Preparation method of diphtheria toxoid vaccine
A technology for diphtheria toxoid and vaccine, which is applied in the field of preparation of diphtheria toxoid vaccine, can solve the problems of simple and extensive, large shear force of bacteria and low quality, and achieves the advantages of reducing antigen damage, reducing pore blockage and improving production efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
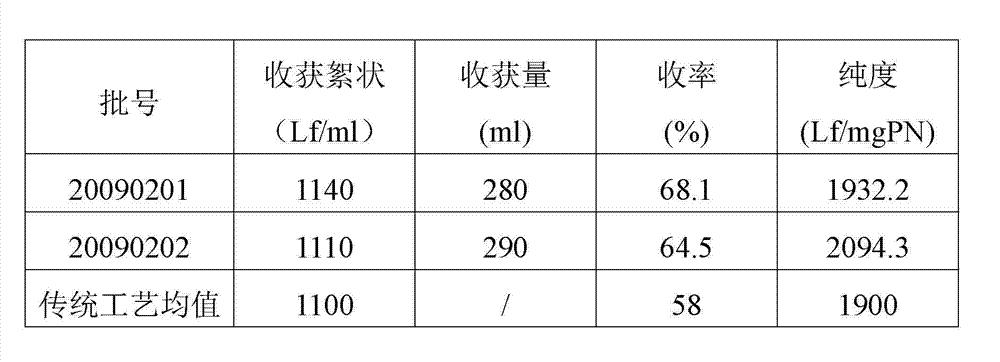
Embodiment 1
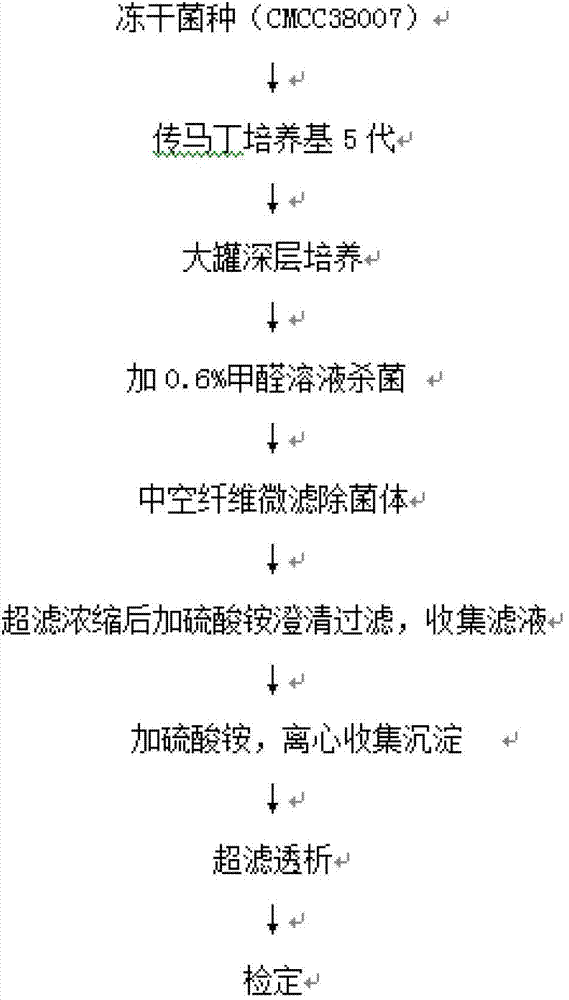
[0015] The preparation method of diphtheria toxoid vaccine comprises the following steps:
[0016] 1) Culture of diphtheria toxoid
[0017] Diphtheria bacillus strain PW8 (CMCC 38007) was used for 5 generations in Martin's medium, then transferred to a large tank with deep stirring, and cultivated at 34-36°C for 48-50 hours by surface aeration method. 0.6% (V / V) of the volume is added to formaldehyde solution, sterilized at 30~35°C for 30 minutes, and transported to the refining tank by pipeline.
[0018] 2) Separation of bacterial liquid
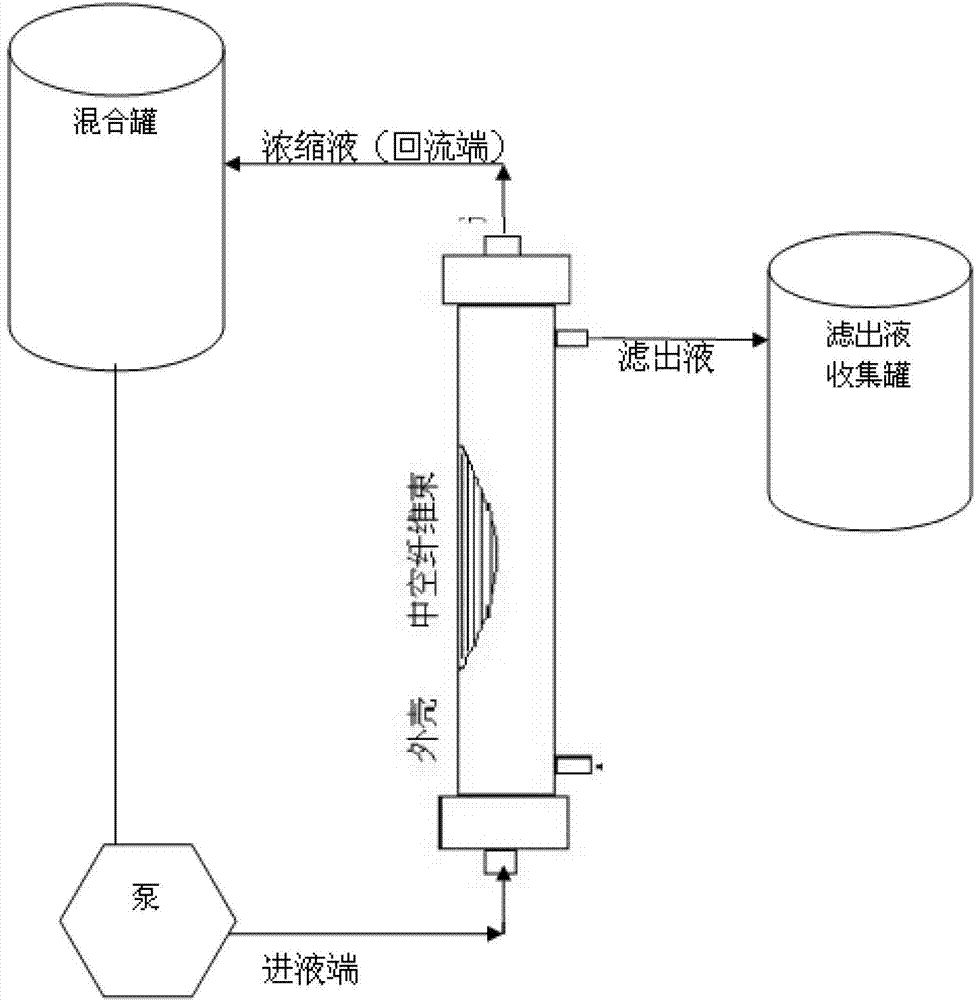
[0019] Take 30L (324Lf / ml) of diphtheria toxoid culture solution, add NaHCO 3 Adjust the pH value of the solution to 6.9, using the microfiltration module in the hollow fiber ultrafiltration system (the system model is MUF-HF7 American PALL, which includes a hollow fiber membrane column, a diaphragm pump, a storage tank, and a necessary filter that can withstand a pressure above 3Bar Pipeline, reflux valve and filter out valve, the pore ...
Embodiment 2
[0038] The preparation method of diphtheria toxoid vaccine comprises the following steps:
[0039] 1) Culture of diphtheria toxoid
[0040] The culture method is the same as in Example 1.
[0041] 2) Separation of bacterial liquid
[0042] Take 30L of fermented liquid, use NaHCO 3 Adjust the pH value of the solution at 7.5, using the microfiltration module in the hollow fiber ultrafiltration system (the system model is MUF-HF7 American PALL, which includes hollow fiber membrane columns, diaphragm pumps, storage tanks, and the necessary filter that can withstand pressures above 3Bar Pipeline, reflux valve and filter out valve, the pore diameter of the hollow fiber membrane column used at this time is 0.45 μm), and the fermentation broth is subjected to tangential flow ultrafiltration to remove bacteria. The tangential flow ultrafiltration steps are:
[0043] Before starting the pump, check the pipeline flow path, open the valves at the filter end and return end of the microf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com