquid and refining and purifying to obtain mixture of diphtheria toxoid and tetanus toxoid.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Fermentation media free of animal-derived components for production of diphtheria toxoids suitable for human vaccine use
ActiveUS9040058B2Speeding drying processAntibacterial agentsBacterial antigen ingredientsMicrobiologyDiptheria toxoid
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Acellular pertussis combined vaccine and preparation method thereof
InactiveCN104707134AClear ingredientsLittle side effectsAntibacterial agentsBacterial antigen ingredientsHemagglutininSide effect
The invention discloses an acellular pertussis combined vaccine and a preparation method thereof and belongs to the technical field of production and preparation of vaccines. The acellular pertussis combined vaccine is prepared from the following raw material components: 5-40Mu g of pertussis toxin, 5-40Mu g of filamentous hemagglutinin, 2-10Mu g of pertussis adhesin, 10-25lf of diphtheria toxoid, 4-10lf of tetanus toxoid, 1.0-2.0mg / ml of aluminium hydroxide and 7.5-9.5g / L of sodium chloride. The preparation method of the acellular pertussis combined vaccine comprises the following steps: preparing monovalent vaccine original fluids, mixing and diluting. The acellular pertussis combined vaccine is clear and definite in ingredients, quality control can be easily realized, the side effect is small, and the safety is high; and the preparation method of the acellular pertussis combined vaccine is simple to operate, convenient in preparation and low in cost, so that the acellular pertussis combined vaccine is applicable to industrial mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
Conserved motif of hepatitis C virus E2/NS1 region
Immunogenic compositions comprising an immunogenic polypeptide and a pharmaceutically acceptable vehicle are described. The immunogenic polypeptide comprises the amino acid sequence Xaa-Thr-Xaa-Val-Thr-Gly-Gly-Xaa-Ala-Ala-Arg-Thr-Thr-Xaa-Gly-Xaa-Xaa-Ser-Leu-Phe-Xaa-Xaa-Gly-Xaa-Ser-Gln-Xaa-Ile-Gln-Leu-Ile (SEQ ID NO:8). The immunogenic polypeptide can be coupled to a pharmaceutically acceptable carrier, such as a diphtheria toxoid.
Owner:CHIRON CORP
Integration of meningococcal conjugate vaccination
ActiveUS9402915B2Antibacterial agentsCarrier-bound antigen/hapten ingredientsCarrier proteinDiphtheria vaccination
Conjugated meningococcal capsular saccharides will be introduced into immunization schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunizing a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunized with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent pneumococcal conjugate vaccine
ActiveCN111821432AAntibacterial agentsBacterial antigen ingredientsPneumococcal serotypesTetanus toxoids
The invention provides a multivalent pneumococcal conjugate vaccine. The vaccine consists of a liquid injection and lyophilized powder; the liquid injection is the conjugate that contains 15 pneumococcal serotypes 1, 2, 3, 4, 5, 6A, 6B, 7F, 9V, 12F, 14, 18C, 19A, 19F and 23F and is prepared by taking diphtheria toxoid as carrier protein; and the lyophilized powder is the conjugate that contains 8pneumococcal serotypes 8, 10A, 11A, 15B, 22F, 24F, 33F and 35B and is prepared by taking tetanus toxoid as the carrier protein. Through the combined using after the lyophilized powder is dissolved inthe liquid injection, the vaccine can be used for preventing the infection caused by the pneumococcus including the serotypes.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Pneumococcal polysaccharide-protein conjugate composition
ActiveUS20190240309A1Strong immune responseImpairing immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus toxoids
The present invention relates to immunogenic compositions comprising a conjugate of a saccharide from Streptococcus pneumoniae serotype 8 and a carrier protein, and a mixture consisting of capsular polysaccharides from Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F individually conjugated to CRM197 carrier protein, or a mixture consisting of capsular polysaccharides from Streptococcus pneumoniae serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F individually conjugated to a carrier protein, wherein the capsular polysaccharides from Streptococcus pneumoniae serotypes 1, 4, 5, 6B, 7F, 9V, 14, and 23F are individually conjugated to protein D, the capsular polysaccharide from Streptococcus pneumoniae serotype 18C is conjugated to tetanus toxoid and the capsular polysaccharide from Streptococcus pneumoniae serotype 19F is conjugated to diphtheria toxoid. Said compositions are useful for the prevention and / or treatment of diseases caused by Streptococcus pneumoniae.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Group-A and group-C meningococcus polysaccharide-adsorption diphtheria tetanus combined vaccine and production process thereof
InactiveCN109513001ALower titerReduce morbidityAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus toxoids
The invention provides a group-A and group-C meningococcus polysaccharide-adsorption diphtheria tetanus combined vaccine and a production process thereof. The combined vaccine contains an adsorption diphtheria tetanus vaccine and a group-A and group-C meningococcus polysaccharide vaccine which are respectively contained in two reagent bottles, wherein the content of diphtheria toxoid in each 1ml of the adsorption diphtheria tetanus vaccine is below 20Lf, the content of tetanus toxoid is below 3Lf, the content of aluminum hydroxide is below 3mg, and the content of sodium chloride is 7.5mg-9mg;and each human dose, namely 0.5ml of the group-A and group-C meningococcus polysaccharide vaccine contains 50 micrograms of group-A meningococcus polysaccharide, 50 micrograms of group-C meningococcuspolysaccharide vaccine and 8mg of lactose. The combined process is mainly used for preventing multiple high-incidence diseases of children of 5 and 6 years old through one injection. The invention further provides the production process of the group-A and group-C meningococcus polysaccharide-adsorption diphtheria tetanus combined vaccine.
Owner:LIAONING MAOKANGYUAN BIO TECH CO LTD +1
Preparation method of type B haemophilus influenzae capsular polysaccharide and combined vaccine
InactiveCN105646726AHigh purityLess impuritiesAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus toxoids
The invention discloses a preparation method of a type B haemophilus influenzae capsular polysaccharide. The preparation method comprises the steps of fermentation culture, sterilization and precipitation, polysaccharide extraction and purification, polysaccharide derivatization and polysaccharide protein conjugate preparation. The invention further discloses a multivalent combined vaccine which is prepared through the steps that an acellular pertussis stock solution, refined diphtheria toxoid and refined tetanus toxoid are added into aluminum hydroxide to be adsorbed and then added into a type B haemophilus influenzae capsular polysaccharide stock solution, and the mixed solution is diluted with normal saline. According to the preparation method, the prior art is optimized, and the high-purity low-impurity-content type B haemophilus influenzae capsular polysaccharide can be obtained; meanwhile, the type B haemophilus influenzae capsular polysaccharide is combined with multiple component vaccines to form the multivalent vaccine, the multivalent vaccine can be immune to multiple diseases simultaneously, the inoculating times are decreased, the medical risk is reduced, and the better immune effect can be obtained through one-time inoculating.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Corynebacterium diphtheriae culture medium and method for preparing diphtheria toxoid by applying same
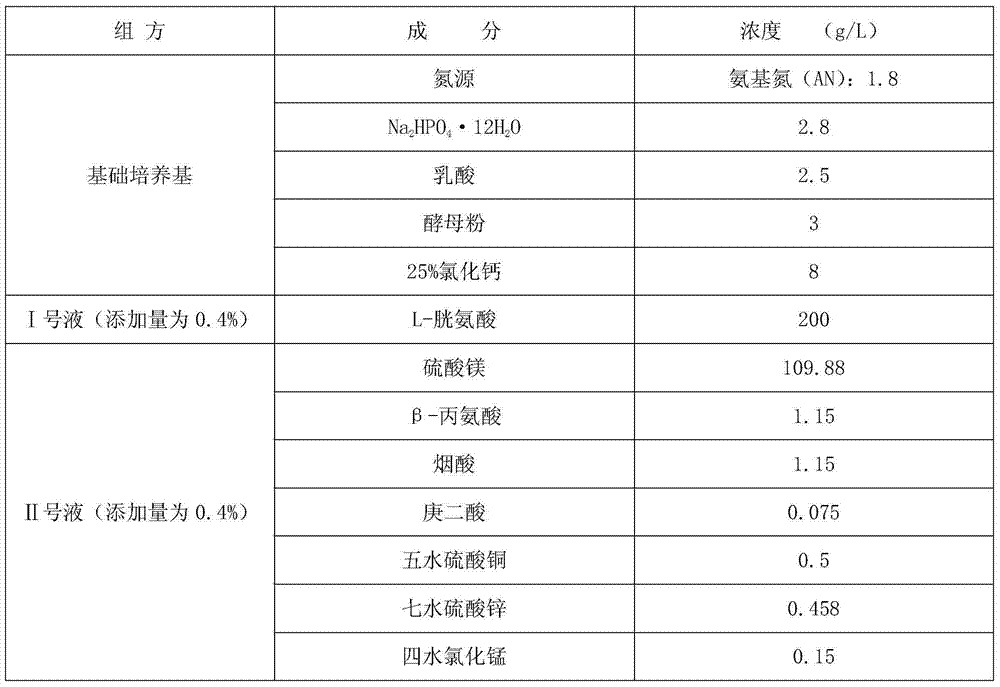
InactiveCN104263678AAvoid potential hazardsEasy to operateBacteriaMicroorganism based processesNiacinDiptheria toxoid
The invention discloses a corynebacterium diphtheriae culture medium. The corynebacterium diphtheriae culture medium consists of a basic culture medium, an I liquid and an II liquid, wherein the basic culture medium consists of the materials of HyPep5630, Amisoy, Na2HPO4.12H2O, lactic acid, yeast powder and calcium chloride; the I liquid is L-cystine solution; the II liquid consists of the materials of magnesium sulfate, beta-alanine, niacin, pimelic acid, copper sulfate pentahydrate, zinc sulfate heptahydrate and manganese chloride tetrahydrate. The method for preparing diphtheria toxoid by applying the corynebacterium diphtheriae culture medium is culture enabling, two-generation culturing, three-generation culturing, four-generation culturing and fermentation tank culturing. The culture medium is the animal-free culture medium; good culture recovery and multiplication culture are carried out during culture passage; and the toxin production can also reach the requirement of the existing pharmacopoeia criterion. The method for preparing the diphtheria toxoid by using the culture medium has the advantages of being simple to operate, convenient to produce, easy in taking of materials and low in cost.
Owner:CHENGDU OLYMVAX BIOPHARM
Ipv-dpt vaccine
InactiveUS20100021497A1Efficient productionAntibacterial agentsBacterial antigen ingredientsProtective antigenTetanus toxoids
The invention provides a process for producing a combined vaccine containing an inactivated Sabin strain of poliovirus, a Bordetella pertussis protective antigen, a diphtheria toxoid and a tetanus toxoid, the process including a step of producing a high-titer Sabin strain poliovirus. The inventive process for producing a combined vaccine, including a step of culturing, in the presence of from about 4 g / L to about 6 g / L of a microcarrier, Vero cells to be inoculated with a Sabin strain of poliovirus, is useful as a process for efficiently producing a combined vaccine containing an inactivated Sabin strain of poliovirus.
Owner:TAKEDA PHARMACEUTICALS CO LTD +1
Method for monitoring tetanus toxoid or diphtheria toxoid
ActiveCN111855826ASolve the problem of lack of monitoring methods for the processQuality improvementComponent separationTetanus toxoidsTarget peptide
The invention discloses a method for monitoring tetanus toxoid or diphtheria toxoid. The method comprises the following steps of: (1) carrying out enzymolysis of a standard substance; (2) carrying outsolid-phase extraction to enrich a target peptide fragment; (3) performing high performance liquid chromatography tandem mass spectrometry analysis; (4) drawing a standard characteristic peptide fragment table to obtain relative response intensity of each characteristic peptide fragment of a standard variety; (5) detecting a sample, and calculating to obtain the relative response intensity and arecovery rate of each characteristic peptide fragment in the sample; and (6) evaluating the detoxification effect according to the number of peptide fragments with good characteristic peptide fragmentrecovery rate. According to the monitoring method of the invention, the method for quantitatively monitoring the process stability of tetanus toxoid and diphtheria toxoid is implemented for the firsttime, the problem that a formaldehyde detoxification protein process lacks a monitoring method is solved, effective quantitative data is provided for production optimization of tetanus vaccine, tetanus-diphtheria bivalent vaccine, pertussis-diphtheria-tetanus triple vaccine, pentavaccine and proteoglycan protein conjugate vaccine and research and development of novel proteoglycan protein conjugate vaccine, and the vaccine quality improvement process and new product research and development are accelerated.
Owner:SHIMADZU (CHINA) CO LTD +1
An immunogenic composition having improved stability, enhanced immunogenicity and reduced reactogenicity and process for preparation thereof
ActiveUS20200206331A1Suitable preventionSuitable treatmentBacterial antigen ingredientsSsRNA viruses positive-senseHepatitis B immunizationAdjuvant
An immunogenic composition comprising of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen, Hepatitis B surface antigen (HBsAg), inactivated whole-cell B. pertussis (wP) antigen, Haemophilus influenzae type B (Hib) capsular saccharide conjugated to a carrier protein, Inactivated Polio Virus (IPV) antigen and additionally one or more antigens and the method of preparing the same. A fully liquid combination vaccine, showing improved immunogenicity, reduced reactogenicity and improved stability. Improved methods of formaldehyde inactivation, improved adsorption profile of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen and Hepatitis B (HepB) surface antigen adsorbed individually onto aluminium phosphate adjuvant, minimum total aluminum content (Al3+) and optimized concentration of 2-phenoxyethanol (2-PE) as preservative.
Owner:SERUM INST OF INDIA PTE LTD
Integration of meningococcal conjugate vaccination
ActiveUS20160303215A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsCarrier proteinMeningococcal carriage
The invention provides a method for immunising a patient, comprising administering multiple conjugates meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Preparation method of diphtheria toxoid vaccine
ActiveCN102961740BAvoid damageEfficient removalAntibacterial agentsBacterial antigen ingredientsFiberUltrafiltration
The invention discloses a preparation method of a diphtheria toxoid vaccine. The diphtheria toxoid vaccine is prepared by taking a diphtheria bacillus strain as a raw material and then carrying out the steps of diphtheria toxoid culture, bacteria-liquid separation, ultrafiltration and concentration, two-time salting out, ultrafiltration and desalination and the like. According to the preparation method, the thalli of the culture solution is removed by using a hollow fiber filter membrane and then refining is carried, so that the pore blockage caused by the accumulation of the thalli and other impurity fragments in the subsequent filter process is prevented; through an improved salting out method, the toxin proteins and other allergens in the culture solution are effectively removed; and a tangential flow ultrafiltration method is used for both the concentration of the culture solution and the desalting method after the salting out, so that the antigen damage caused by the shearing to the toxin proteins is decreased and the precipitation of the proteins is avoided. According to the preparation method, the preparation time of the diphtheria toxoid vaccine is shortened and the production efficiency is improved.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
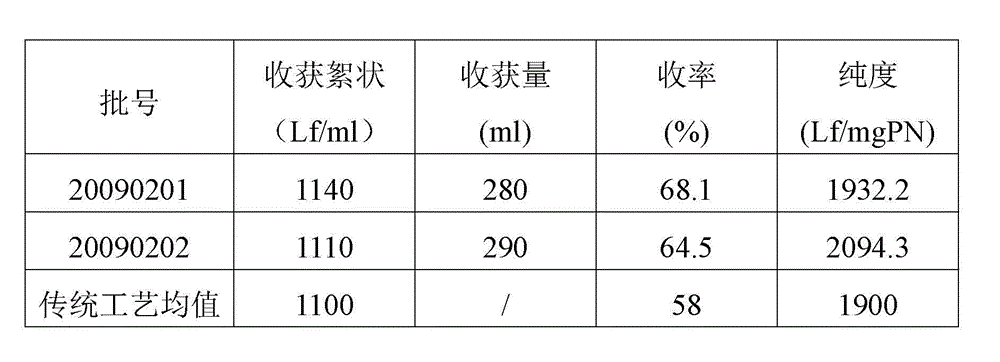
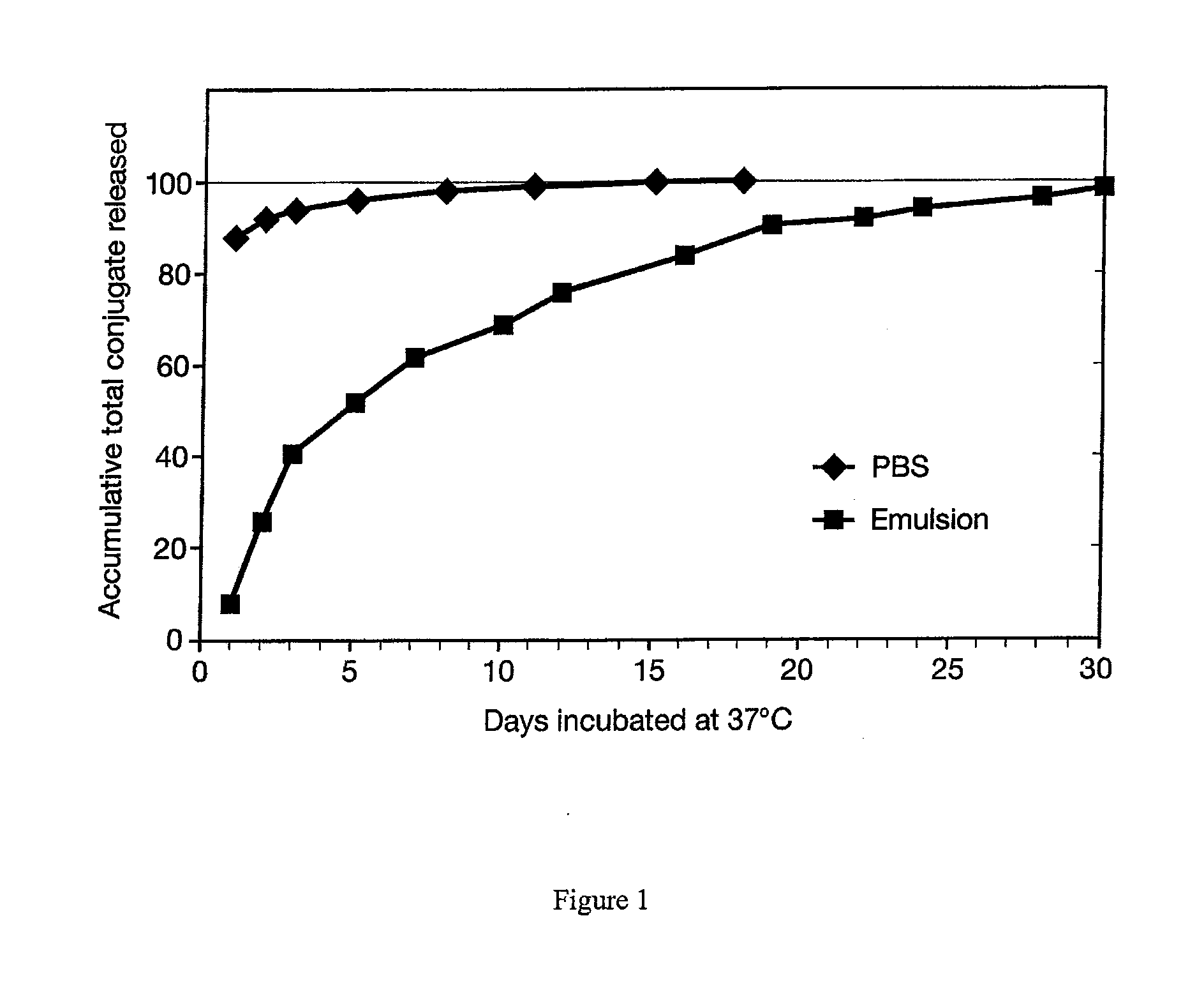
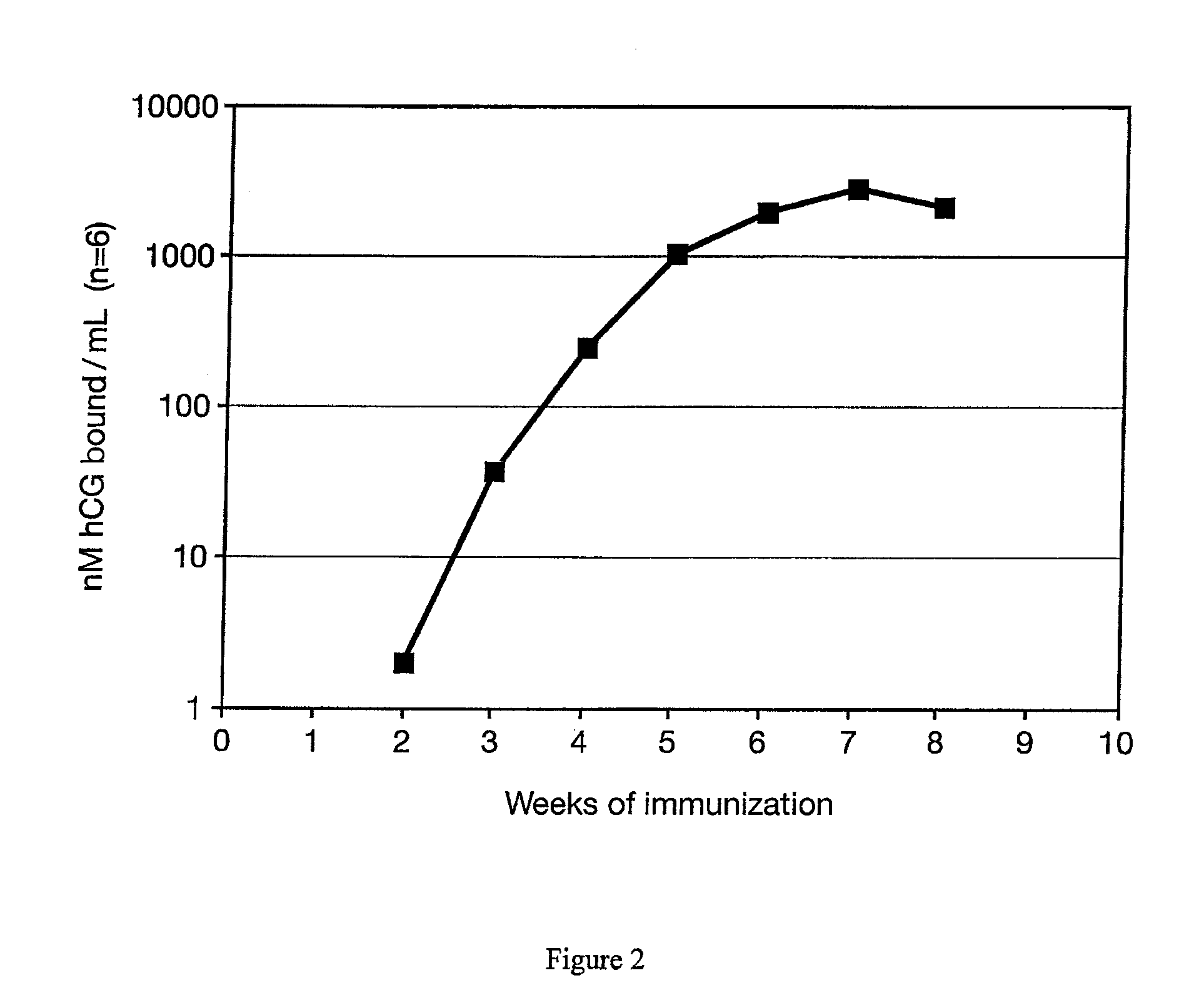
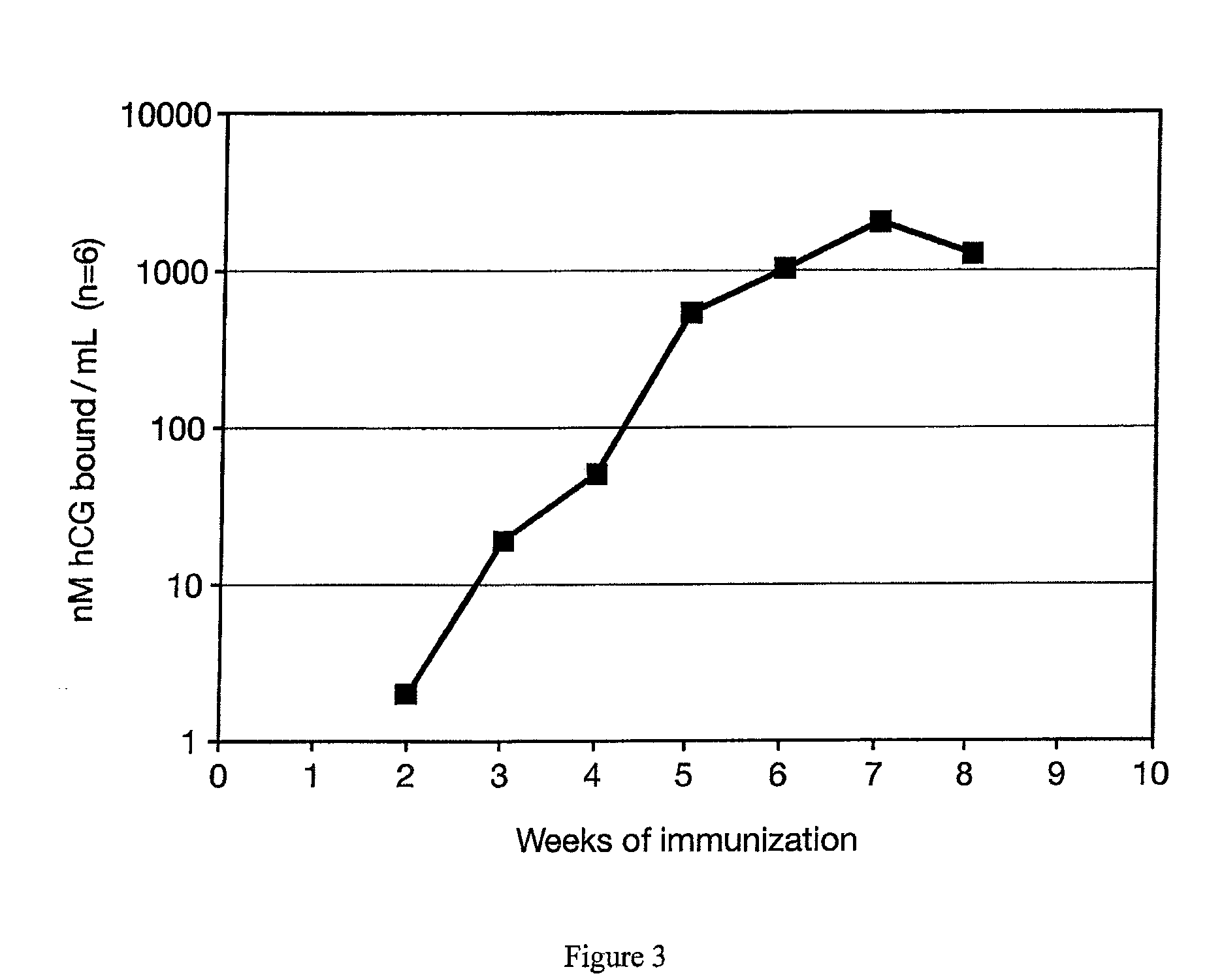
METHOD FOR DELIVERING A HUMAN CHORIONIC GONADOTROPIN (Hcg) VACCINE FOR LONG-ACTING ANTIBODY PROTECTION
This invention comprises a method of immunization in which human chorionic gonadotropin (hCG) vaccine antigens are incorporated into an inorganic salt / biopolymer complex using a simple manufacturing process. The resulting solid matrix is administered to human subjects in the form of microparticles. The method comprises suspending microparticles in an emulsion of a natural oil and water containing an adjuvant compound acceptable for human use and injecting a pharmaceutical dose of the suspension intramuscularly. The vaccine antigens are conjugates of peptide fragments of the beta subunit of hCG with a carrier protein, diphtheria toxoid. Vaccine antigens delivered in this formulation are effective for eliciting antibodies in recipients for the treatment of cancer or hormone-related diseases.
Owner:ROYER BIOMEDICAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com