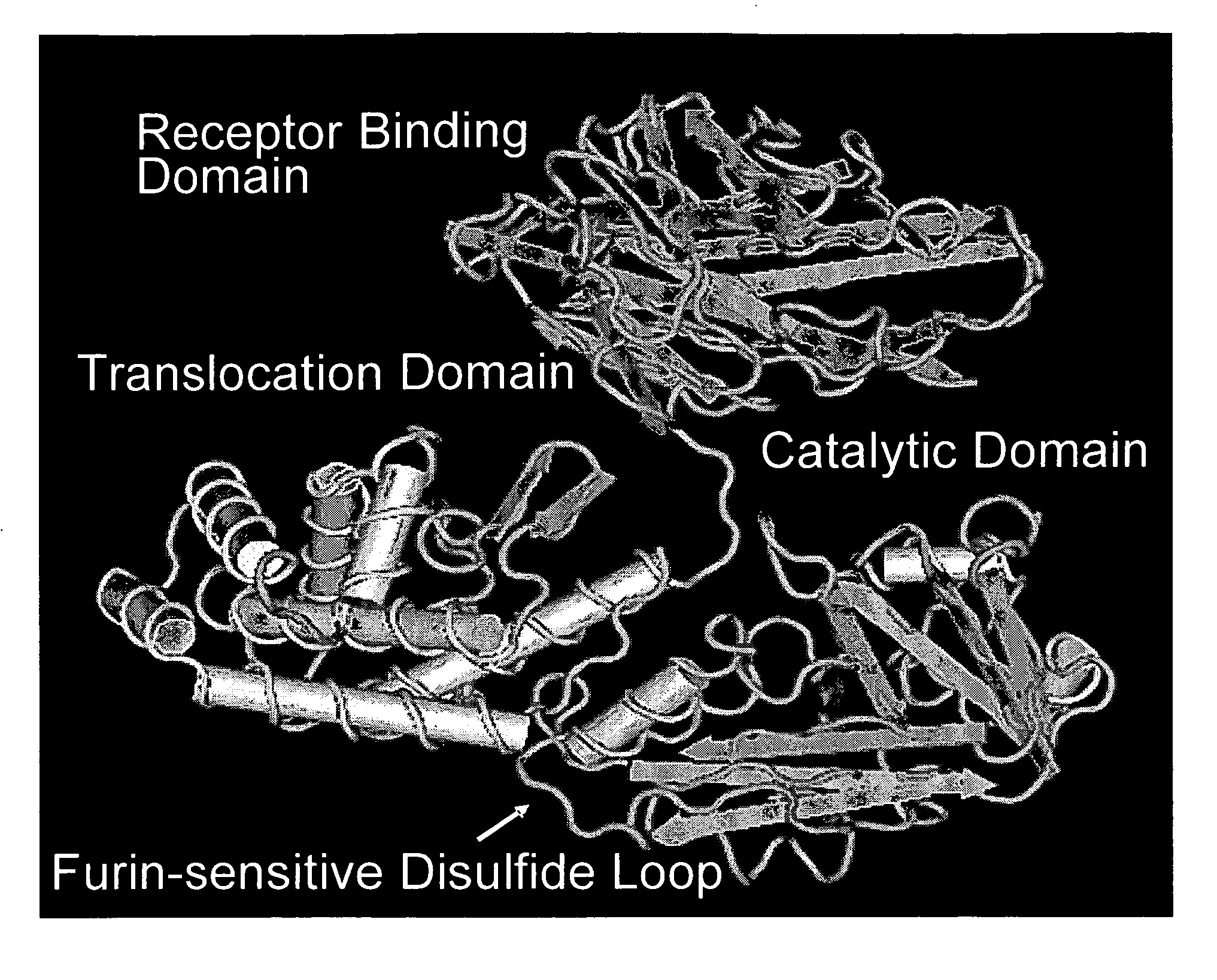
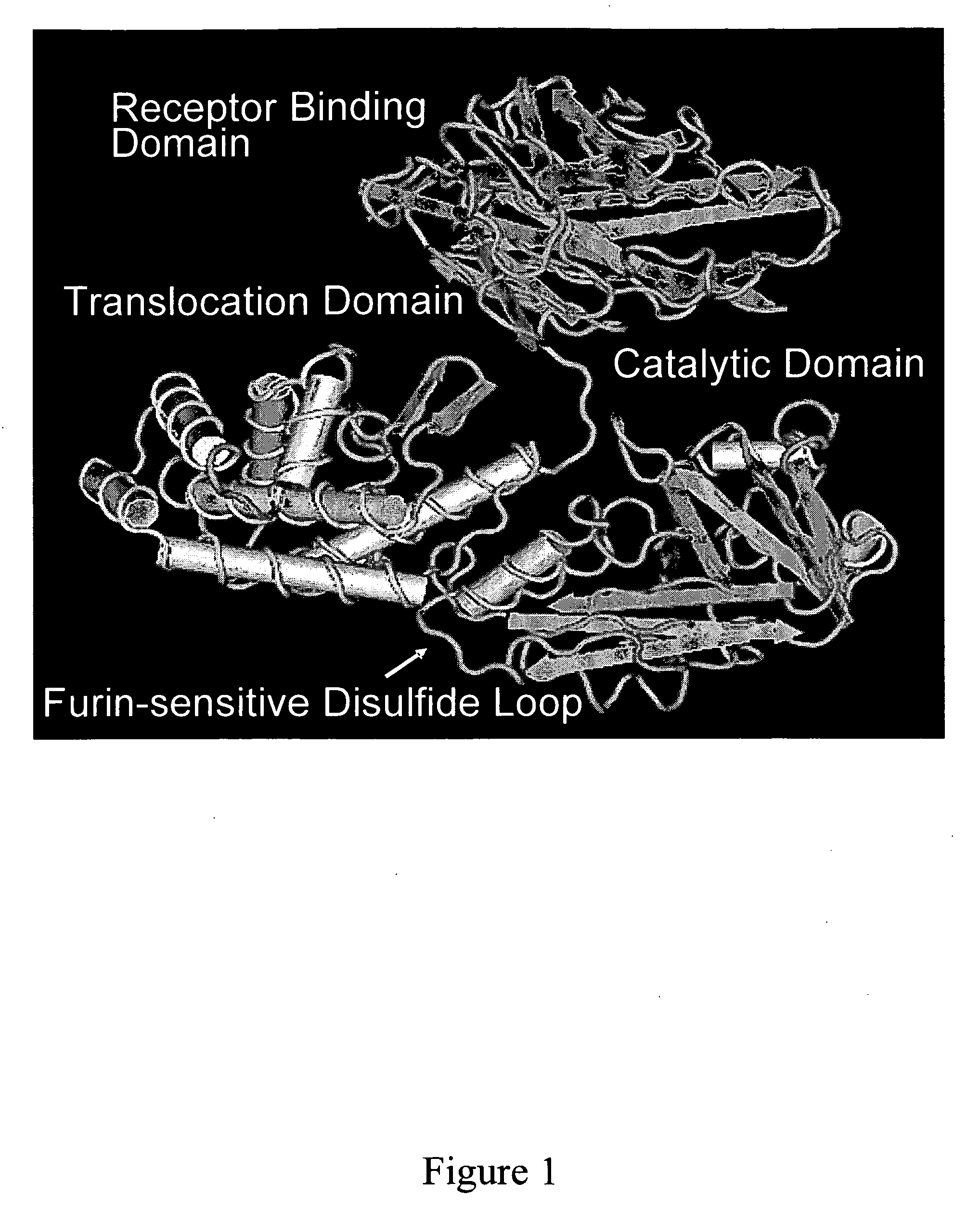
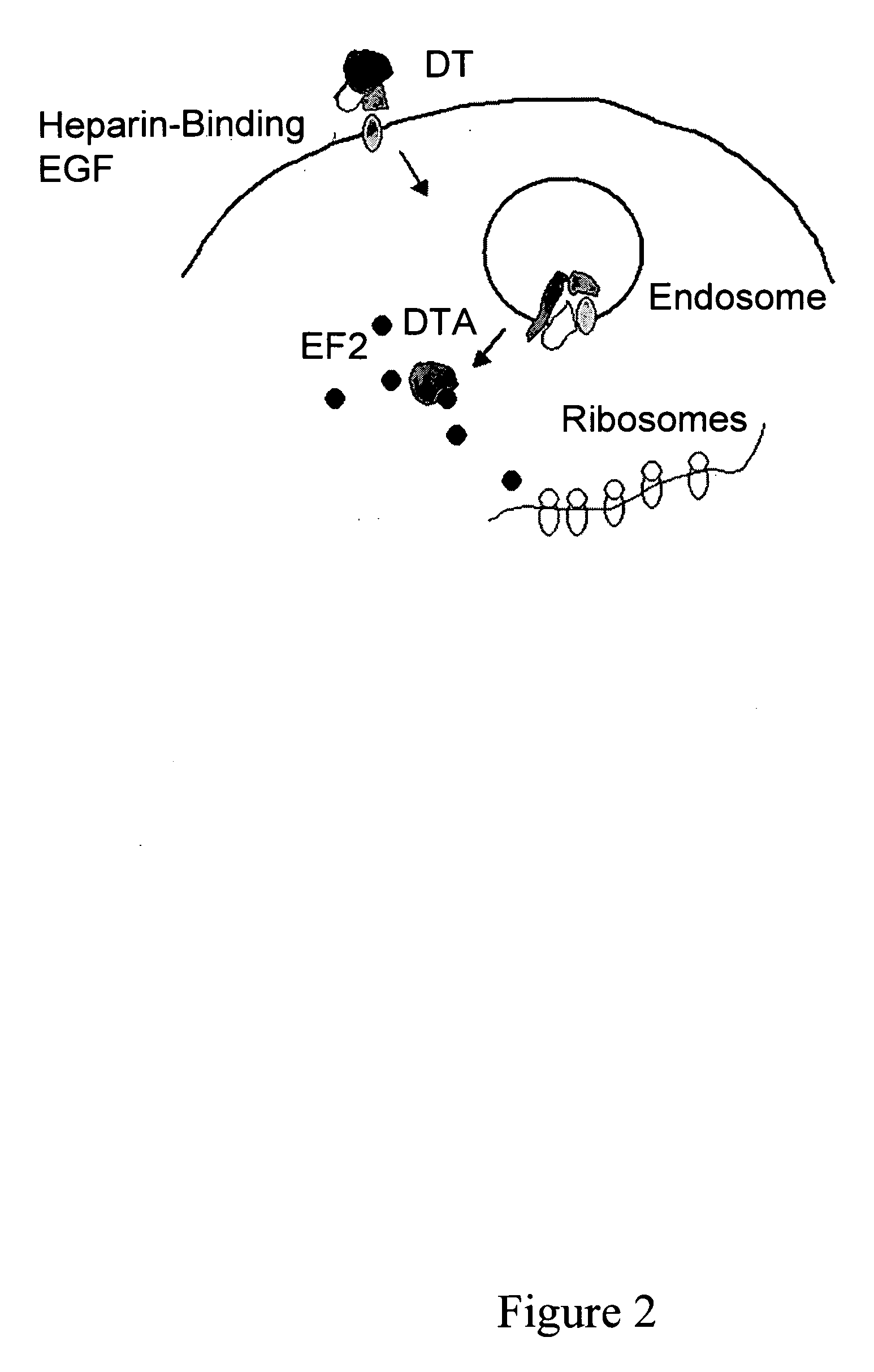
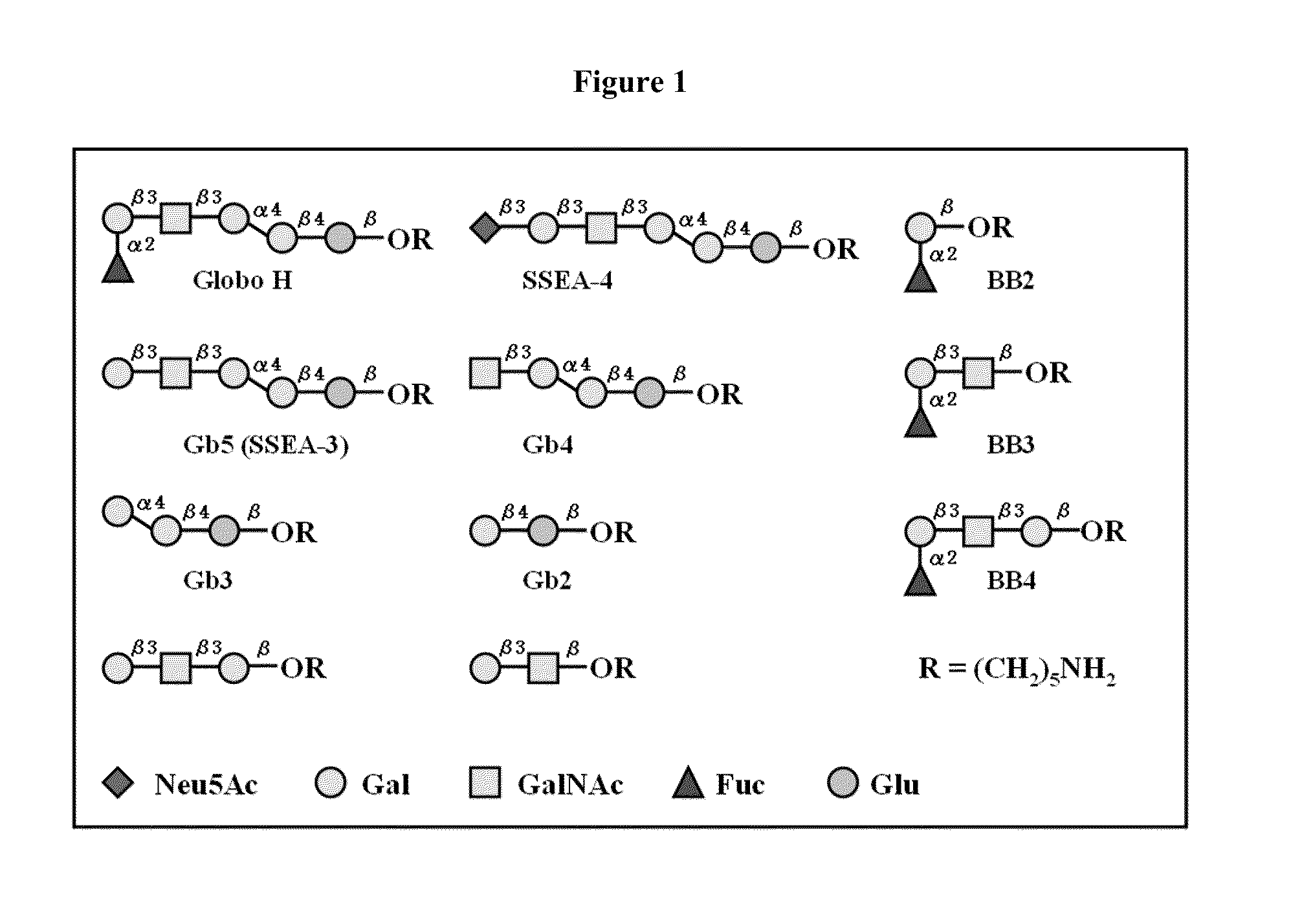
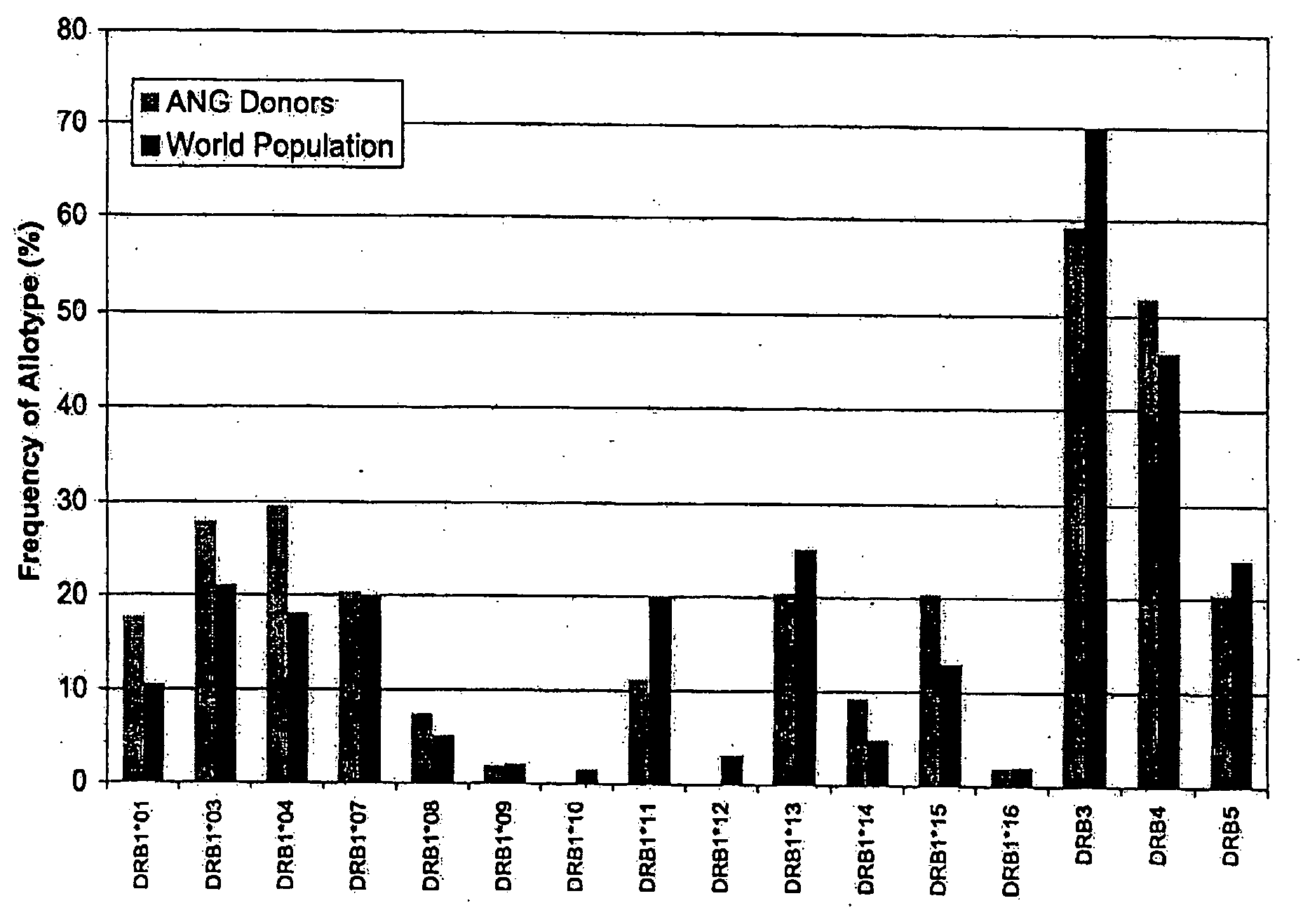
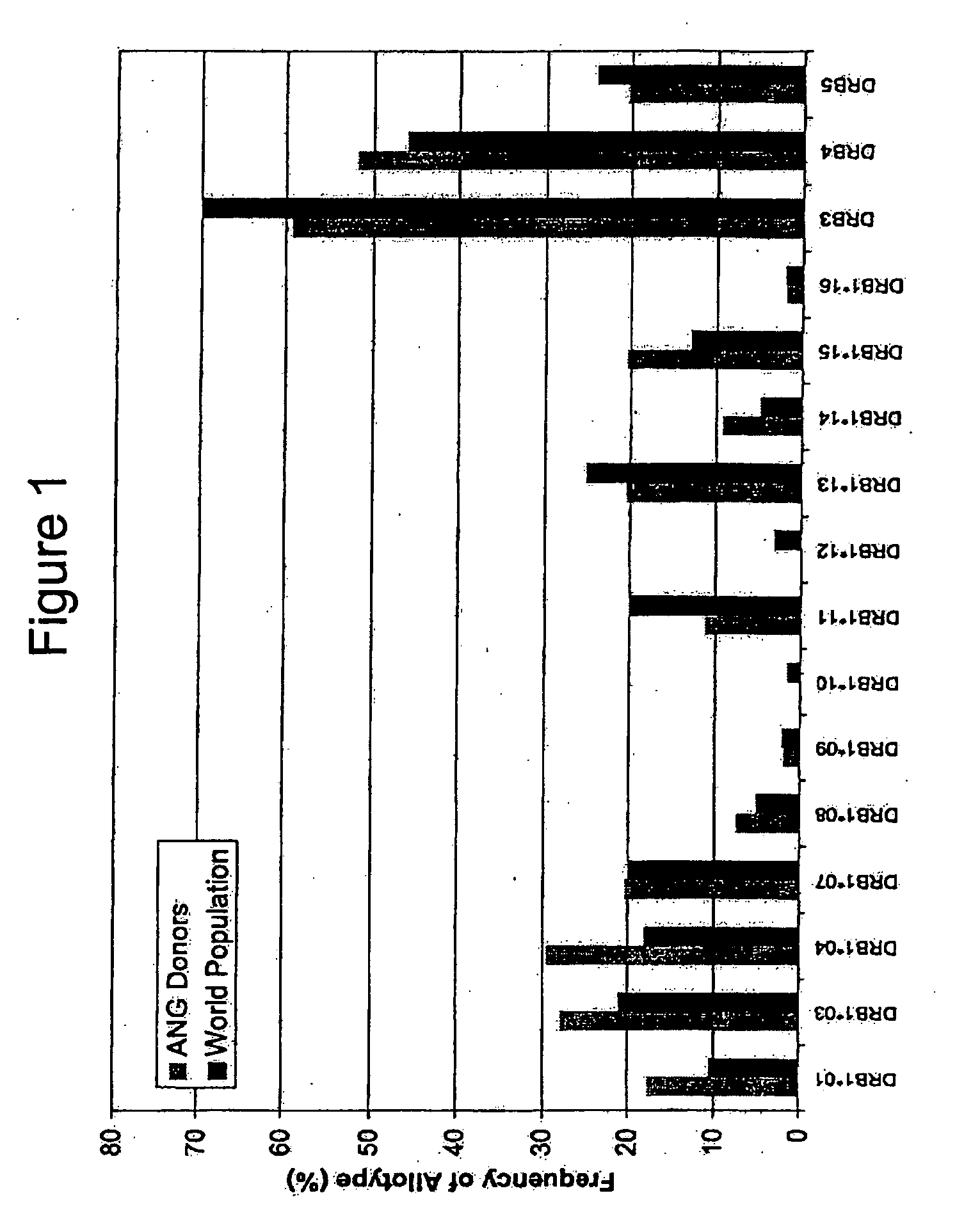
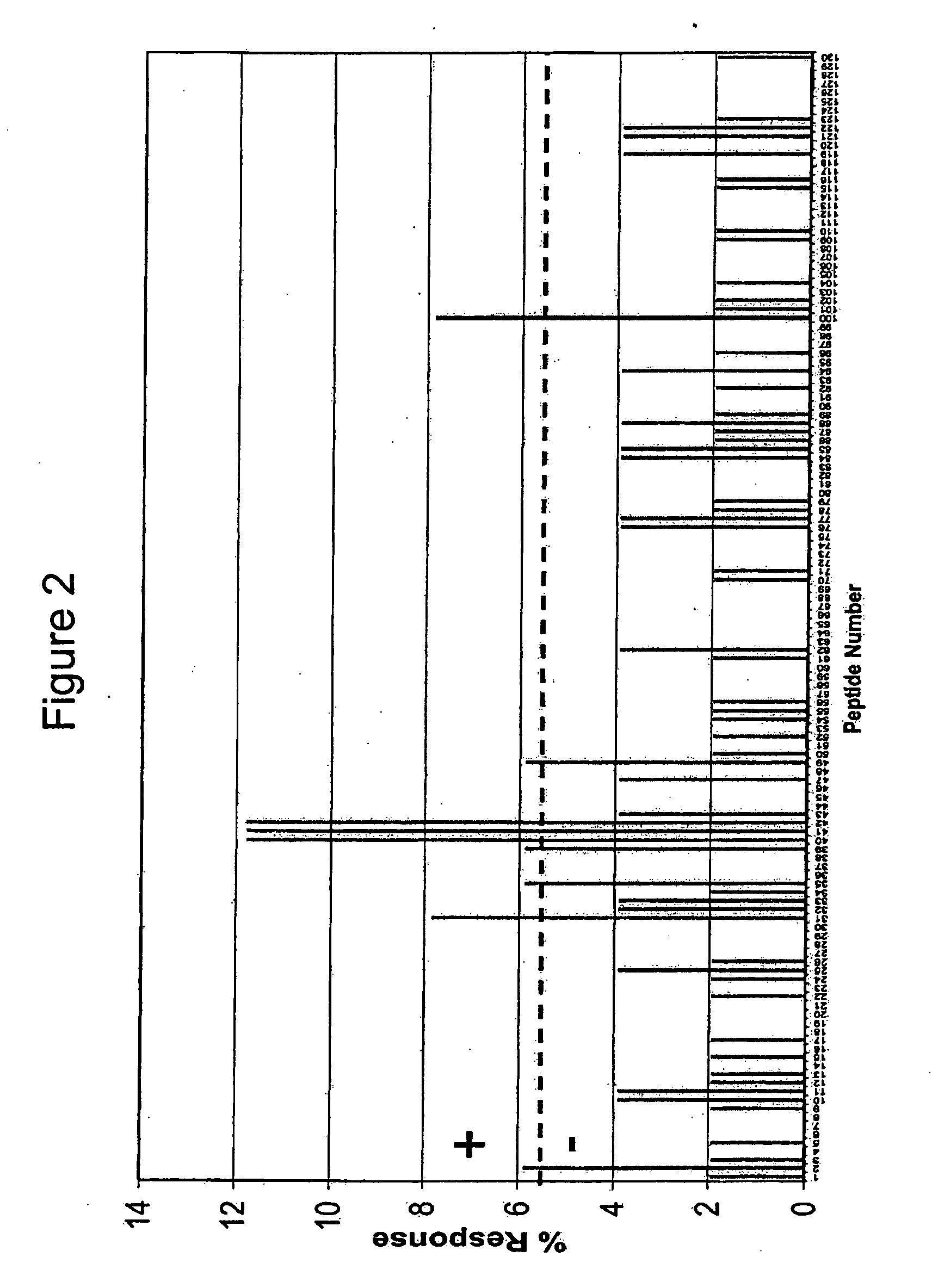
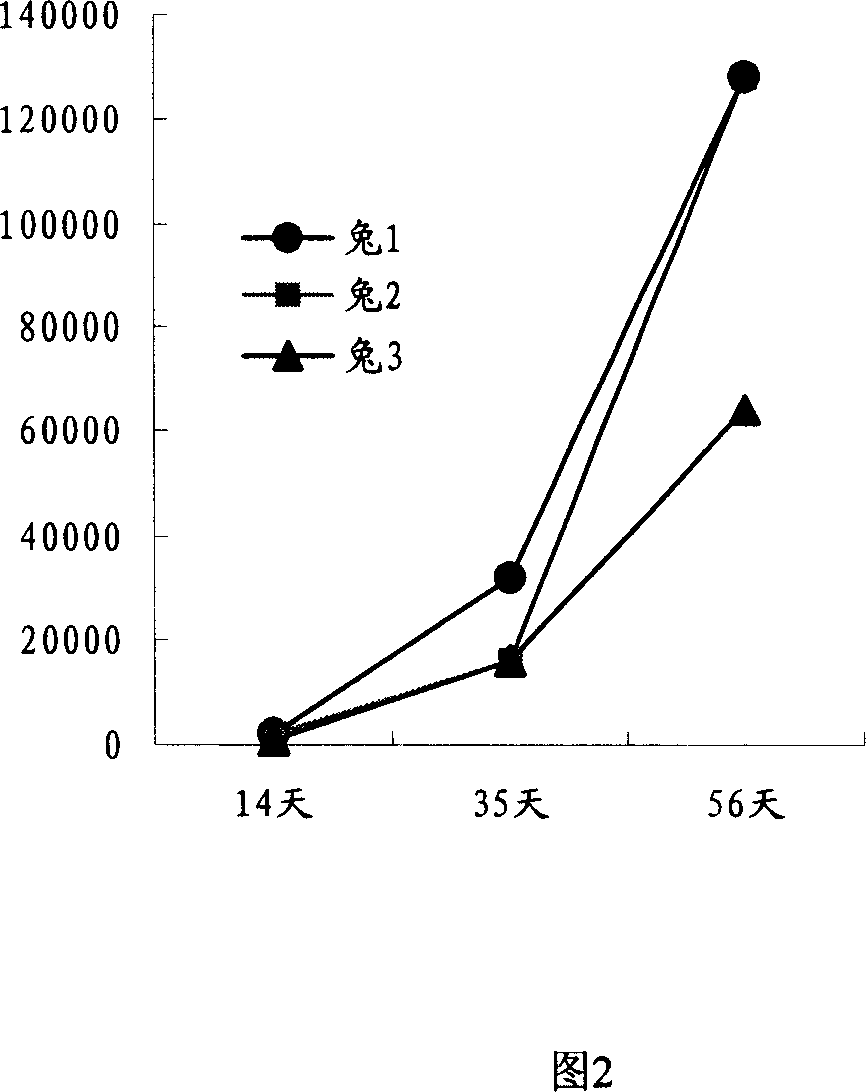
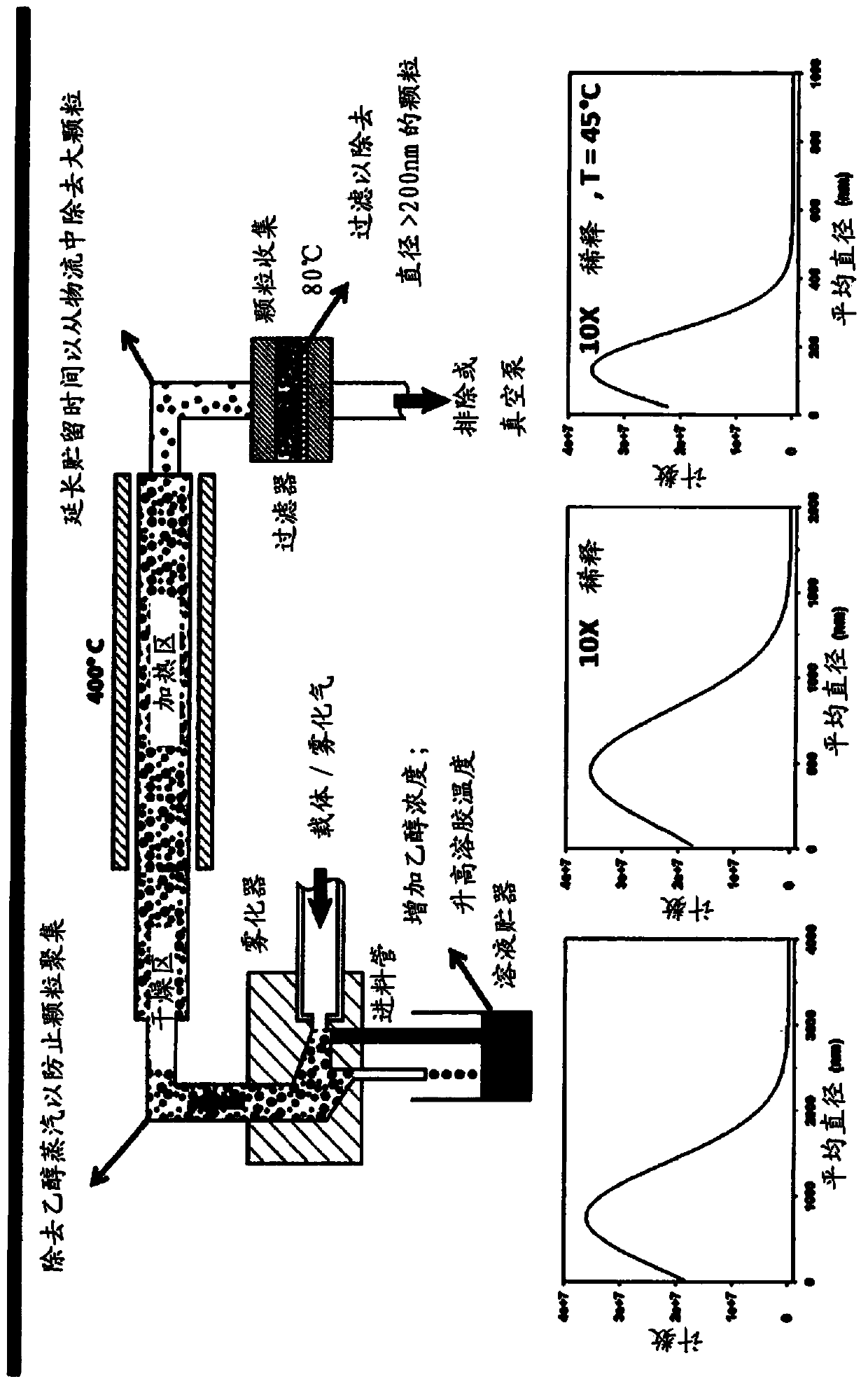
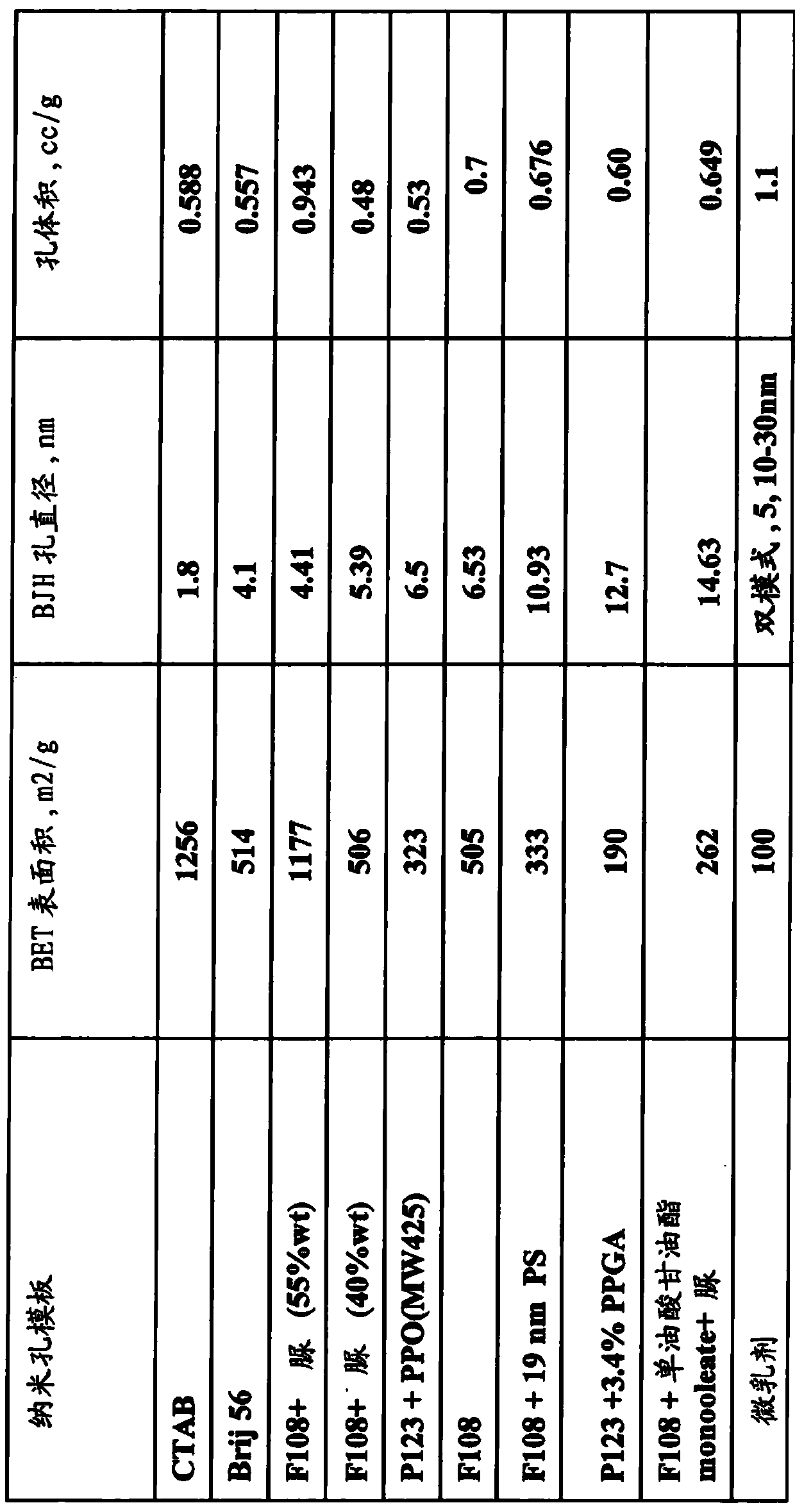
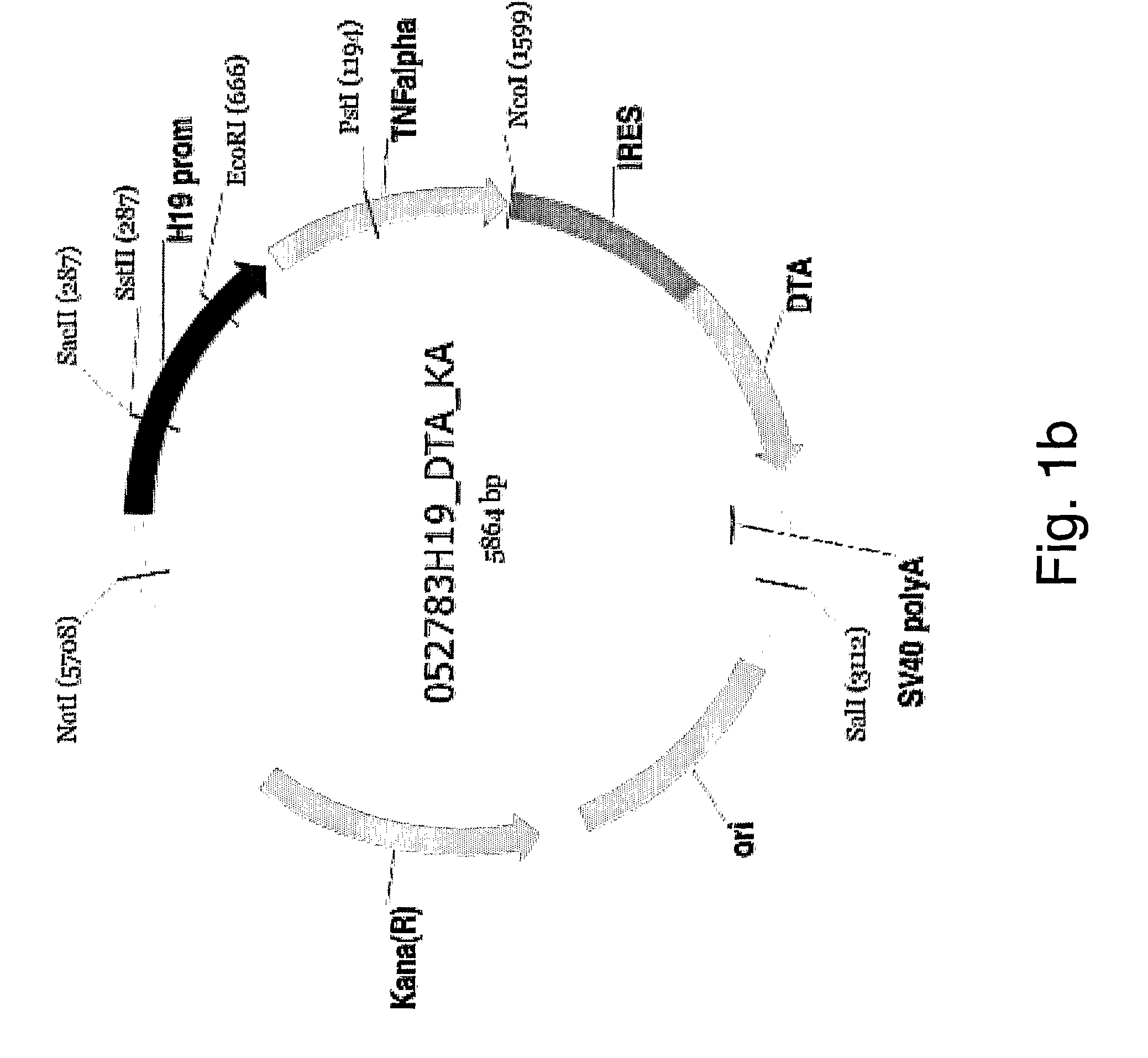
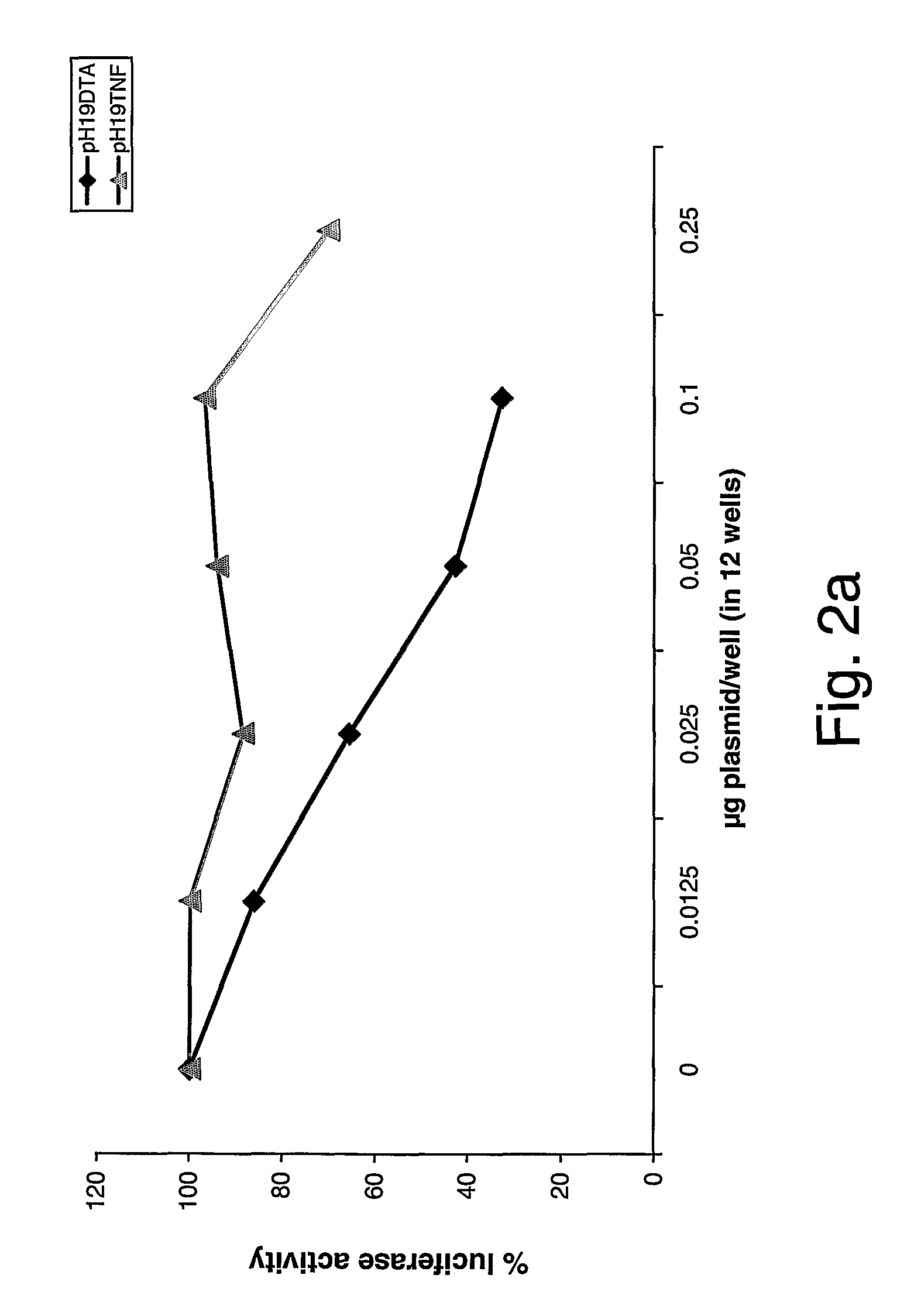
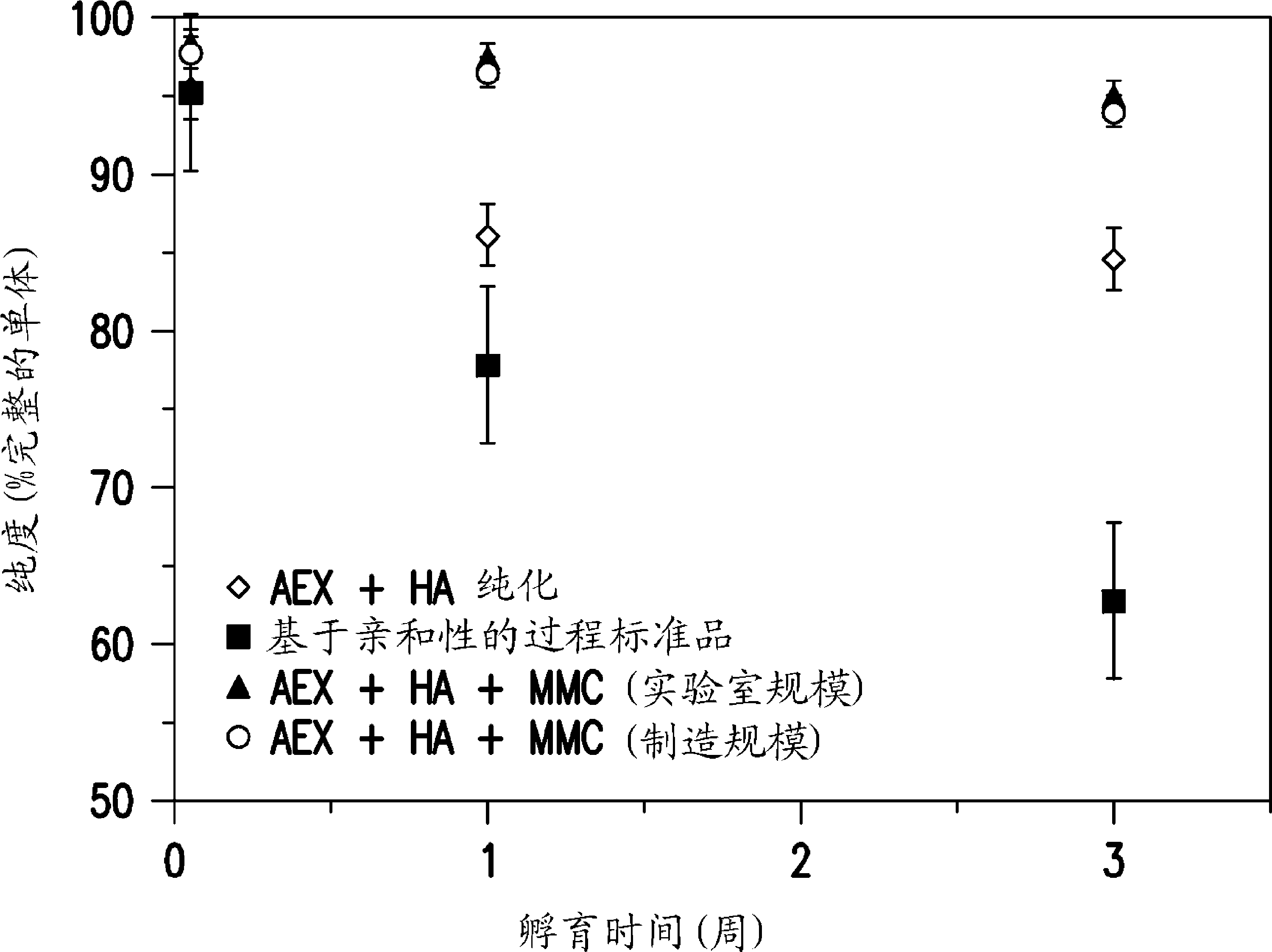
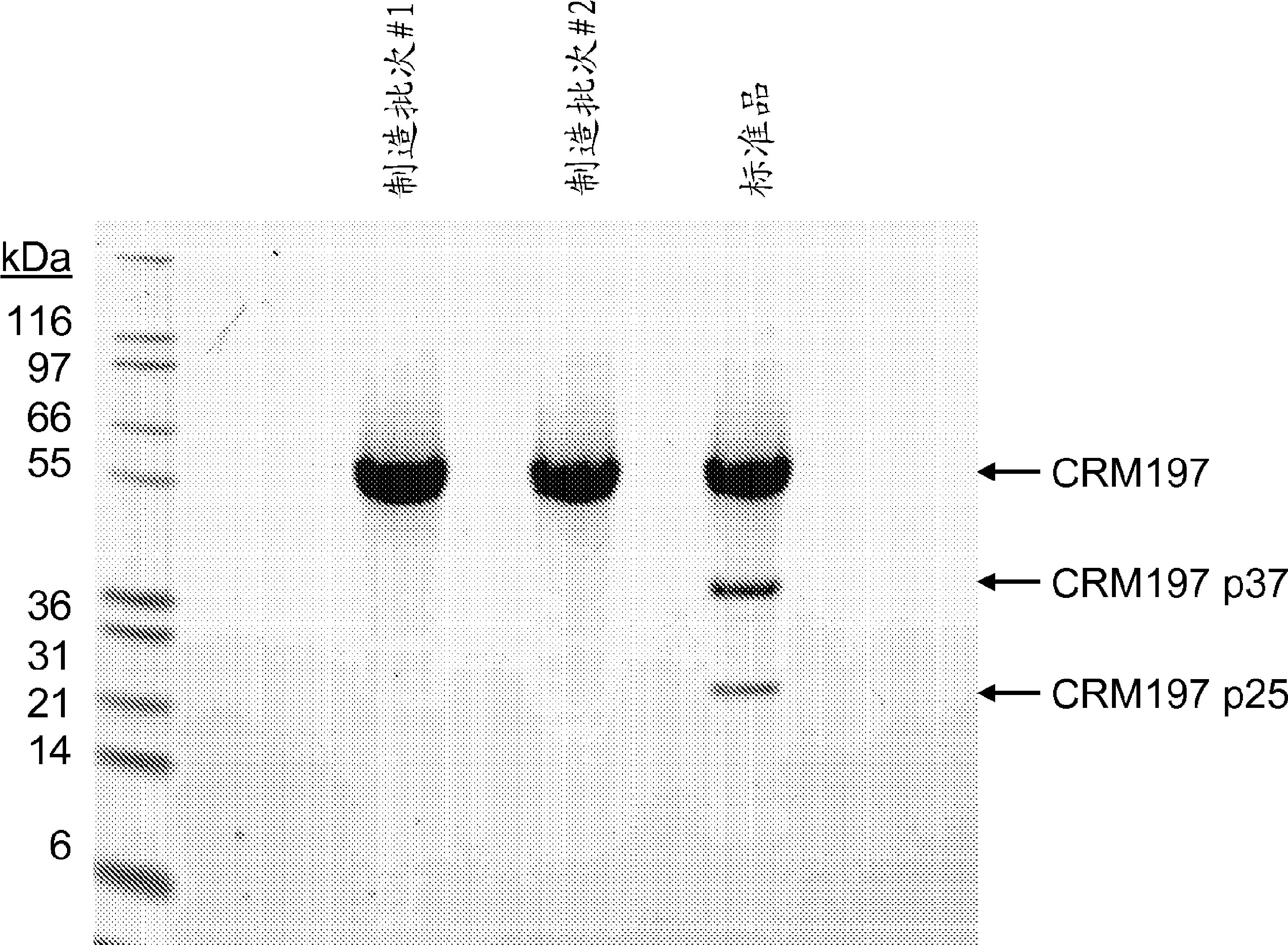
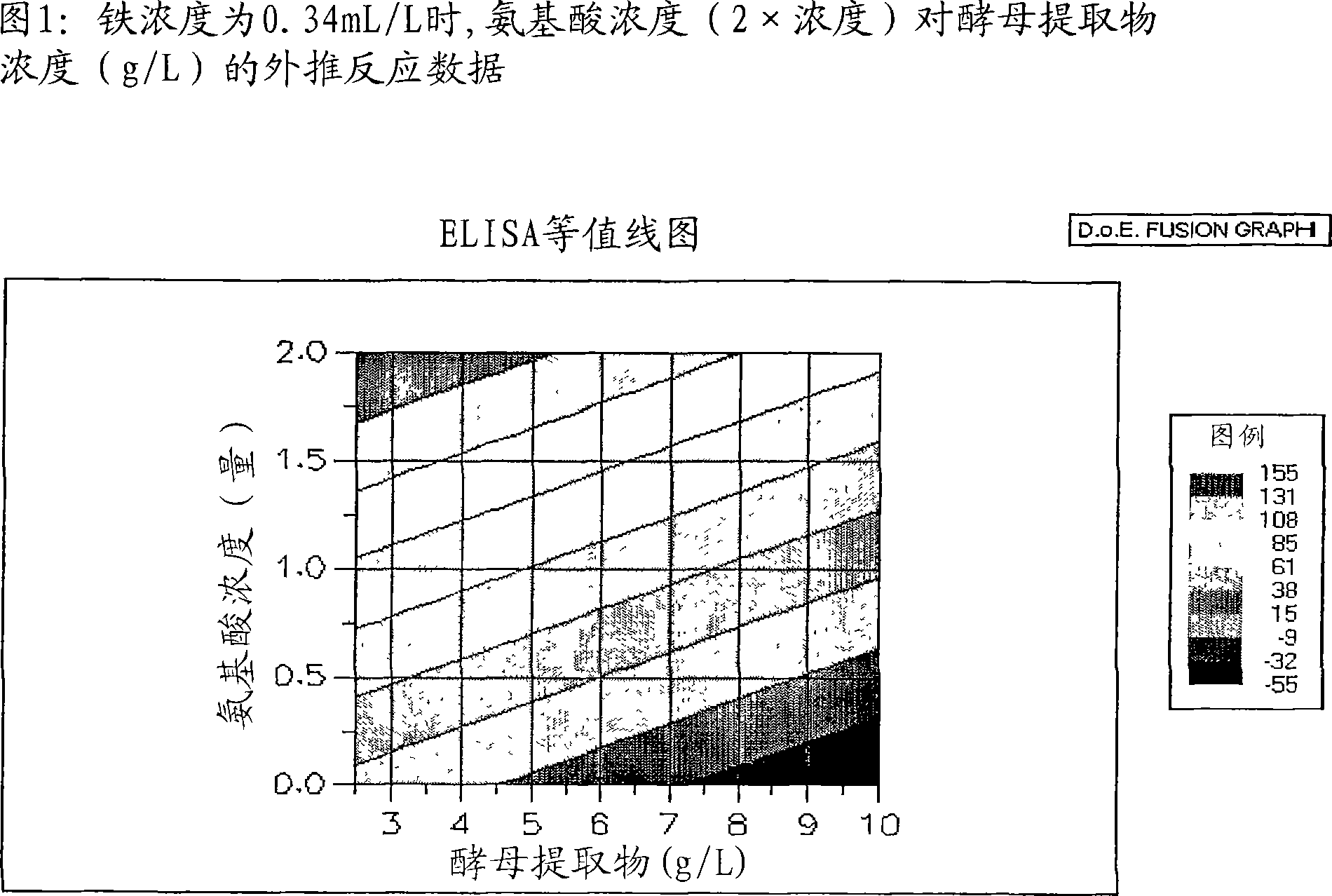
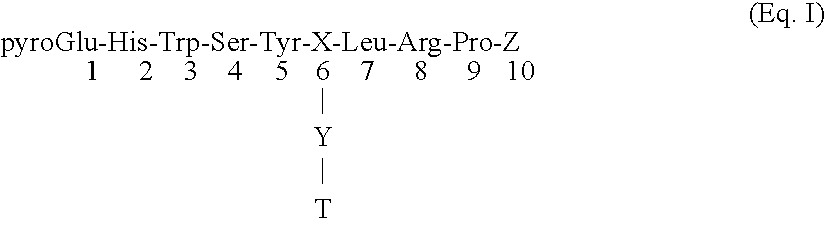Patents
Literature
130 results about "Diphtheria toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
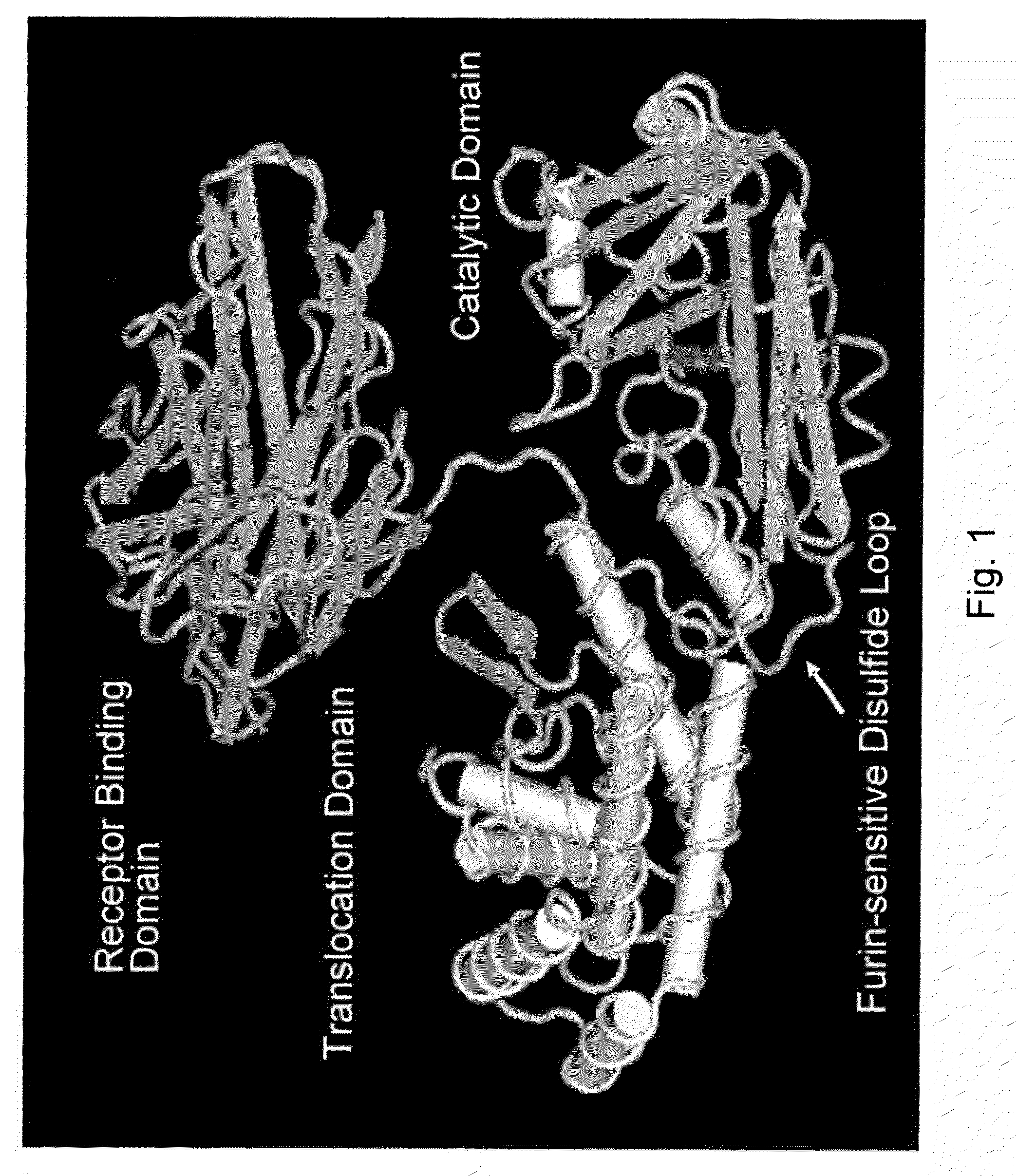
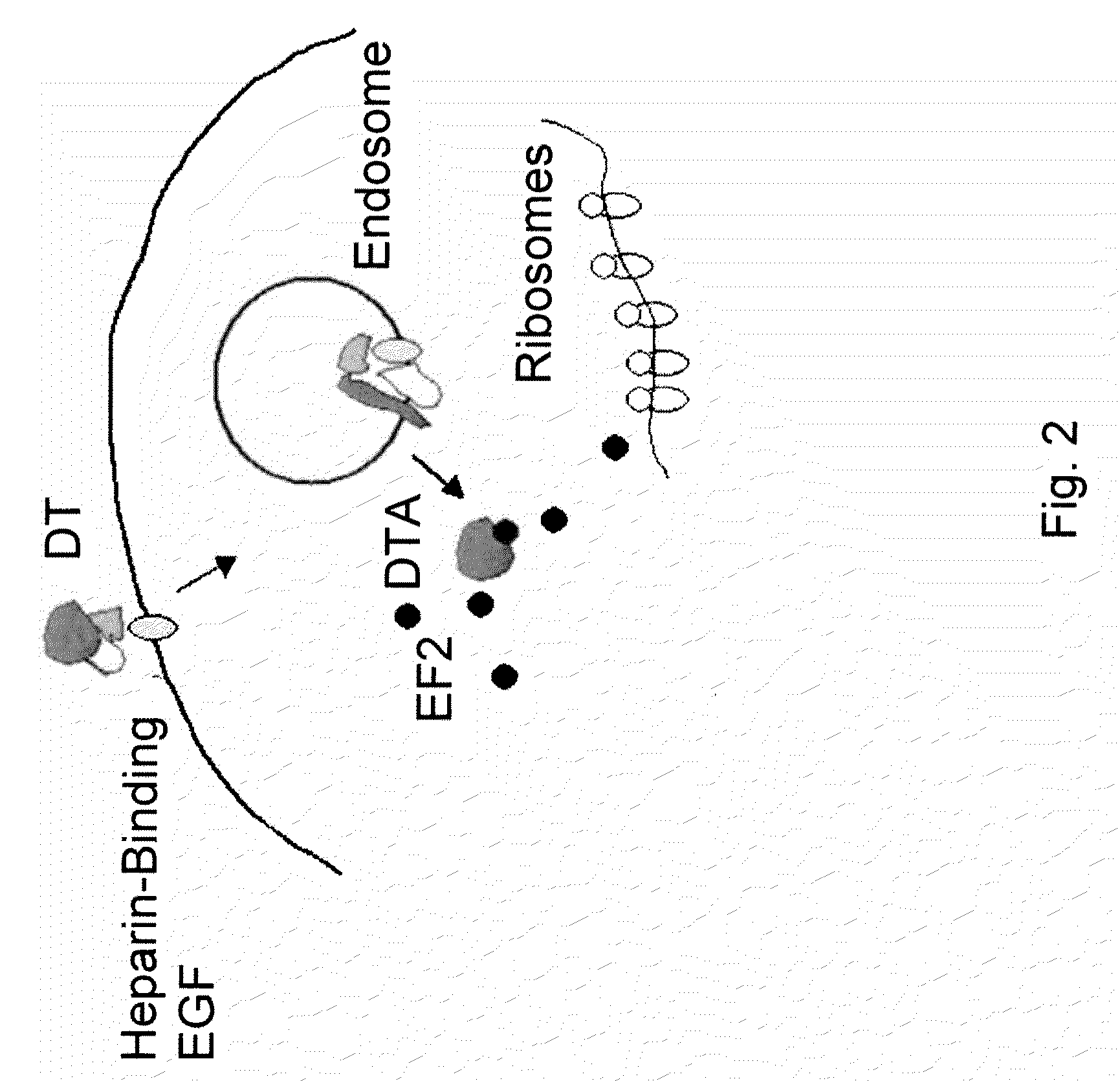
Diphtheria toxin is an exotoxin secreted by Corynebacterium, the pathogenic bacterium that causes diphtheria. The toxin gene is encoded by a prophage (a virus that has inserted itself into the genome of the host bacterium). The toxin causes the disease in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.
Methods and compositions based on diphtheria toxin-interleukin-3 conjugates
ActiveUS20080138313A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationBiocidePeptide/protein ingredientsCancer cellWhite blood cell
The present invention provides methods for inhibiting interleukin-3 receptor-expressing cells, and, in particular, inhibiting the growth of such cells by using a diphtheria toxin-human interleukin-3 conjugate (DT-IL3) that is toxic to cells expressing the interleukin-3 receptor. In preferred embodiments, the DT-IL3 conjugate is a fusion protein comprising amino acids 1-388 of diphtheria toxin fused via a peptide linker to full-length, human interleukin-3. In certain embodiments, the methods of the present invention relate to the administration of a DT-IL3 conjugate to inhibit the growth of cancer cells and / or cancer stem cells in humans, which cells express one or more subunits of the interleukin-3 receptor. Exemplary cells include myeloid leukemia cancer stem cells. In other embodiments, the methods of the present invention relate to ex vivo purging of bone marrow or peripheral blood to remove cells that express one or more subunits of the interleukin-3 receptor such that the purged bone marrow or peripheral blood is suitable, e.g., for autologous stem cell transplantation to restore hematopoietic function.
Owner:SCOTT & WHITE MEMORIAL HOSPITAL
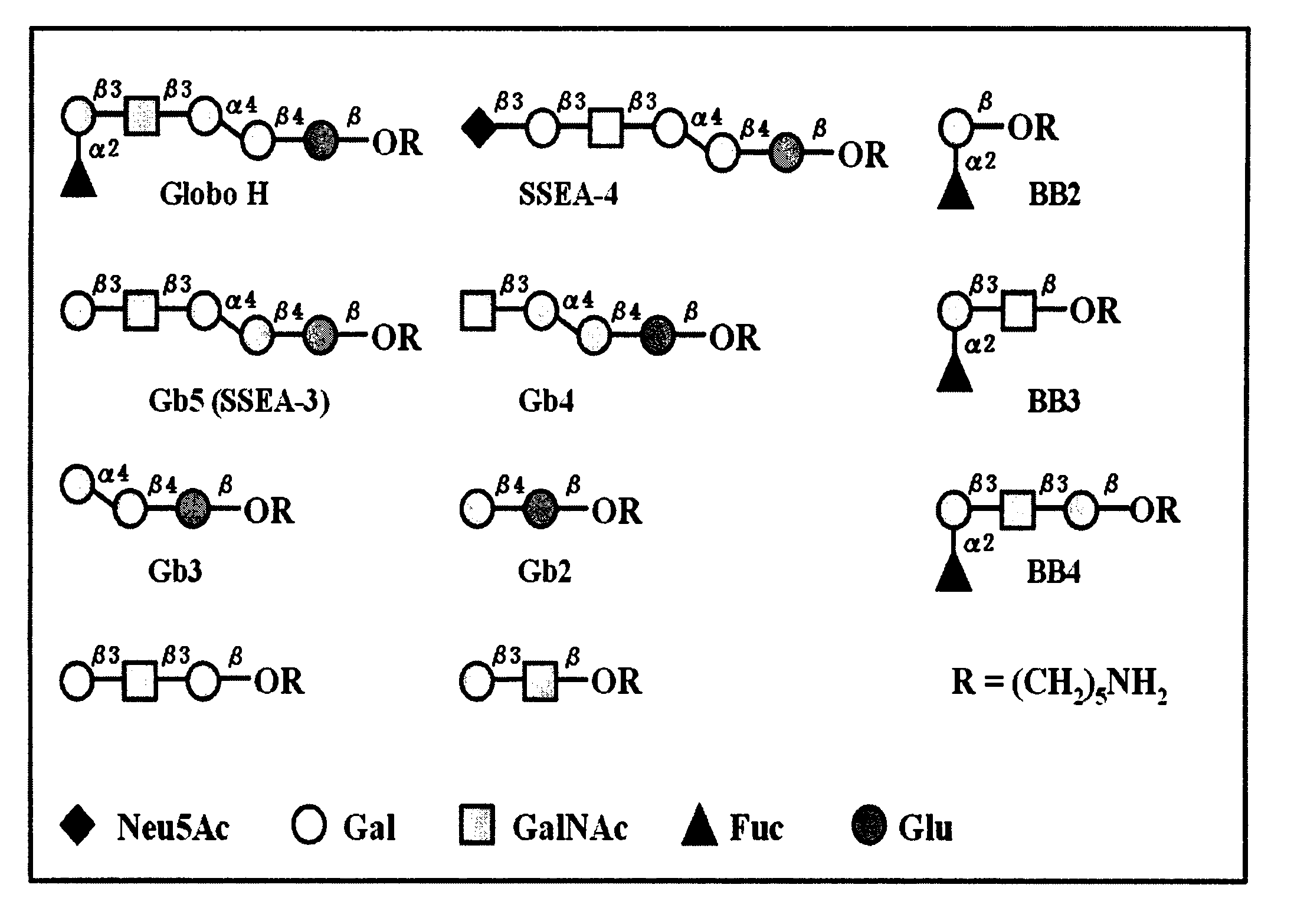
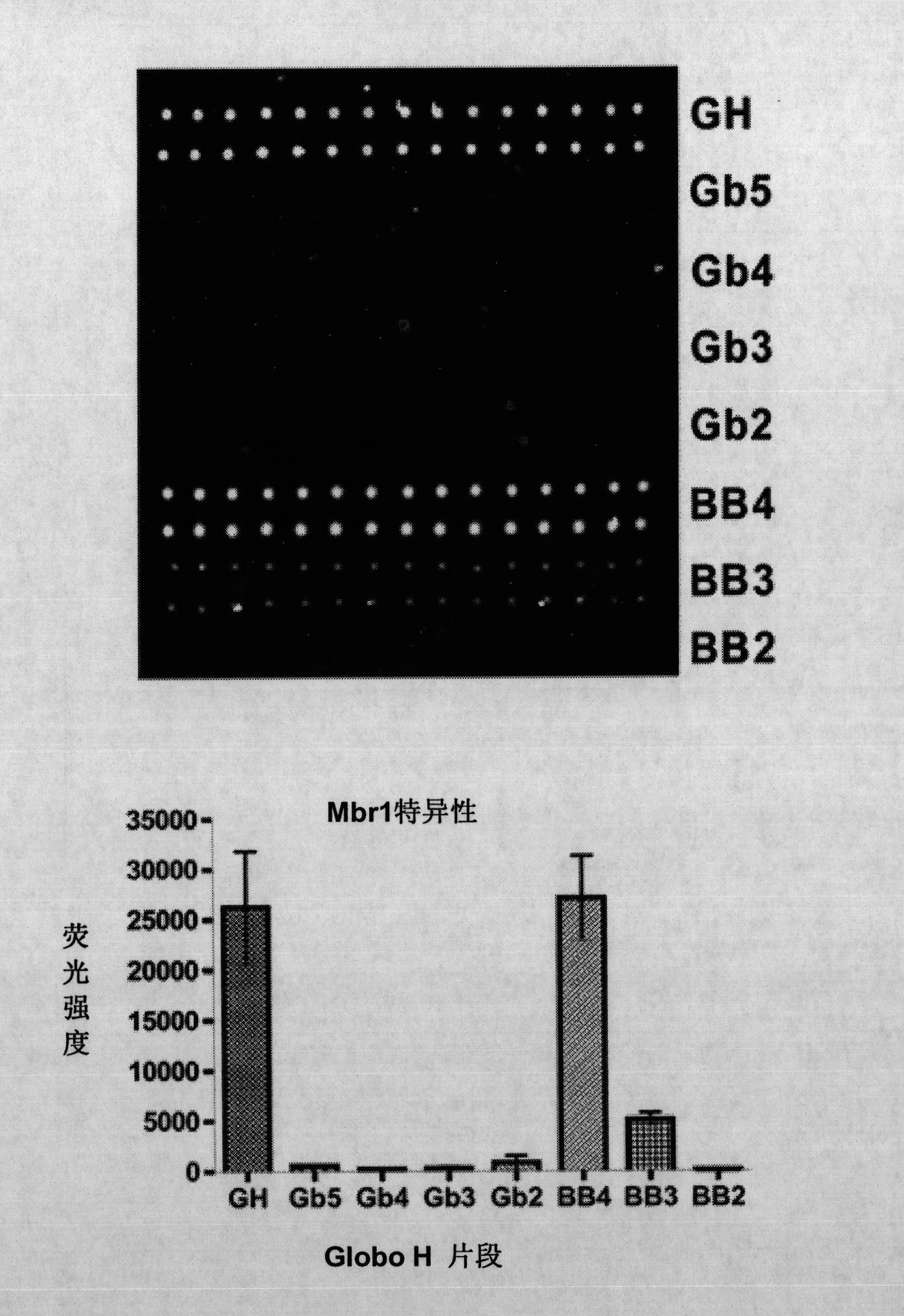
Globo h and related Anti-cancer vaccines with novel glycolipid adjuvants
ActiveUS20100136042A1Shrink tumorInhibit tumor growthOrganic active ingredientsSugar derivativesDendritic cellAdjuvant
Owner:ACAD SINIC
Overexpression porcine co-stimulatory 4-1BB vector and application thereof
InactiveCN105087620AHigh copy numberLower activation thresholdVector-based foreign material introductionAnimal husbandryInteinEmbryo
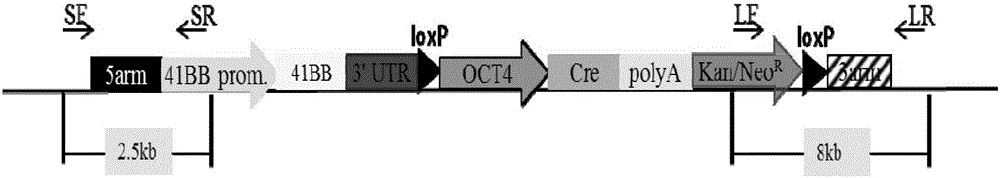
The invention provides an overexpression porcine co-stimulatory 4-1BB vector and application thereof. PCR (polymerase chain reaction) amplification is performed on a left homologous arm and a right homologous arm of an intron 1 of a rosa26 gene, a 4-1BB regulatory sequence and an OCT4 specific promoter; the left homologous arm, a 4-1BB expression cassette, LoxP locus-contained Cre and Neo expression cassettes, the right homologous arm and negative selection DTA diphtheria toxin are connected in sequence to obtain a 4-1BB homologous recombinant vector p4BOCNDR; the vector and a CRISPR / Cas9 (clustered regularly interspaced short palindromic repeats / CRISPR-associated) targeting vector of sgRNA (small guide ribonucleic acid) containing the intron 1 of the specific targeting porcine rosa26 gene are transferred together into a porcine fetus fibroblast; by taking a positive cell as a donor cell and an oocyte as a recipient cell, a cloned embryo is obtained through a somatic cell nuclear transfer technique; the cloned embryo is transplanted into a porcine uterus for fetation to obtain a transgenic pig integrating a 4-1BB gene at the fixed point of a first intron of the rosa26 gene and automatically deleting a marker gene.
Owner:CHINA AGRI UNIV
RNA cancer vaccines
PendingUS20190351040A1Balanced immune responseOrganic active ingredientsAntibody ingredientsTetanusAdjuvant
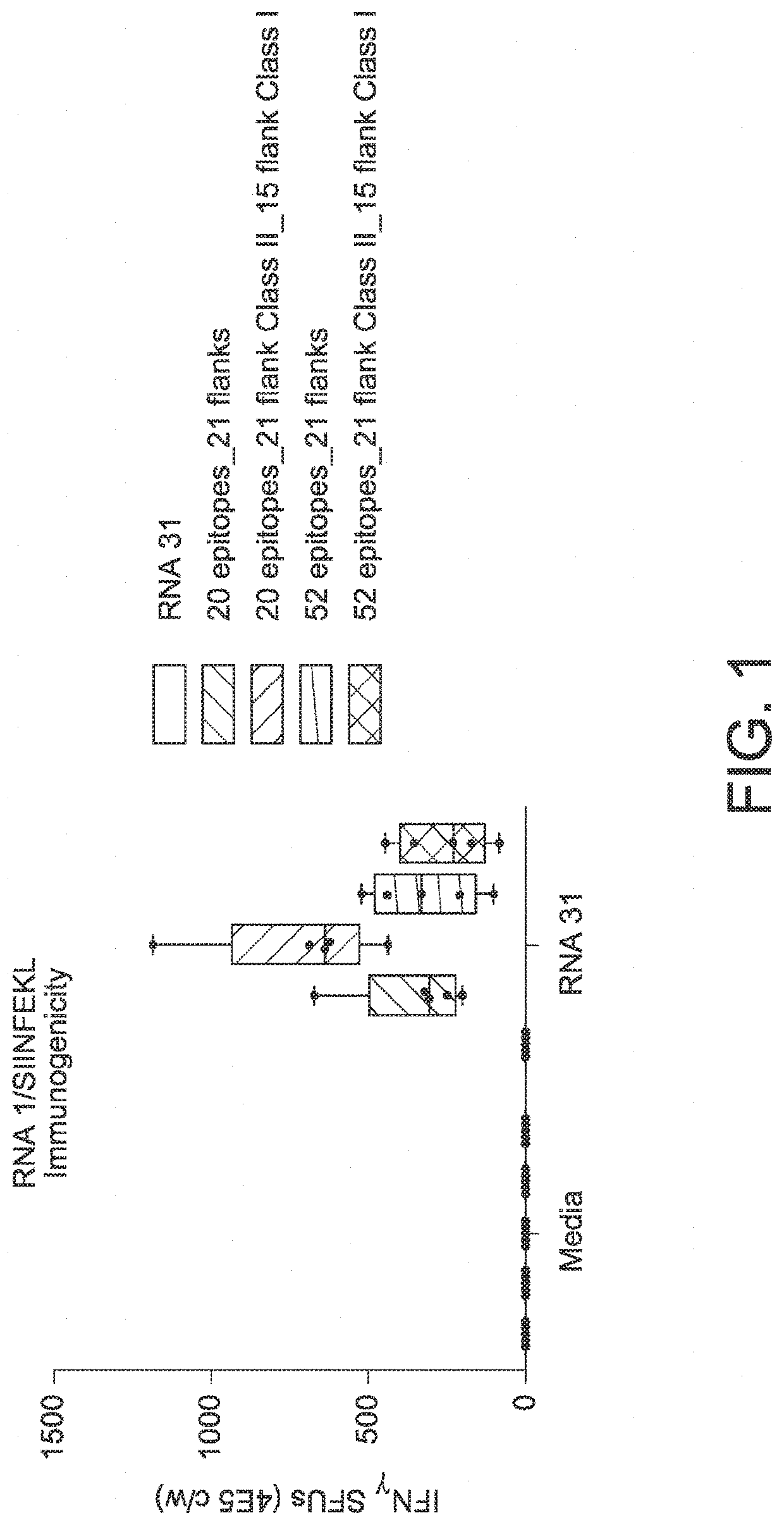
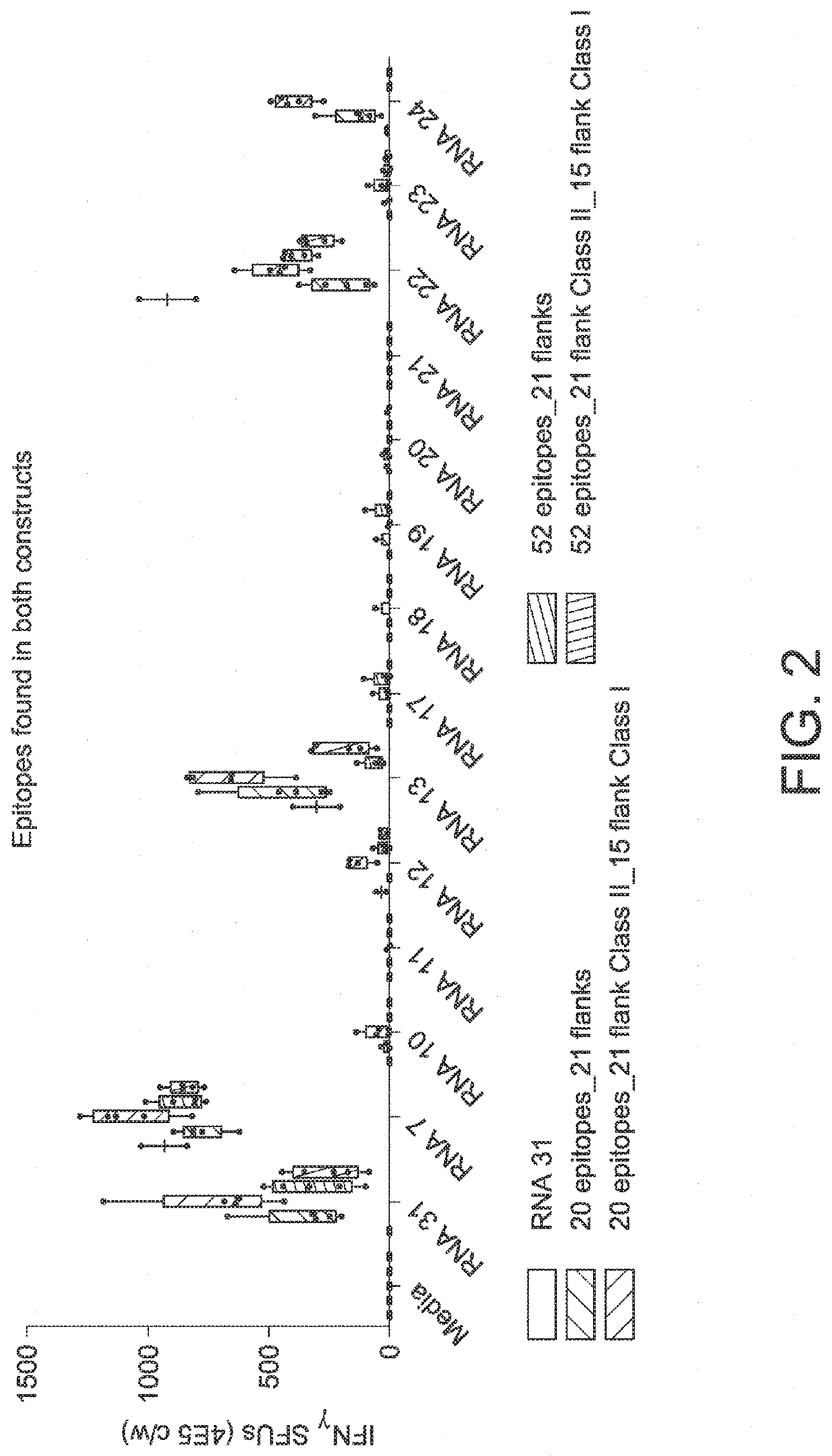
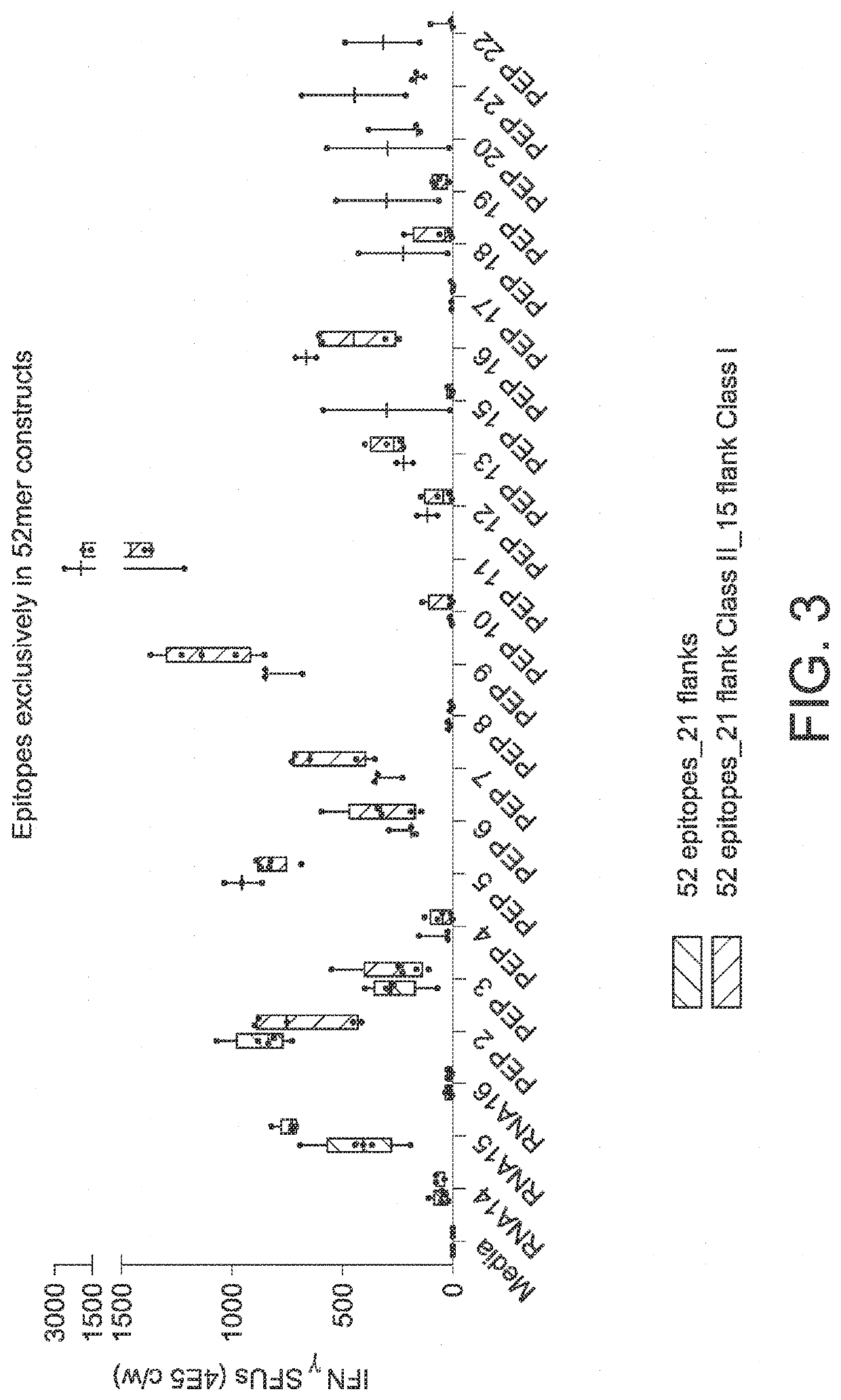
The disclosure relates to cancer ribonucleic acid (RNA) vaccines, as well as methods of using the vaccines and compositions comprising the vaccines. In particular, the disclosure relates to concatemeric mRNA cancer vaccines encoding several cancer epitopes on a single mRNA construct, i.e. poly-epitope mRNA constructs or poly-neo-epitope constructs. The disclosure further relates to p53 and KRAS mutations, as well as incorporation of immune enhancers such as STING, e.g. mRNA constructs further encoding an immune stimulator or adjuvant. The disclosure further relates to inclusion of universal T cell epitopes, such as tetanus or diphtheria toxins to elicit an enhanced immune response.
Owner:MODERNATX INC
Modified diphtheria toxins
InactiveUS20090010966A1High anticancer activityReduced binding activityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseCell binding
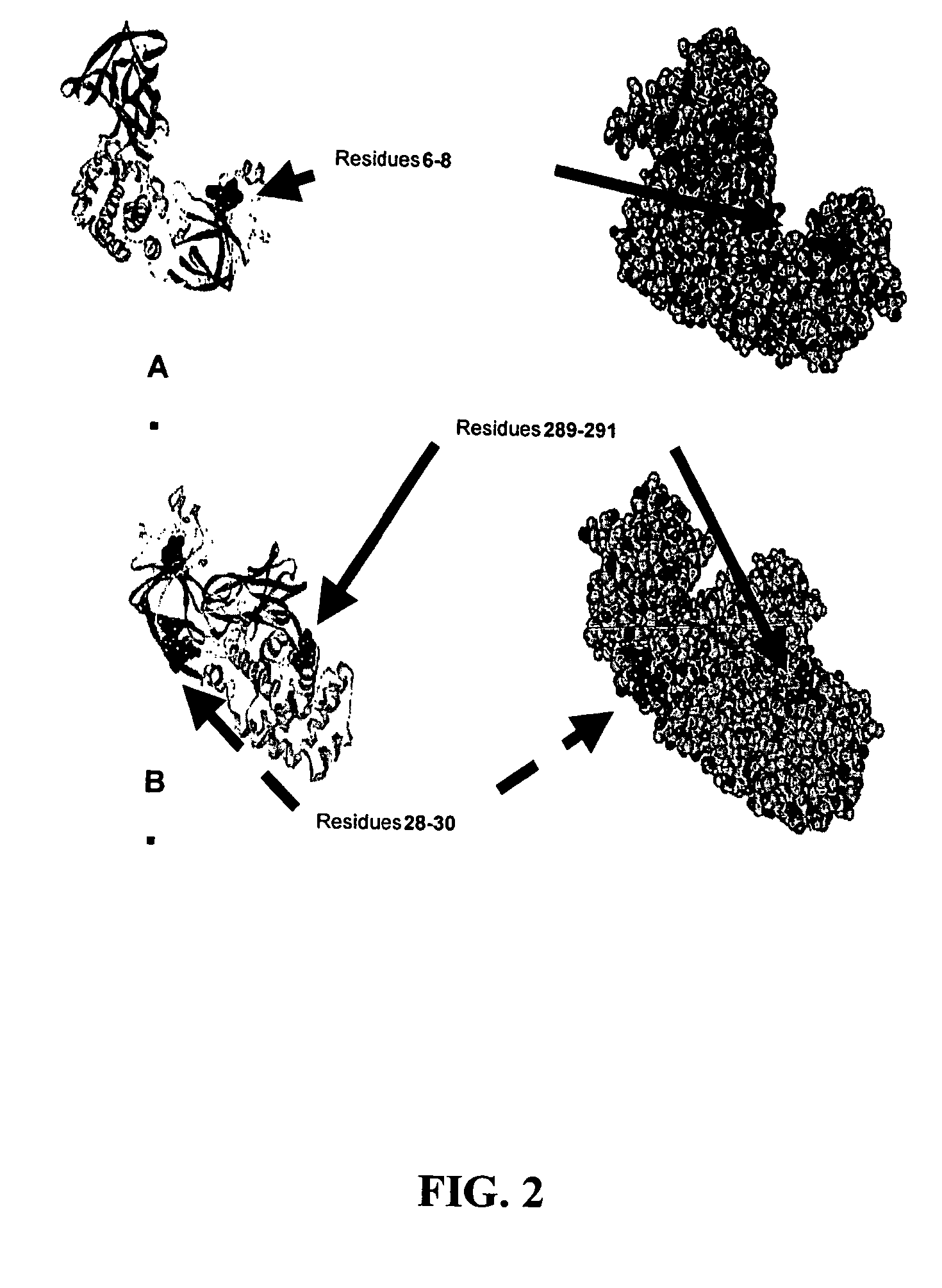
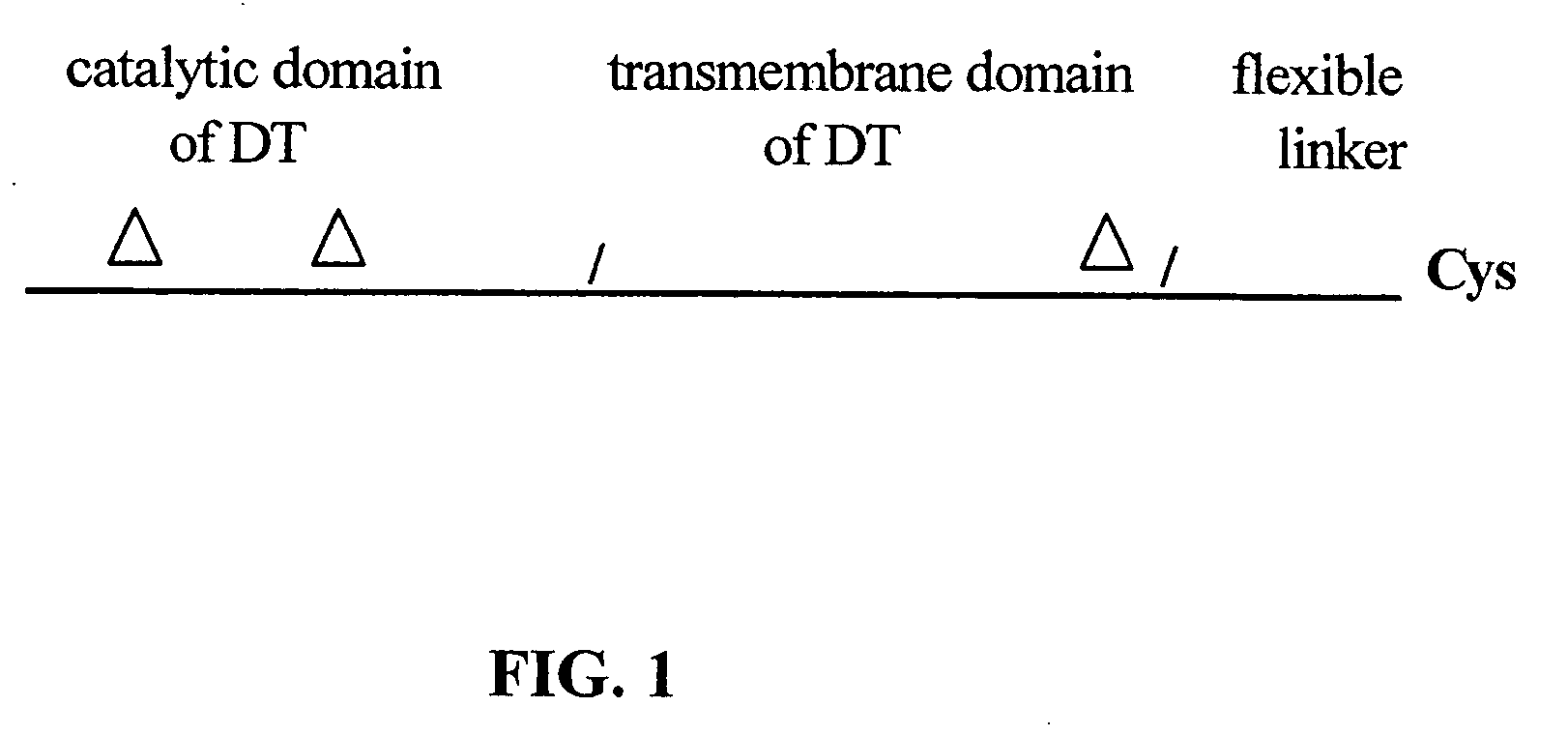
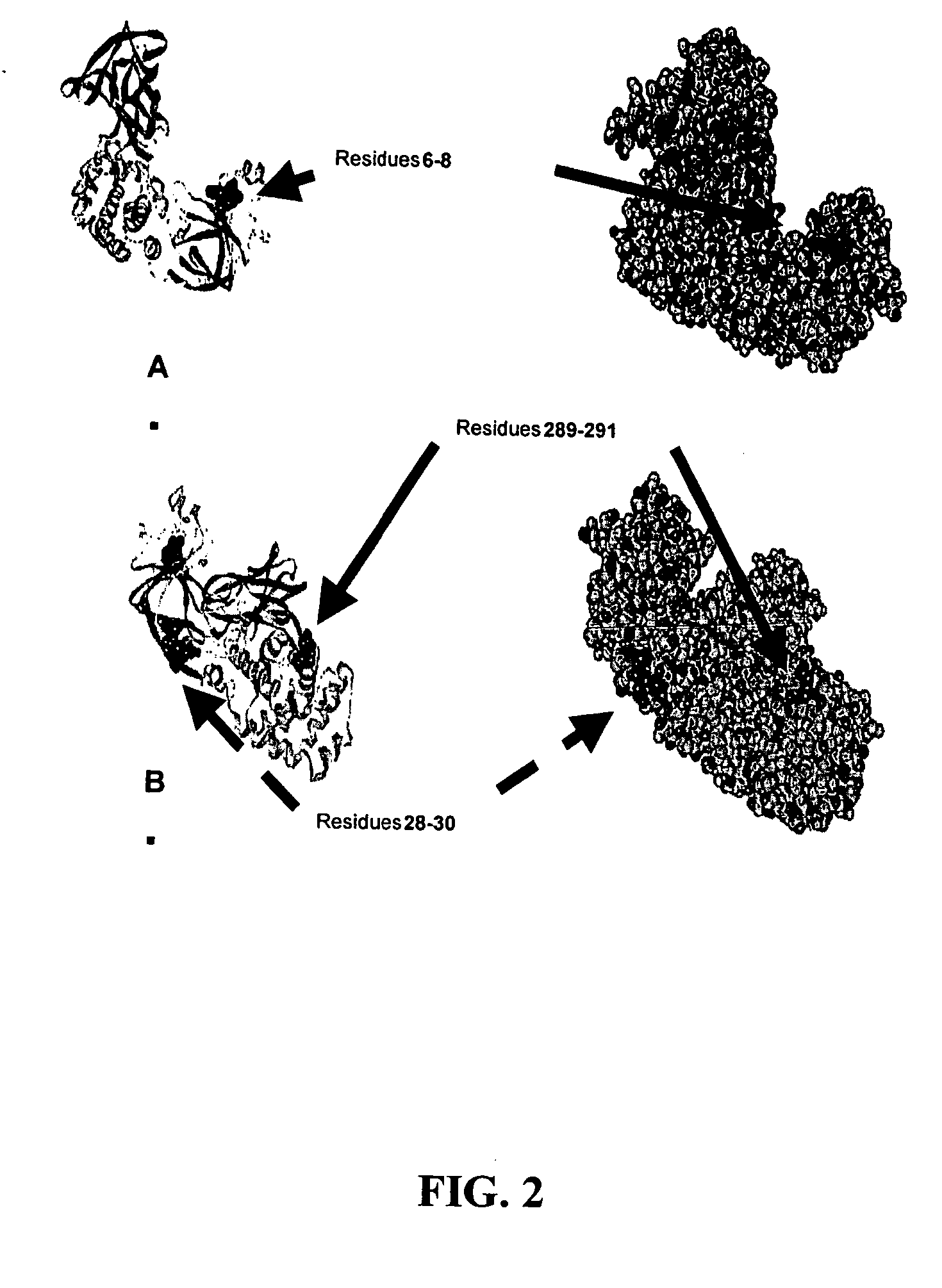
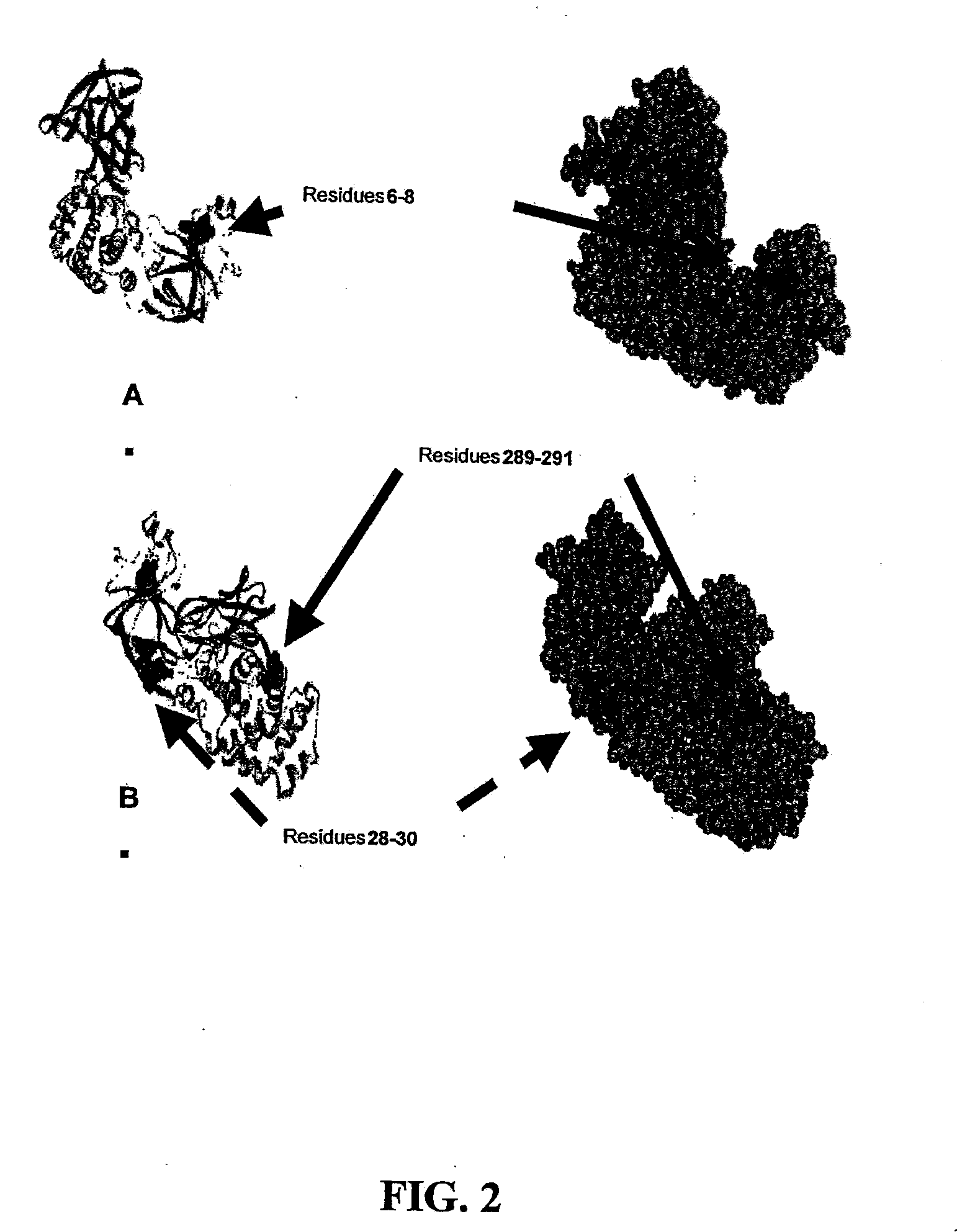
The present application relates to compositions of modified diphtheria toxin and fusion proteins containing modified diphtheria toxin that reduce binding to vascular endothelium or vascular endothelial cells, and therefore, reduce the incidence of Vascular Leak Syndrome, as well as methods of making the compositions. The present application also relates to a polypeptide toxophore from a modified diphtheria toxin, where the modification is at least one amino acid residue at the amino acid residues 6-8, 28-30 or 289-291 of an unmodified native diphtheria toxin. Also described are fusion proteins which contain a modified diphtheria toxin and a non-diphtheria toxin fragment which contains a cell binding portion. The modified diphtheria toxins described can be used for the treatment of a malignant disease or a non-malignant disease.
Owner:ANGELICA THERAPEUTICS
High level expression of recombinant toxin proteins
ActiveUS20110287443A1High yieldQuality improvementAntibody mimetics/scaffoldsBacteria peptidesBacteroidesHigh level expression
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PFENEX
Porous nanoparticle-supported lipid bilayers (protocells) for targeted delivery including transdermal delivery of cargo and methods thereof
InactiveUS20150272885A1Large specific surface areaAmenable to high capacity loadingOrganic active ingredientsPowder deliveryCancers diagnosisBinding peptide
The present invention is directed to protocells for specific targeting of hepatocellular and other cancer cells which comprise a nanoporous silica core with a supported lipid bilayer; at least one agent which facilitates cancer cell death (such as a traditional small molecule, a macromolecular cargo (e.g. siRNA or a protein toxin such as ricin toxin A-chain or diphtheria toxin A-chain) and / or a histone-packaged plasmid DNA disposed within the nanoporous silica core (preferably supercoiled in order to more efficiently package the DNA into protocells) which is optionally modified with a nuclear localization sequence to assist in localizing protocells within the nucleus of the cancer cell and the ability to express peptides involved in therapy (apoptosis / cell death) of the cancer cell or as a reporter, a targeting peptide which targets cancer cells in tissue to be treated such that binding of the protocell to the targeted cells is specific and enhanced and a fusogenic peptide that promotes endosomal escape of protocells and encapsulated DNA. Protocells according to the present invention may be used to treat cancer, especially including hepatocellular (liver) cancer using novel binding peptides (c-MET peptides) which selectively bind to hepatocellular tissue or to function in diagnosis of cancer, including cancer treatment and drug discovery.
Owner:STC UNM +1
Modified toxins
ActiveUS20090041797A1Low immunogenicityPeptide/protein ingredientsBacteria peptidesDiseaseVascular endothelium
Owner:ANGELICA THERAPEUTICS
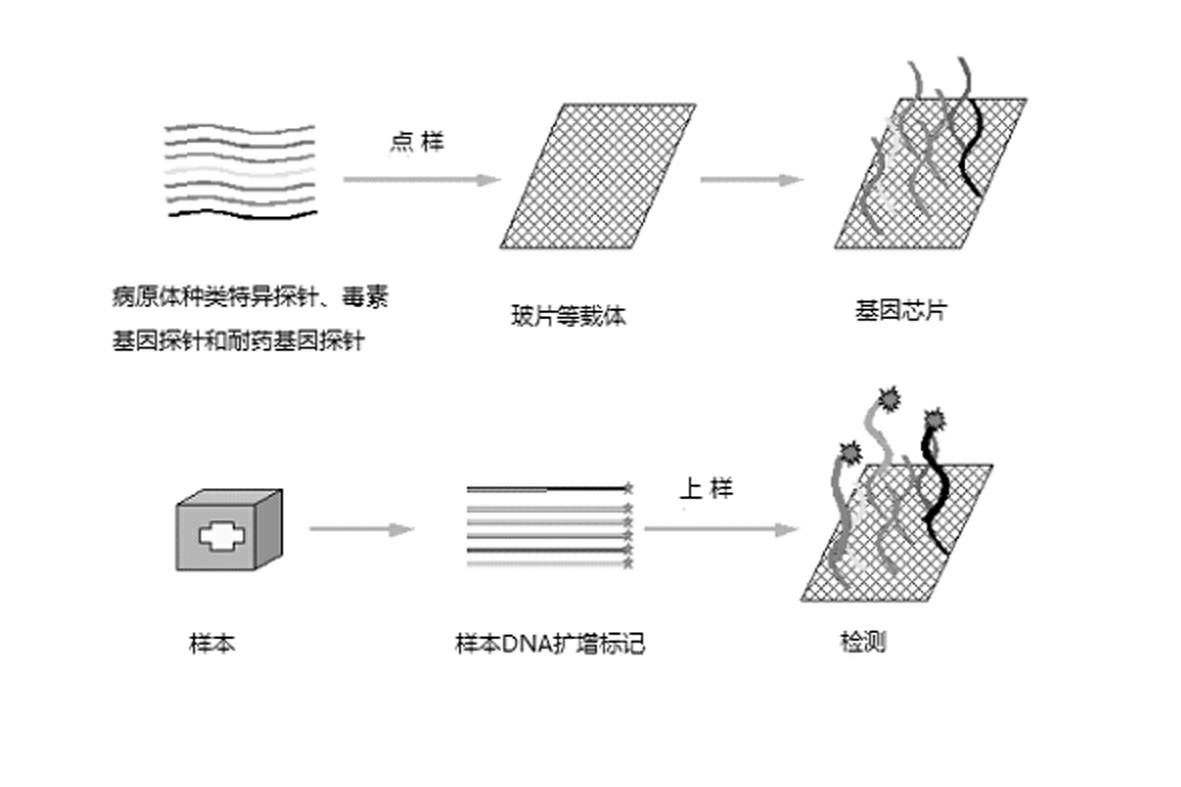
Gene chip for high-flux detection of pathogens and application thereof
InactiveCN102534013AStrong specificityDetermine the typeMicrobiological testing/measurementAgainst vector-borne diseasesYersinia pestisBrucella
The invention relates to a gene chip for high-flux detection of pathogens and application thereof. The gene comprises (1) a combination of 174 oligonucleotide probes of pathogen variety specific genes, toxin genes and drug-resistant genes; and (2) a probe array, which is formed by curing the oligonucleotide probes on a carrier material by arm molecules. The gene chip comprises 174 gene probes, namely 32 pathogen variety specific gene probes of the following 8 pathogens of Burkholderia mallei, Burkholderia pseudomallei, Brucella, salmonella, Yersinia pestis, Bacillus anthracis, comma bacillus and the like, 25 toxin gene probe of the following 7 toxins of diphtheria toxin, Shiga toxin, staphylococcus enterotoxin, choleratoxin and the like, and 117 drug-resistant gene probes of 17 drug-resistant genes of extended-spectrum beta-lactamase, cephalosporinase, carbapenemase, integrase gene, common gene engineering carrier drug-resistant gene and the like. The gene chip can be used to detect multiple pathogen variety specific genes, toxin genes and drug-resistant genes.
Owner:李越希
Diphtheria toxin variant
InactiveUS7585942B2Reduce the binding forceReduce morbidityPolypeptide with localisation/targeting motifBacterial antigen ingredientsVascular endotheliumCancer research
The present invention relates to methods and compositions of modified variants of diphtheria toxin (DT) that reduce binding to vascular endothelium or vascular endothelial cells, and therefore, reduce the incidence of Vascular Leak Syndrome. One aspect of the present invention relates to a polypeptide toxophore from a modified DT, wherein the mutation is the substitution or deletion at least one amino acid residue at the amino acid residues 6-8, 28-30 or 289-291 of native DT. Another aspect of the present invention relates to a fusion protein which comprises a modified DT and a non-DT fragment. Another aspect of the present invention relates to the use of modified DT for the treatment of cancer.
Owner:ANJIN
Diphtheria toxin variant
InactiveUS20060159708A1Reduce the binding forceReduce morbidityPolypeptide with localisation/targeting motifBacterial antigen ingredientsVascular endotheliumDiphtheria toxin
The present invention relates to methods and compositions of modified variants of diphtheria toxin (DT) that reduce binding to vascular endothelium or vascular endothelial cells, and therefore, reduce the incidence of Vascular Leak Syndrome. One aspect of the present invention relates to a polypeptide toxophore from a modified DT, wherein the mutation is the substitution or deletion at least one amino acid residue at the amino acid residues 6-8, 28-30 or 289-291 of native DT. Another aspect of the present invention relates to a fusion protein which comprises a modified DT and a non-DT fragment. Another aspect of the present invention relates to the use of modified DT for the treatment of cancer.
Owner:ANJIN
High level expression of recombinant toxin proteins
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PELICAN TECH HLDG INC
Immunogen by using mutant CRM197 of diphtheria toxin as carrier, preparation method, and application
ActiveCN101050236AGood effectHigh recovery ratePeptide/protein ingredientsPeptide preparation methodsN-HydroxysuccinimideCarrier protein
This invention relates to a method for preparing immunogen G17CRM197 containing diphtherin mutant CRM197 as carrier. The method comprises: crosslinking diphtherin mutant CRM197 with gastrin G17 through epsiv-maleimidyl caproic acid N-hydroxysuccinimide eater (EMCS). Diphtherin mutant CRM197 is used as a carrier, and has such advantages as high recovery rate and easy purification. The method has such advantages as high product crosslinking rate, short preparation time, and low cost. Immunogen G17CRM197 can be used as an effective component in therapeutic vaccines, or used in anti-tumor drugs with appropriate immunoadjuvants.
Owner:QILU PHARMA HAINAN
Porous nanoparticle-supported lipid bilayers (protocells) for targeted delivery including transdermal delivery of cargo and methods thereof
The present invention is directed to protocells for specific targeting of hepatocellular and other cancer cells which comprise a nanoporous silica core with a supported lipid bilayer; at least one agent which facilitates cancer cell death (such as a traditional small molecule, a macromolecular cargo (e.g. siRNA or a protein toxin such as ricin toxin A-chain or diphtheria toxin A-chain) and / or a histone-packaged plasmid DNA disposed within the nanoporous silica core (preferably supercoiled in order to more efficiently package the DNA into protocells) which is optionally modified with a nuclear localization sequence to assist in localizing protocells within the nucleus of the cancer cell and the ability to express peptides involved in therapy (apoptosis / cell death) of the cancer cell or as a reporter, a targeting peptide which targets cancer cells in tissue to be treated such that binding of the protocell to the targeted cells is specific and enhanced and a fusogenic peptide that promotes endosomal escape of protocells and encapsulated DNA. Protocells according to the present invention may be used to treat cancer, especially including hepatocellular (liver) cancer using novel binding peptides (c-MET peptides) which selectively bind to hepatocellular tissue or to function in diagnosis of cancer, including cancer treatment and drug discovery.
Owner:STC UNM +1
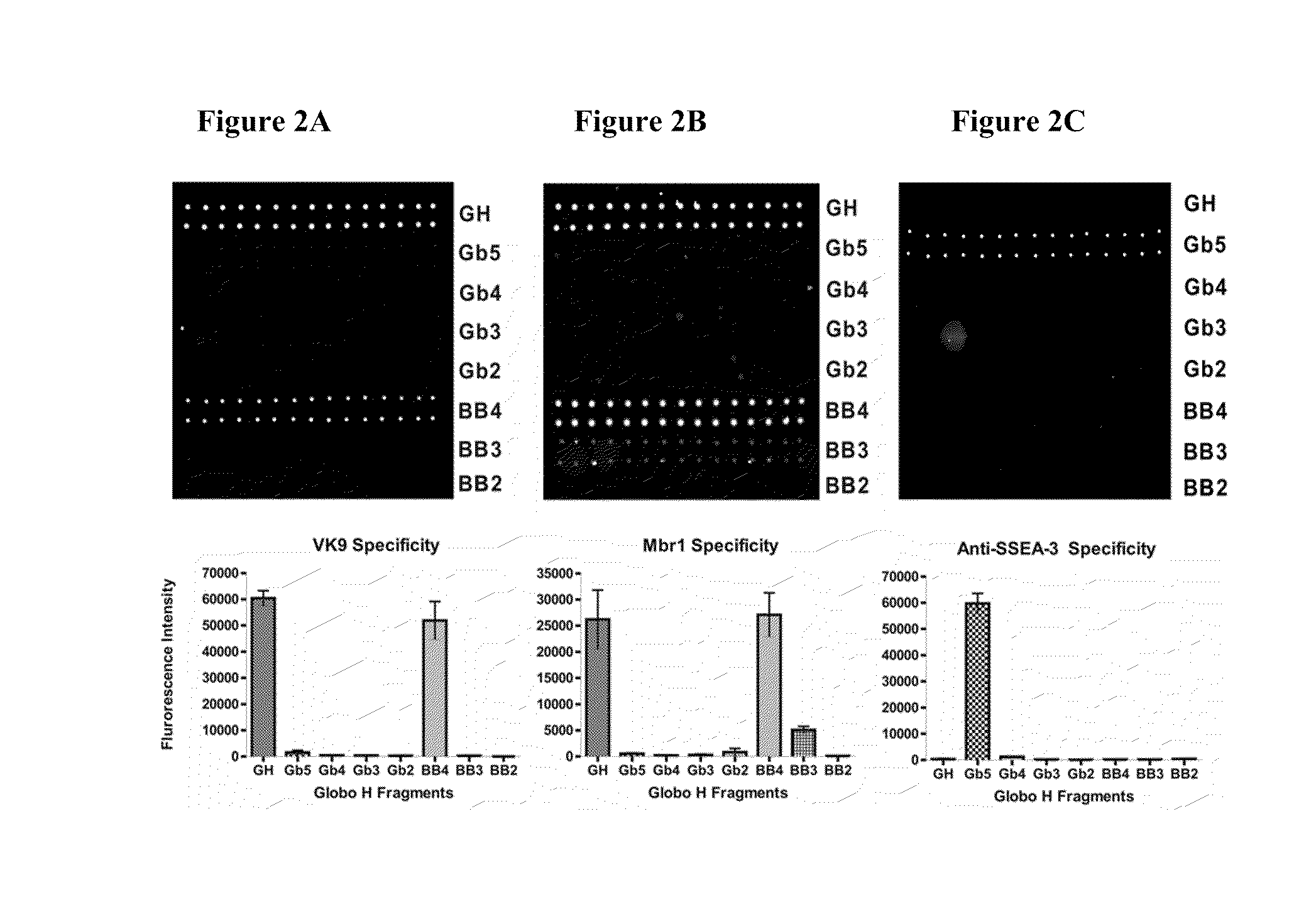
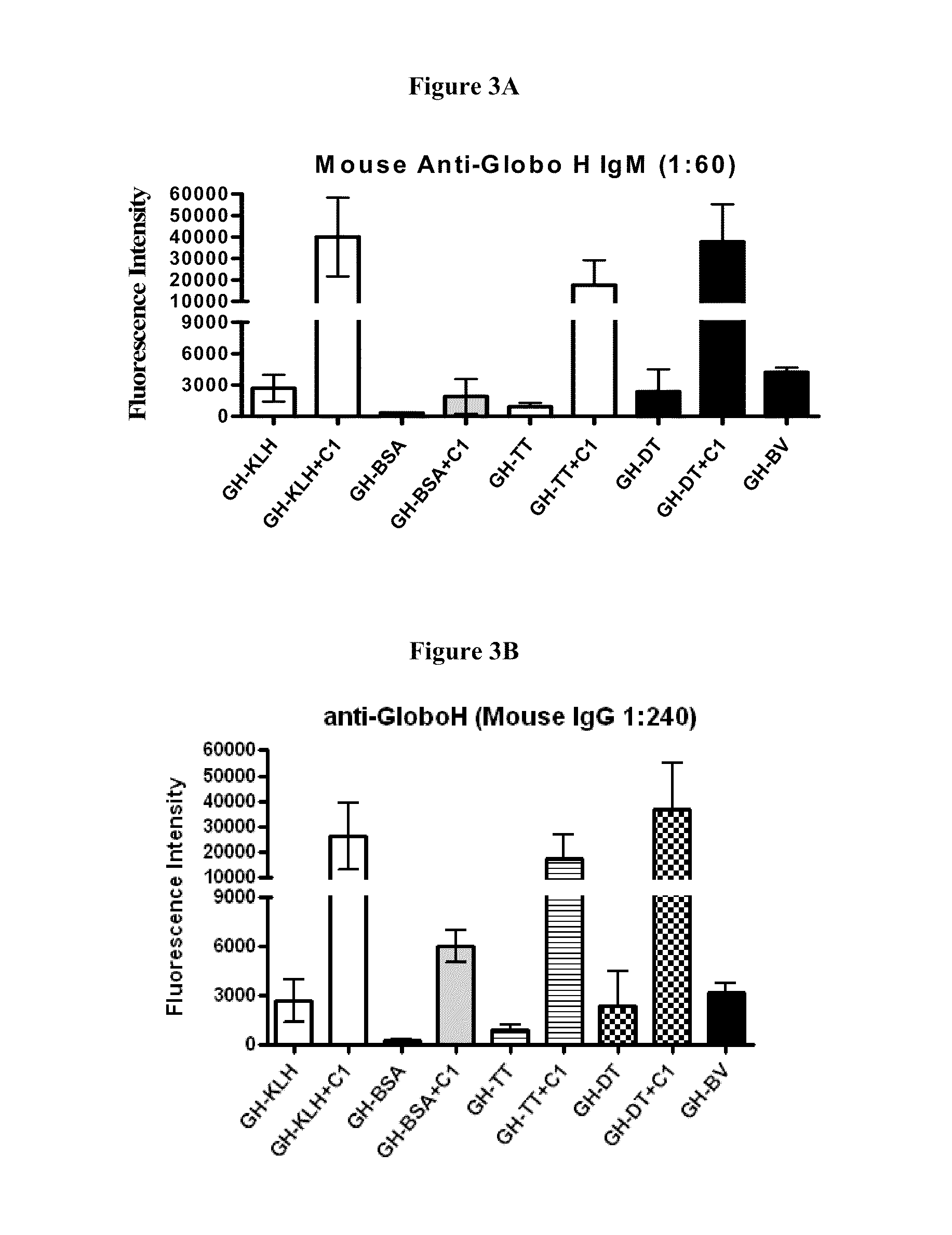
Globo h and related anti-cancer vaccines with novel glycolipid adjuvants
Immunogenic compositions, cancer vaccines and methods for treating cancer are provided. Compositions comprising: (a) a glycan such as Globo H or an immunogenic fragment thereof, wherein the glycan is conjugated with a carrier protein by a linker such as para-nitrophenyl; and (b) an adjuvant comprising glycolipid capable of binding CDId on a dendritic cell, such as an a-galactosyl-ceramide derivative, wherein the immunogenic composition induces an immune response that induces a higher relative level of IgG isotype antibodies as compared to IgM isotype antibodies, are provided. Immunogenic compositions comprising the carrier protein diphtheria toxin cross-reacting material 197 (DT-CRM 197) and the adjuvant C34 are provided. Antibodies generated by immunogenic compositions disclosed herein further neutralize at least one of the antigens Globo H, stage-specific embryonic antigen-3 (SSEA-3) and stage-specific embryonic antigen-4 (SSEA-4). Therapeutics against breast cancer stem cells comprising immunogenic compositions comprising Globo H, SSEA-3 or SSEA-4 conjugated with DT-CRM 197.
Owner:ACAD SINIC
Porous nanoparticle-supported lipid bilayers (protocells) for targeted delivery and methods of using same
InactiveUS20160106671A1Inhibit growthBiocideHeavy metal active ingredientsLipid formationBinding peptide
The present invention is directed to protocells for specific targeting of hepatocellular and other cancer cells which comprise a nanoporous silica core with a supported lipid bilayer; at least one agent which facilitates cancer cell death (such as a traditional small molecule, a macromolecular cargo (e.g. siRNA or a protein toxin such as ricin toxin A-chain or diphtheria toxin A-chain) and / or a histone-packaged plasmid DNA disposed within the nanoporous silica core (preferably supercoiled in order to more efficiently package the DNA into protocells) which is optionally modified with a nuclear localization sequence to assist in localizing protocells within the nucleus of the cancer cell and the ability to express peptides involved in therapy (apoptosis / cell death) of the cancer cell or as a reporter, a targeting peptide which targets cancer cells in tissue to be treated such that binding of the protocell to the targeted cells is specific and enhanced and a fusogenic peptide that promotes endosomal escape of protocells and encapsulated DNA. Protocells according to the present invention may be used to treat cancer, especially including hepatocellular (liver) cancer using novel binding peptides (c-MET peptides) which selectively bind to hepatocellular tissue or to function in diagnosis of cancer, including cancer treatment and drug discovery.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC +1
Diphtheria toxin variant
InactiveUS20090156502A1Reduce the binding forceReduce morbidityPolypeptide with localisation/targeting motifBacterial antigen ingredientsVascular endotheliumCancer research
Owner:ANJIN
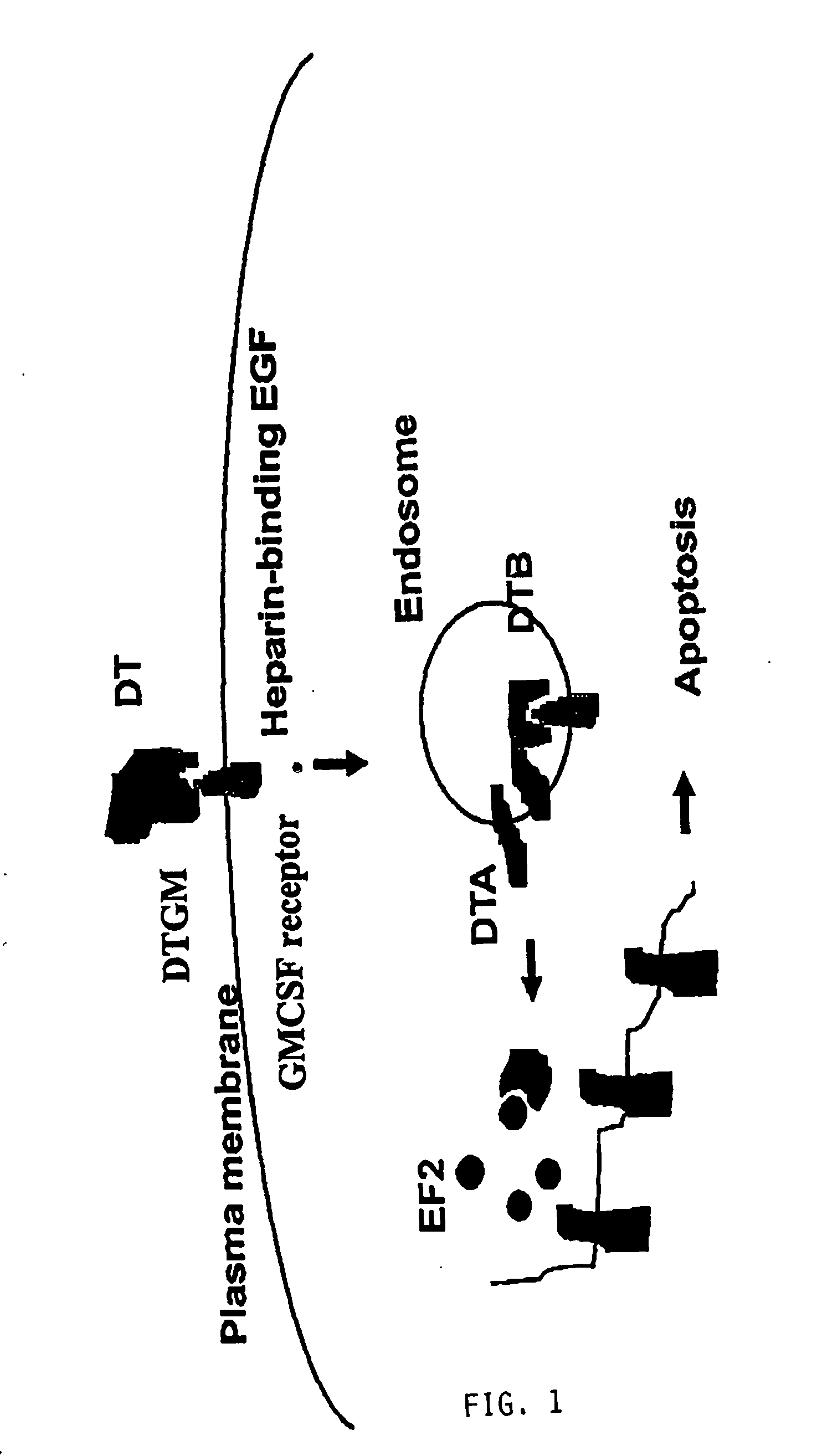
Interfusion protein between diphtheria toxin and GM CSF mutant, coded gene and application
This invention discloses fusion protein of diphtherin and GM-CSF mutant, its coding gene, and its application. The fusion protein is selected from: (a) the protein shown in SEQ ID No.2; (b) the protein derived from SEQ ID No.2 by substituting, deleting and / or adding one or more amino acid residues, which can kill acute myeloid leukemia cells. The fusion protein can kill target cells, and has a high expression level.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Nucleic acid constructs, pharmaceutical compositions and methods of using same for treating cancer
InactiveUS8034914B2Sugar derivativesGenetic material ingredientsNucleic acid sequencingDiphtheria toxin
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Methods of purification of native or mutant forms of diphtheria toxin
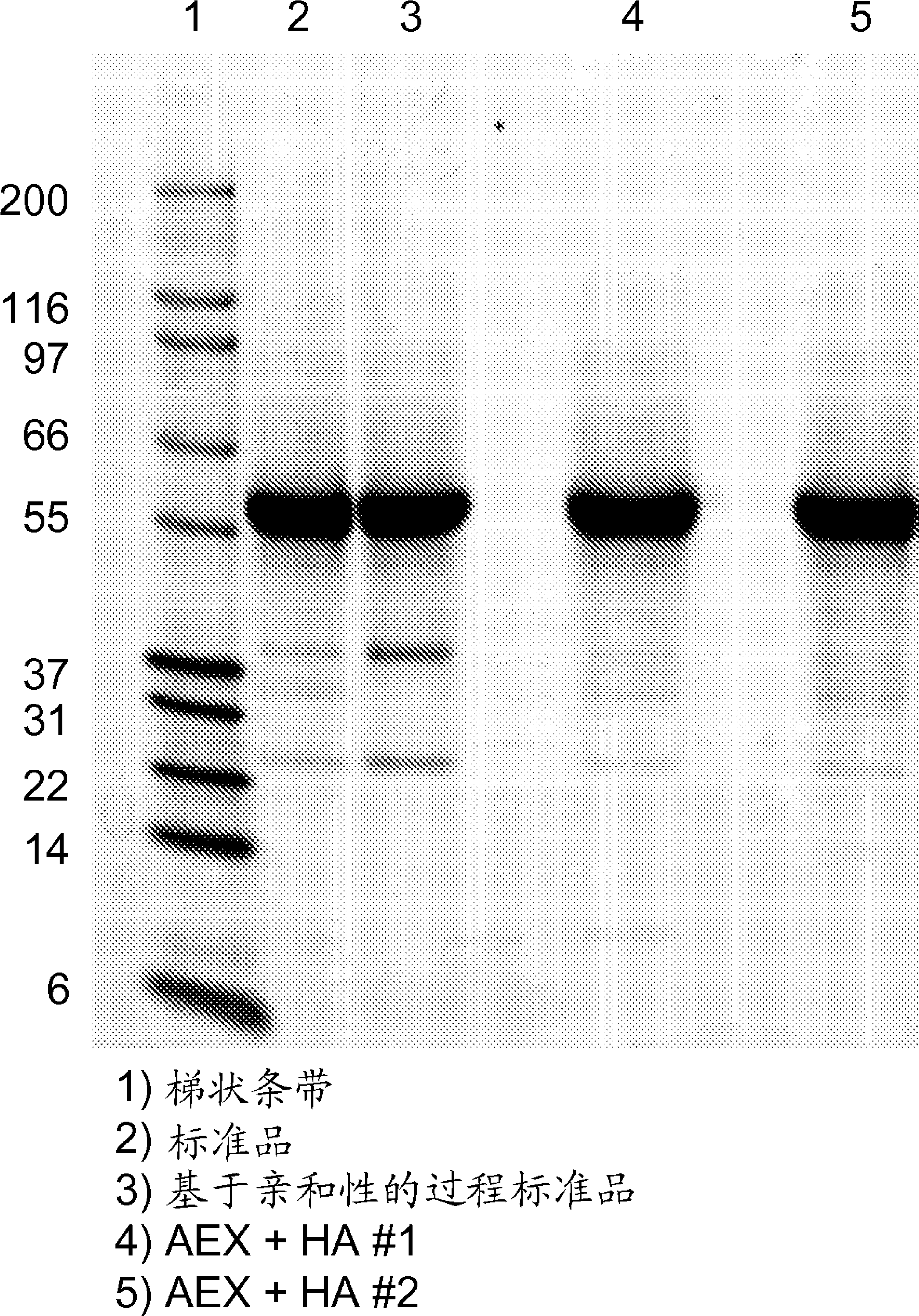
The present invention relates to the use of hydroxyapatite chromatography and multimodal chromatography, for purification of diphtheria toxin, or a mutant form thereof, from a mixture, for example, a host cell fermentation mixture containing impurities such as host cell proteins and DNA. This invention further relates to the integration of such a method into a multi-step procedure with other fractionation methods for purification of diphtheria toxin suitable for in vitro and in vivo applications.
Owner:MERCK & CO INC
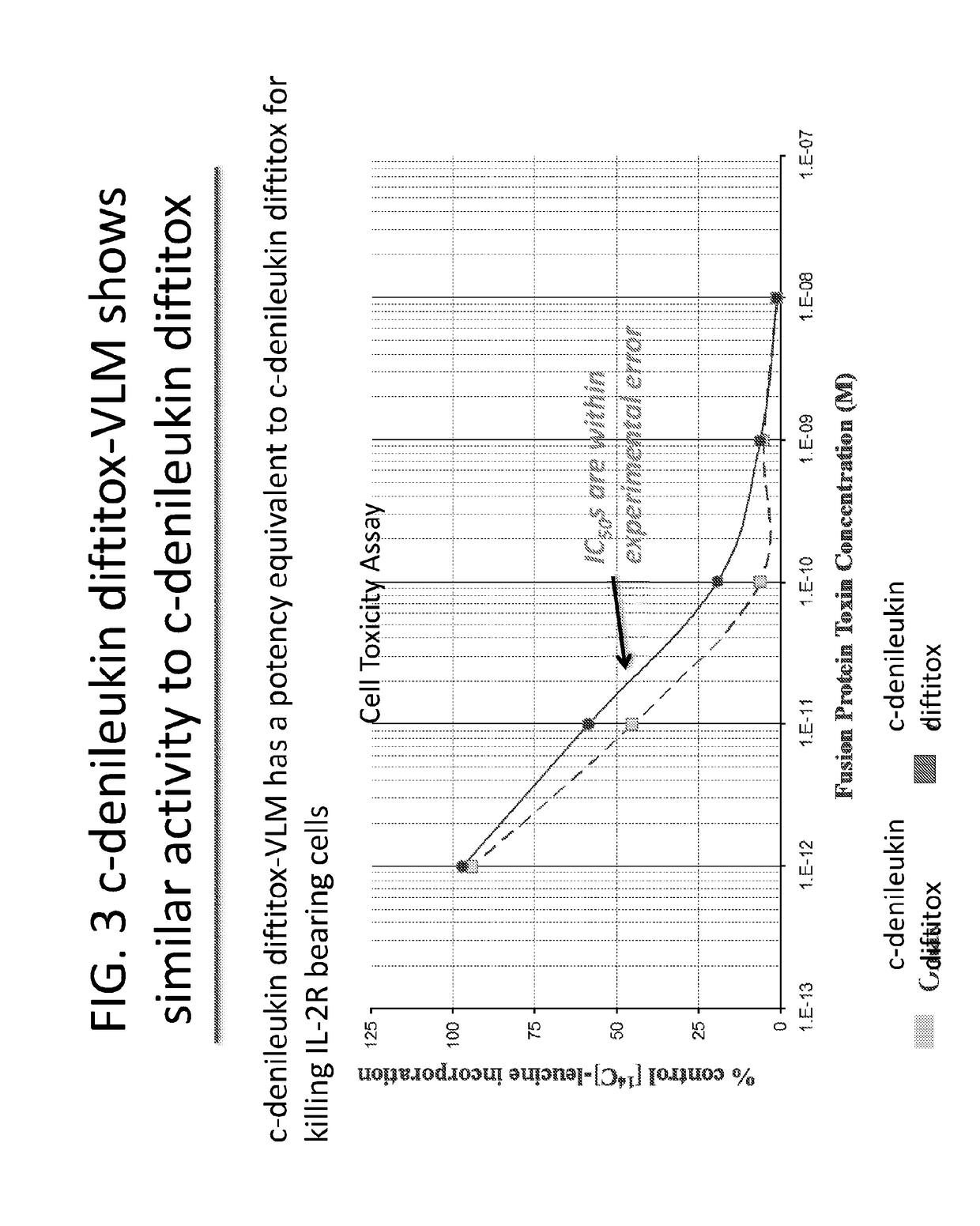
Methods of Producing Aggregate-Free Monomeric Diphtheria Toxin Fusion Proteins and Therapeutic Uses
ActiveUS20190071472A1Antibacterial agentsPeptide/protein ingredientsSequence signalRegulation of gene expression
The present invention is a DNA expression vector comprising: a toxP; a mutant toxO that blocks Fe-mediated regulation of gene expression; and a DNA sequence encoding a protein, wherein the toxP and the mutant toxO regulate expression of the DNA segment encoding the protein. It is preferred that DNA expression vectors of the present invention include DNA sequences encoding a signal peptide so that a protein expressed is attached to the signal peptide prior to processing. Novel proteins are produced off of the DNA expression vector of the present invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
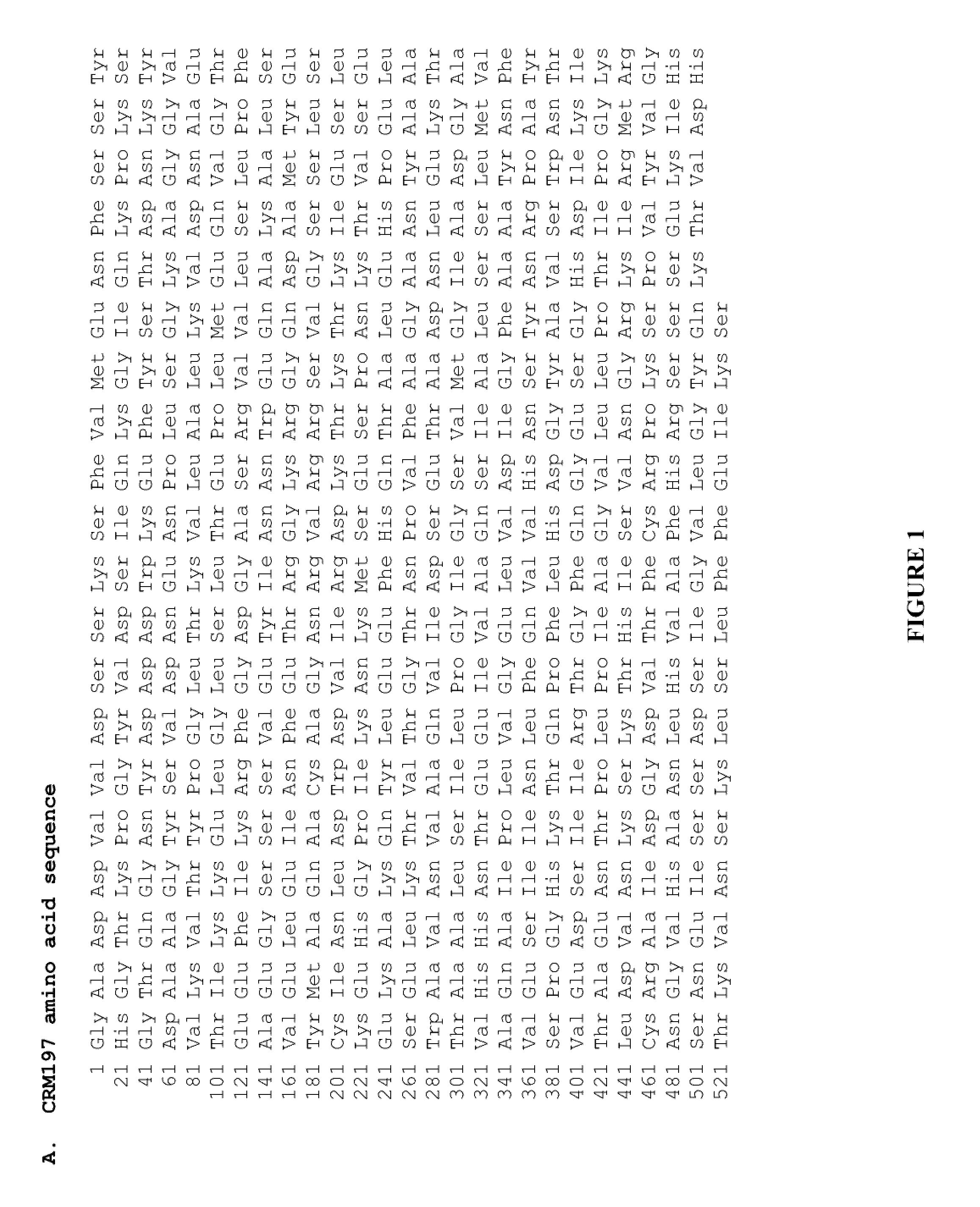
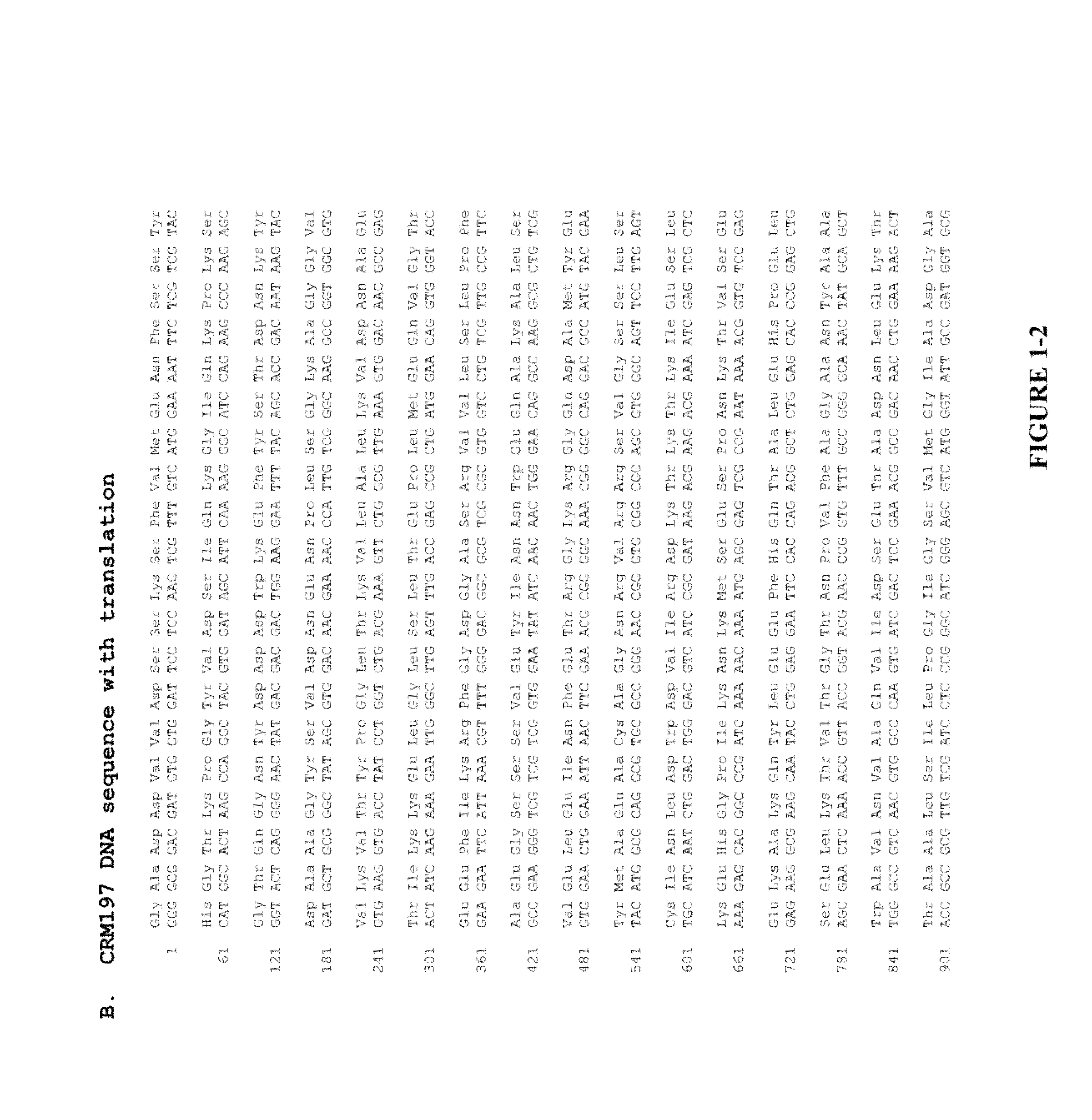
Methods and compositions relating to crm197
The present invention provides novel methods of producing diphtheria toxin. In particular, the present invention provides novel methods of producing nontoxic forms of diphtheria toxin, e.g., CRM197. The present invention also provides novel compositions comprising diphtheria toxin or nontoxic forms of diphtheria toxin, e.g., CRM197.
Owner:EMPA EIDGENOESSISCHE MATERIALPRFUNGS & FORSCHUNGSANSTALT +1
Recombined diphtheria toxin, preparation method, and application
InactiveCN101003568AAntigenicity cannot affectEasy to stretchPeptide/protein ingredientsBacteria peptidesDiseaseEscherichia coli
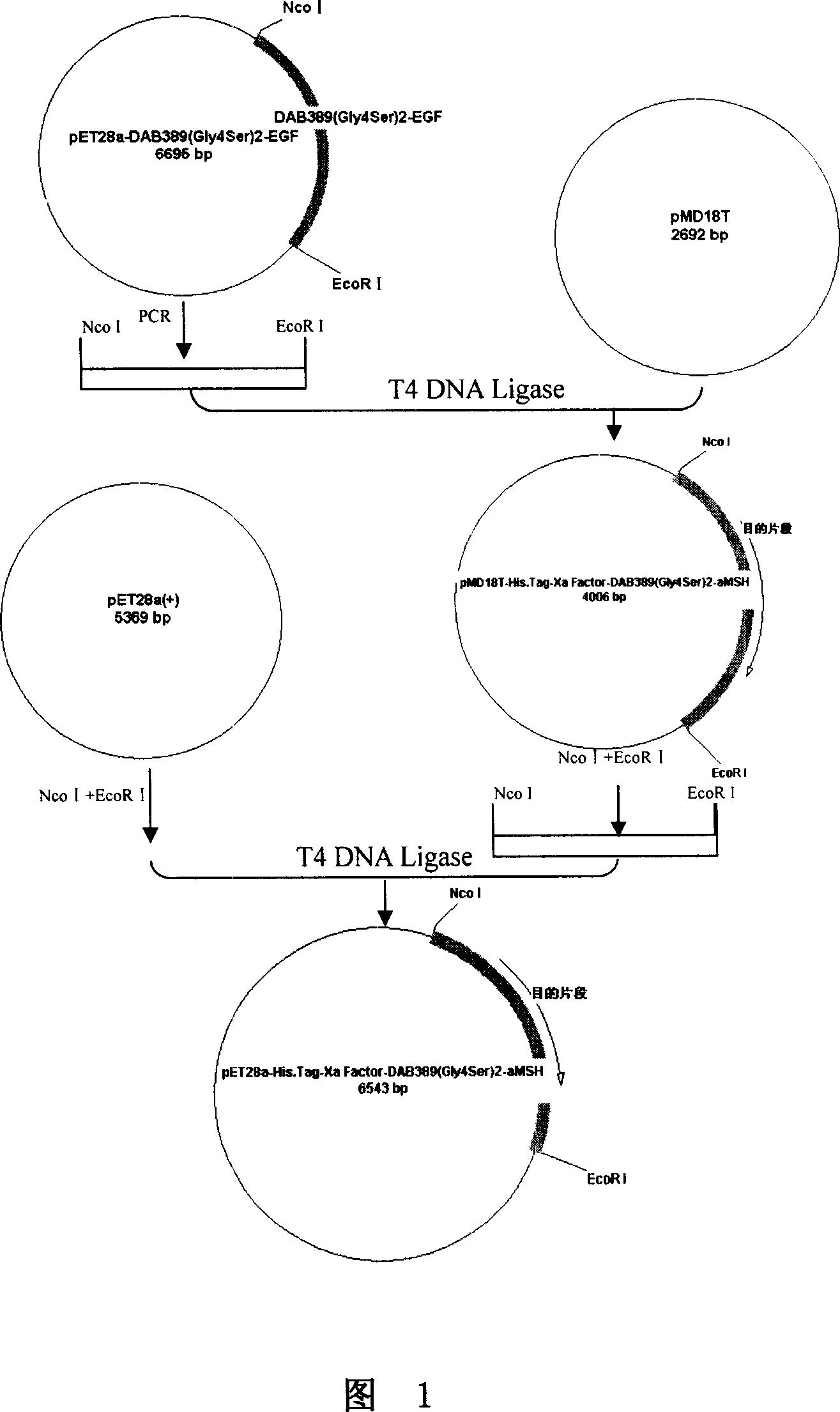
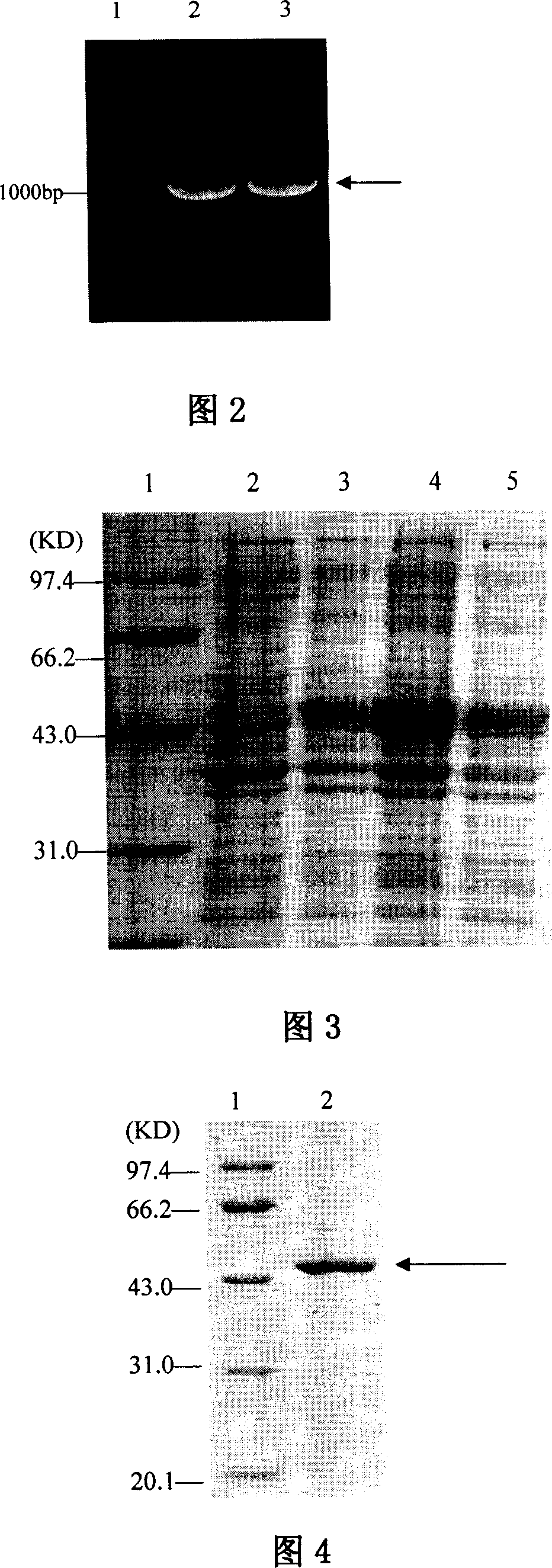
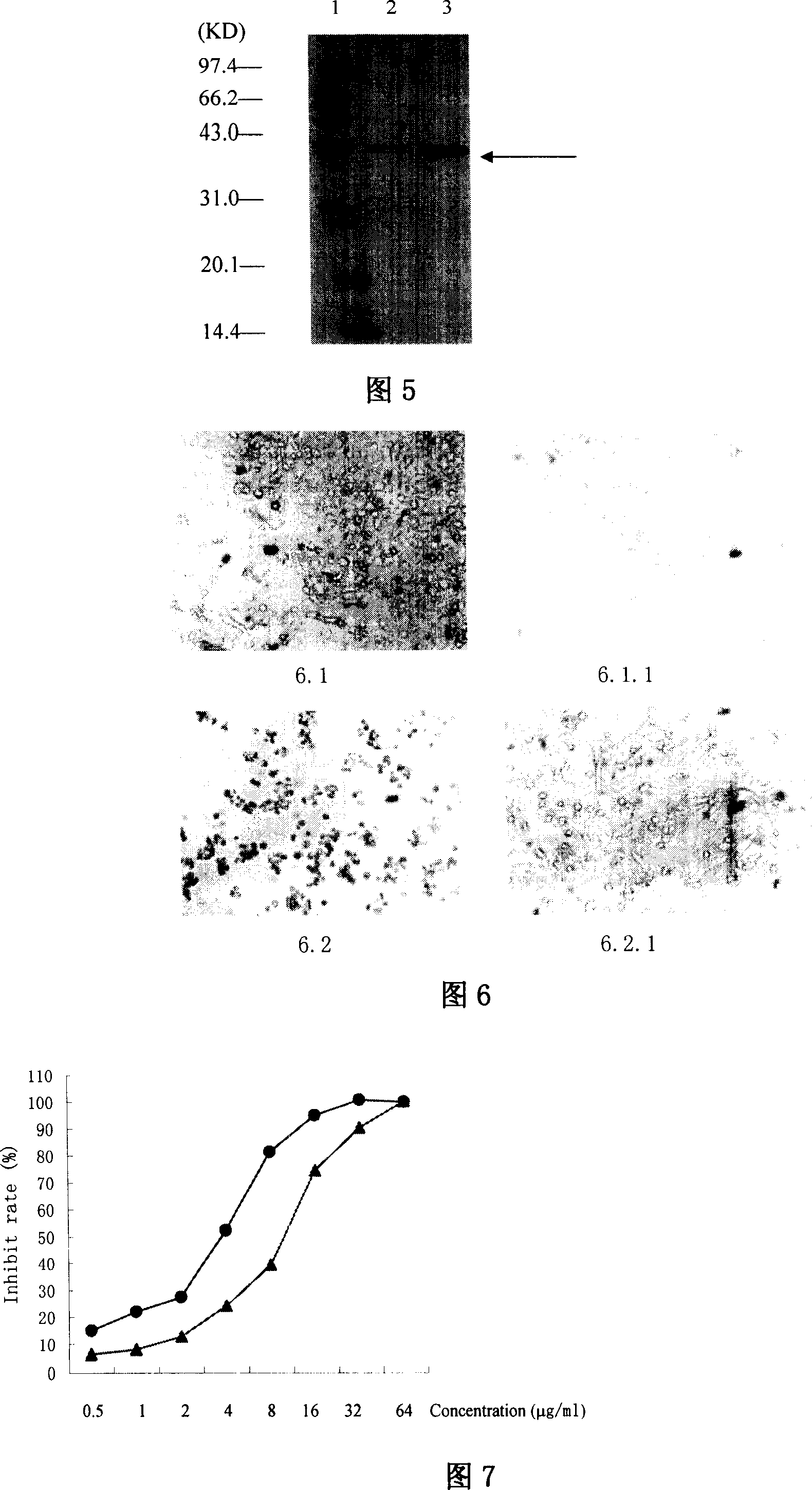
This invention discloses a method for preparing recombinant diphtherin and its application. The recombinant diphtherin comprises diphtherin DAB389, a linker peptide (Gly4Ser) 2, and alpha-melanocyte stimulating hormone. The method comprises: (1) preparing a gene fragment of DAB389(Gly4Ser)2-alpha-MSH and a leading peptide, constructing an expression plasmid containing the gene fragment, and transforming Escherichia coli; (2) inducing with IPTG to obtain the target protein, purifying, digesting with Xa factor, and separating to obtain DAB389(Gly4Ser)2-alpha-MSH fusion protein. The method has such advantages as easy purification, and high yield. The recombinant diphtherin has high activity, and can attack and kill malignant melanoma without injuring normal cells, thus can be used to treat malignant melanoma.
Owner:JILIN AGRICULTURAL UNIV
Production of diphtheria toxin
InactiveCN1894399AAntibacterial agentsBacterial antigen ingredientsNitrogen sourceDiphtheria vaccination
A Corynebacterium diphtheriae culture medium for the production of diphtheria toxin and methods for producing the toxin are provided. The medium is substantiallty free of animal-derived products and comprises water, a carbohydrate source, a nitrogen source and a number of free amino acids in an initial concentration wherein the initial concentration of each free amino acid is not limiting for the production of the toxin.
Owner:圣诺菲·帕斯图尔公司
Preparation method of group C meningococcal capsular polysaccharide conjugate vaccine
InactiveCN105879020AHarm reductionReduce hydrolytic denaturationAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineMeningococcal carriage
Owner:北京成大天和生物科技有限公司
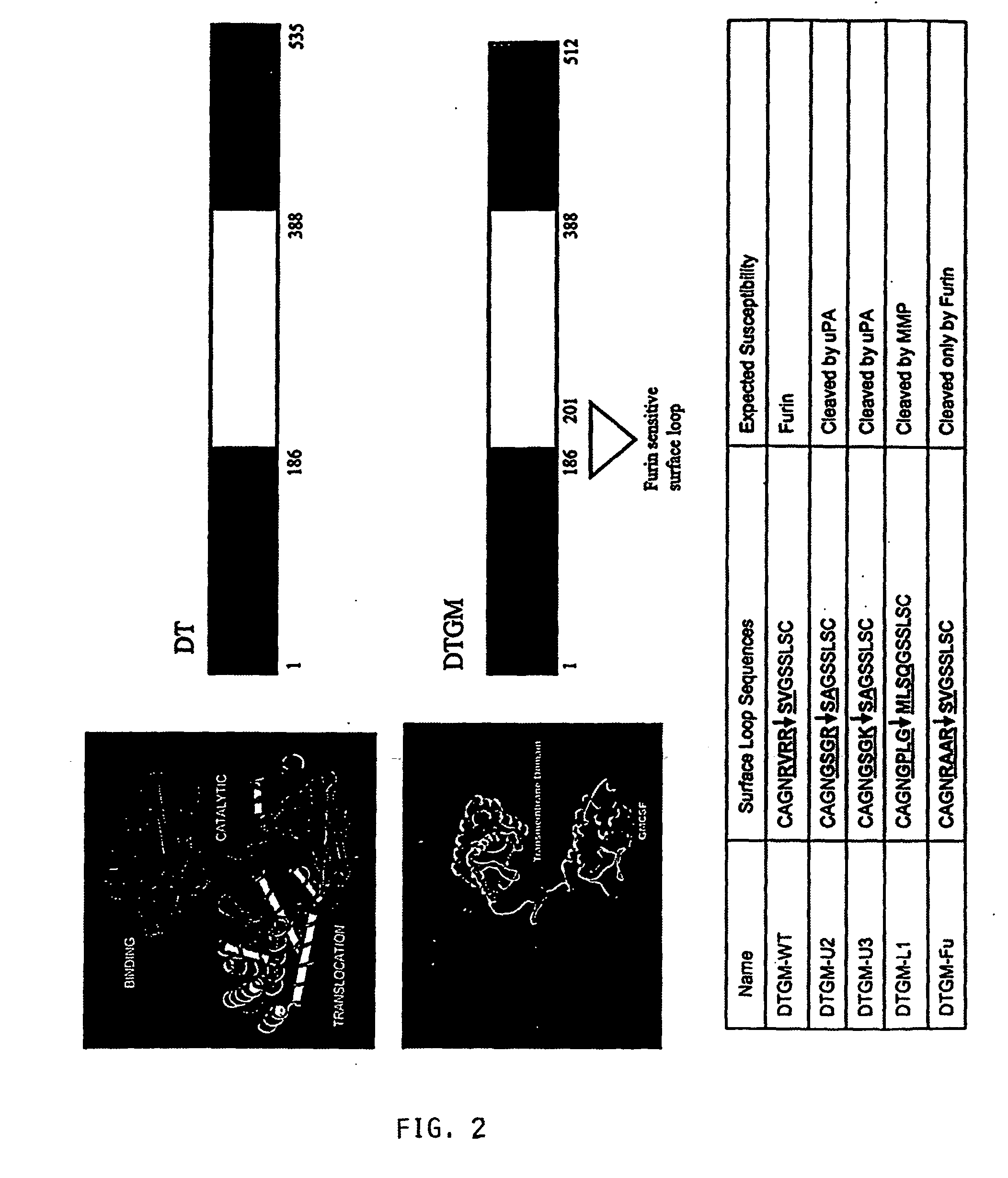
Activation of Recombinant Diphtheria Toxin Fusion Proteins by Specific Proteases Highly Expressed on the Surface of Tumor Cells
The present invention provides compositions and methods for inhibiting abnormal cell growth. In particular, the invention provides nucleic acids encoding Diphtheria toxin fusion proteins comprising residues 1-388 of Diphtheria toxin, wherein the native furin cleavage site has been substituted for a matrix metalloproteinase or plasminogen activator cleavage site, and a heterologous polypeptide and the polypeptides encoded by such nucleic acids. In addition, the invention provides methods of treating cancer by administering such polypeptides.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Production of diphtheria toxin
InactiveUS20110097359A1Control concentrationAntibacterial agentsBacterial antigen ingredientsNitrogen sourceSaxitoxin
A Corynebacterium diphtheriae culture medium for the production of diphtheria toxin and methods for producing the toxin are provided. The medium is substantially free of animal-derived products and comprises water, a carbohydrate source, a nitrogen source and a number of free amino acids in an initial concentration wherein the initial concentration of each free amino acid is not limiting for the production of the toxin.
Owner:LEE TIM
Methods and compositions based on diphtheria toxin-interleukin-3 conjugates
ActiveUS20090232767A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationPeptide/protein ingredientsMammal material medical ingredientsCancer cellWhite blood cell
The present invention provides methods for inhibiting interleukin-3 receptor-expressing cells, and, in particular, inhibiting the growth of such cells by using a diphtheria toxin-human interleukin-3 conjugate (DT-IL3) that is toxic to cells expressing the interleukin-3 receptor. In preferred embodiments, the DT-IL3 conjugate is a fusion protein comprising amino acids 1-388 of diphtheria toxin fused via a peptide linker to full-length, human interleukin-3. In certain embodiments, the methods of the present invention relate to the administration of a DT-IL3 conjugate to inhibit the growth of cancer cells and / or cancer stem cells in humans, which cells express one or more subunits of the interleukin-3 receptor. Exemplary cells include myeloid leukemia cancer stem cells. In other embodiments, the methods of the present invention relate to ex vivo purging of bone marrow or peripheral blood to remove cells that express one or more subunits of the interleukin-3 receptor such that the purged bone marrow or peripheral blood is suitable, e.g., for autologous stem cell transplantation to restore hematopoietic function.
Owner:SCOTT & WHITE MEMORIAL HOSPITAL
Human rotavirus VP8 recombinant protein and human rotavirus vaccine using same
The invention provides a human rotavirus VP8 recombinant protein. The amino acid sequences of the VP8 recombinant protein include an amino acid sequence of a human rotavirus VP8 protein (hereinafter referred to as VP8 protein) and an amino acid sequence of exogenous polypeptide. The isoelectric point of the exogenous polypeptide is less than or equal to 5. The invention also provides a human rotavirus vaccine prepared based on the VP8 recombinant protein. Compared with the prior art, the VP8 recombinant protein provided by the invention can be expressed by fusing the VP8 protein with negativecharge-containing amino acid polypeptide fragments in tetanus toxin and / or diphtheria toxin proteins, and the water solubility and yield of the rotavirus VP8 protein expressed by escherichia coli areimproved; and moreover, the obtained fused protein has good immune ability, and the prepared vaccine has good effect.
Owner:CHENGDU MAXVAX BIOTECHNOLOGY LLC
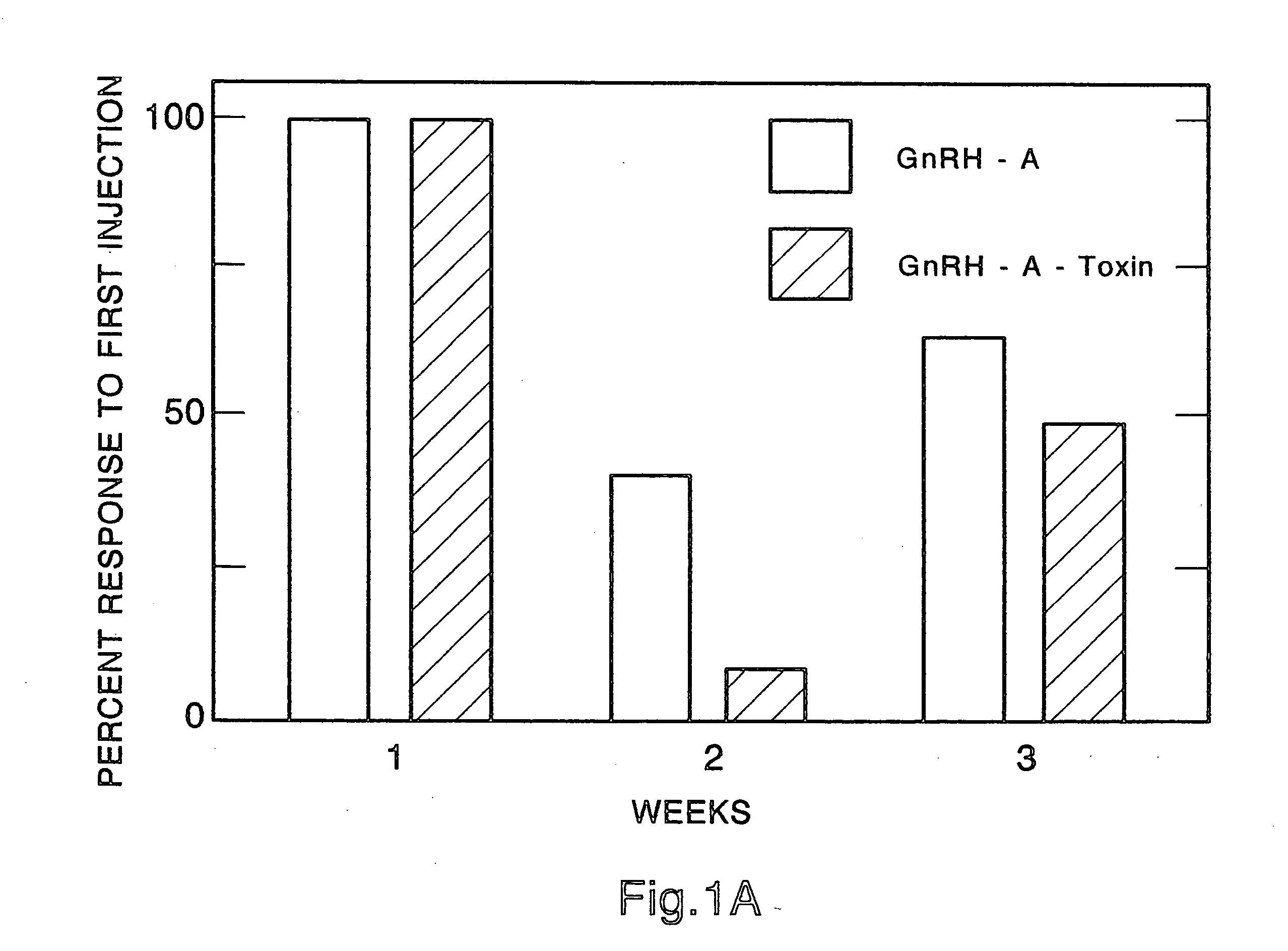
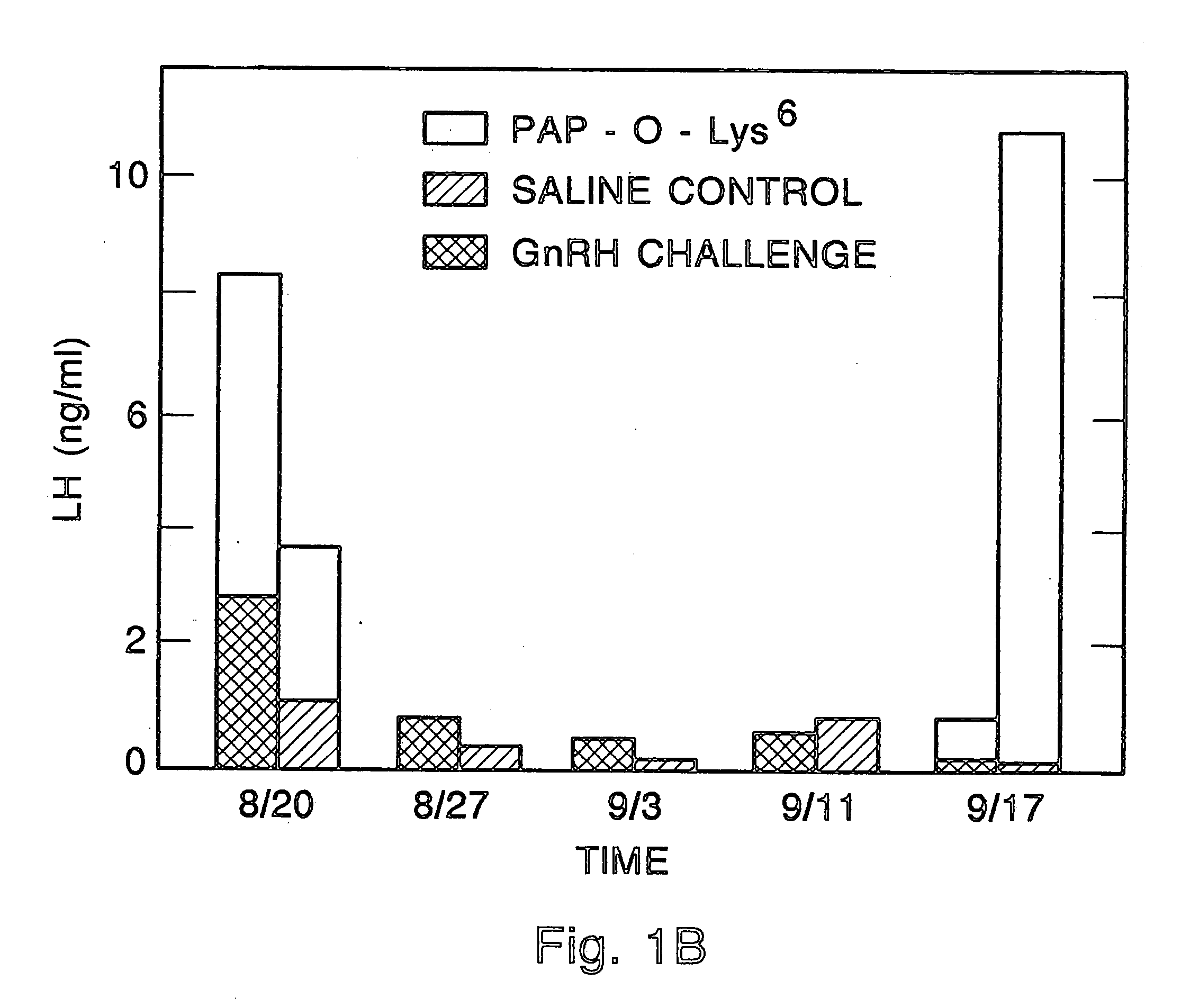
Method for inactivating gonadotrophs
InactiveUS20050277582A1Easy to transportImprove solubilityPeptide/protein ingredientsDepsipeptidesGeloninPseudomonas aeruginosa exotoxin A
Certain toxic compounds (T) such as, for example, compounds based upon diphtheria toxin, ricin toxin, pseudomonas exotoxin, .alpha.-amanitin, pokeweed antiviral protein (PAP), ribosome inhibiting proteins, especially the ribosome inhibiting proteins of barley, wheat, corn, rye, gelonin and abrin, as well as certain cytotoxic chemicals such as, for example, melphalan and daunomycin can be conjugated to certain analogs of gonadotropin-releasing hormone to form a class of compounds which, when injected into an animal, destroy the gonadotrophs of the animal's anterior pituitary gland. Hence such compounds may be used to sterilize such animals and / or to treat certain sex hormone related diseases.
Owner:NETT TORRANCE +3
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com