Pneumococcus polysaccharide protein coupling vaccine and its preparing method
A pneumococcal polysaccharide and protein coupling technology, applied in the field of biomedicine, can solve the problems of T cell memory, low titer, toxicity of pneumolysin, that is, hemolytic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
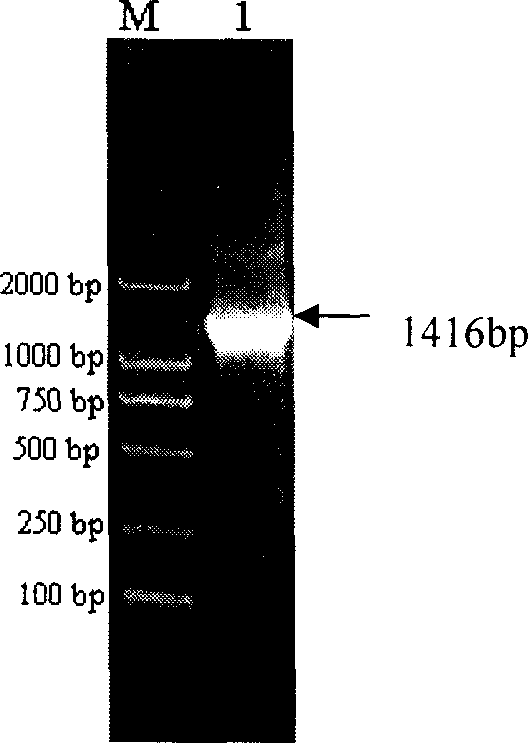
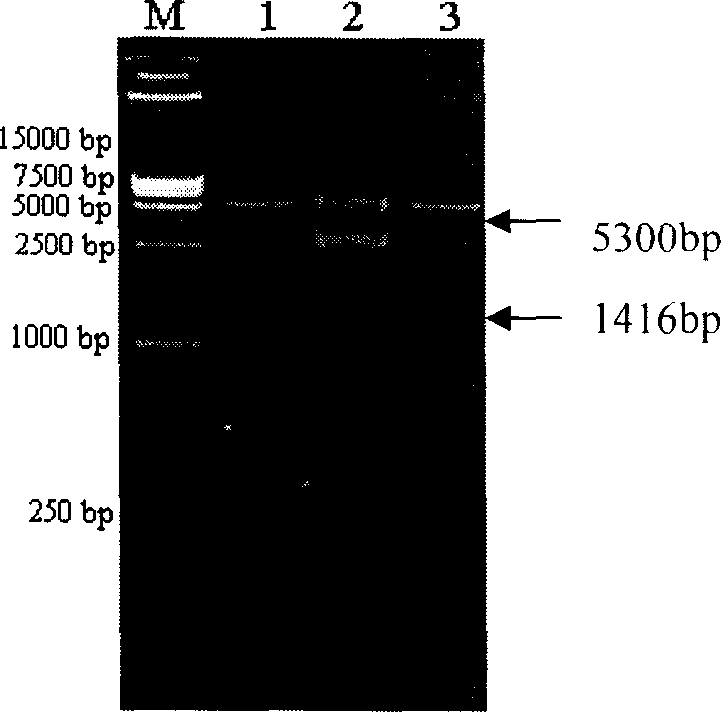
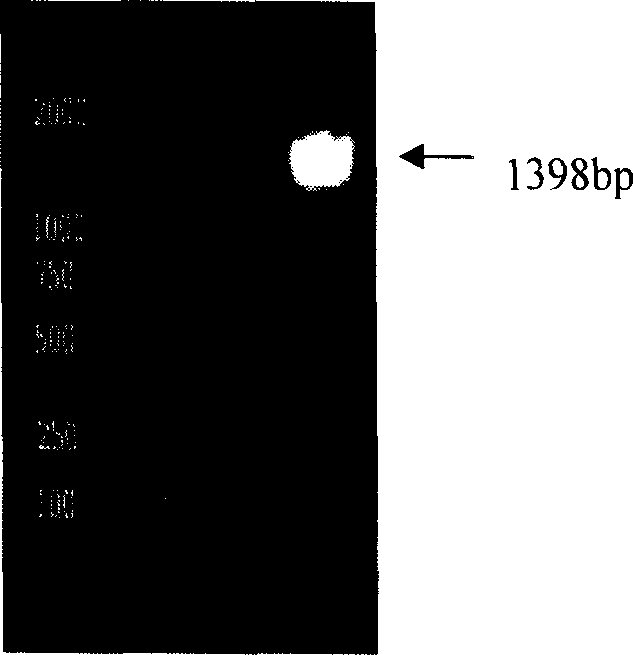
[0021] The (pneumococcal) hemolysin protein produced by pneumococcus itself is very small. The present invention uses genetic engineering methods to clone and obtain the pneumolysin gene from Streptococcus pneumoniae. After modification, the pneumolysin protein is expressed in E. It is purified.
[0022] 1. The present inventor has initially designed a pneumolysin gene sequence (see SEQ ID NO.3 in the sequence listing) (GenBank accession No.X52474) according to the report of Walker et al. in 1987. CATATG ) and XhoI (C TCGAG ) of the primer sequence 4 (SEQ ID NO.4) and primer sequence 5 (SEQ ID NO.5) of the recognition site, using PCR technology to amplify from the chromosomal DNA of pneumococcus to obtain pneumococcal hemolytic bacteria with the sequence of SEQ ID NO.3 pneumolysin gene, and the pneumolysin gene and the prokaryotic expression vector (plasmid) pET-21a were digested with restriction endonucleases NdeI and XhoI respectively, and a gene recombinant plasmid capab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com