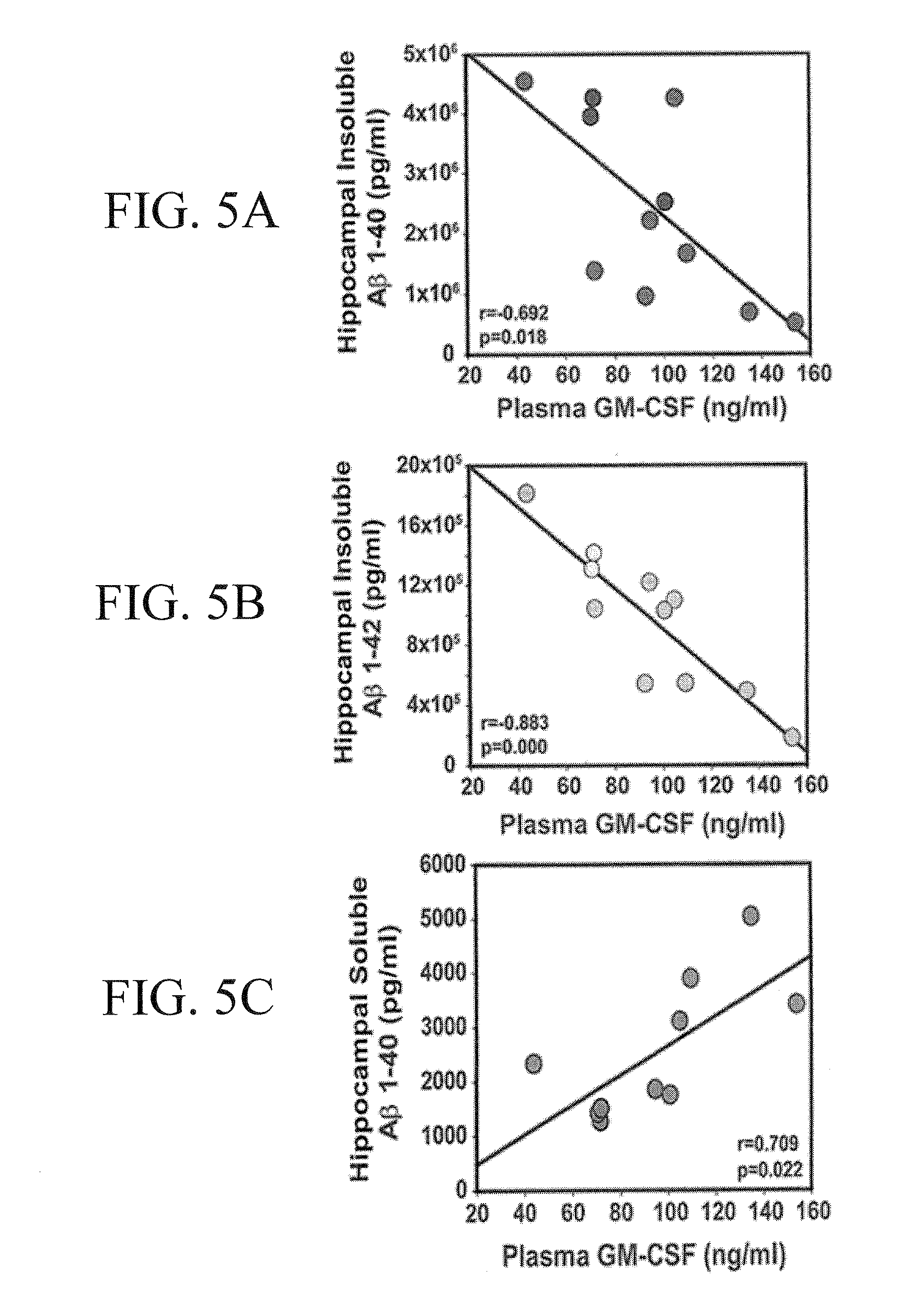
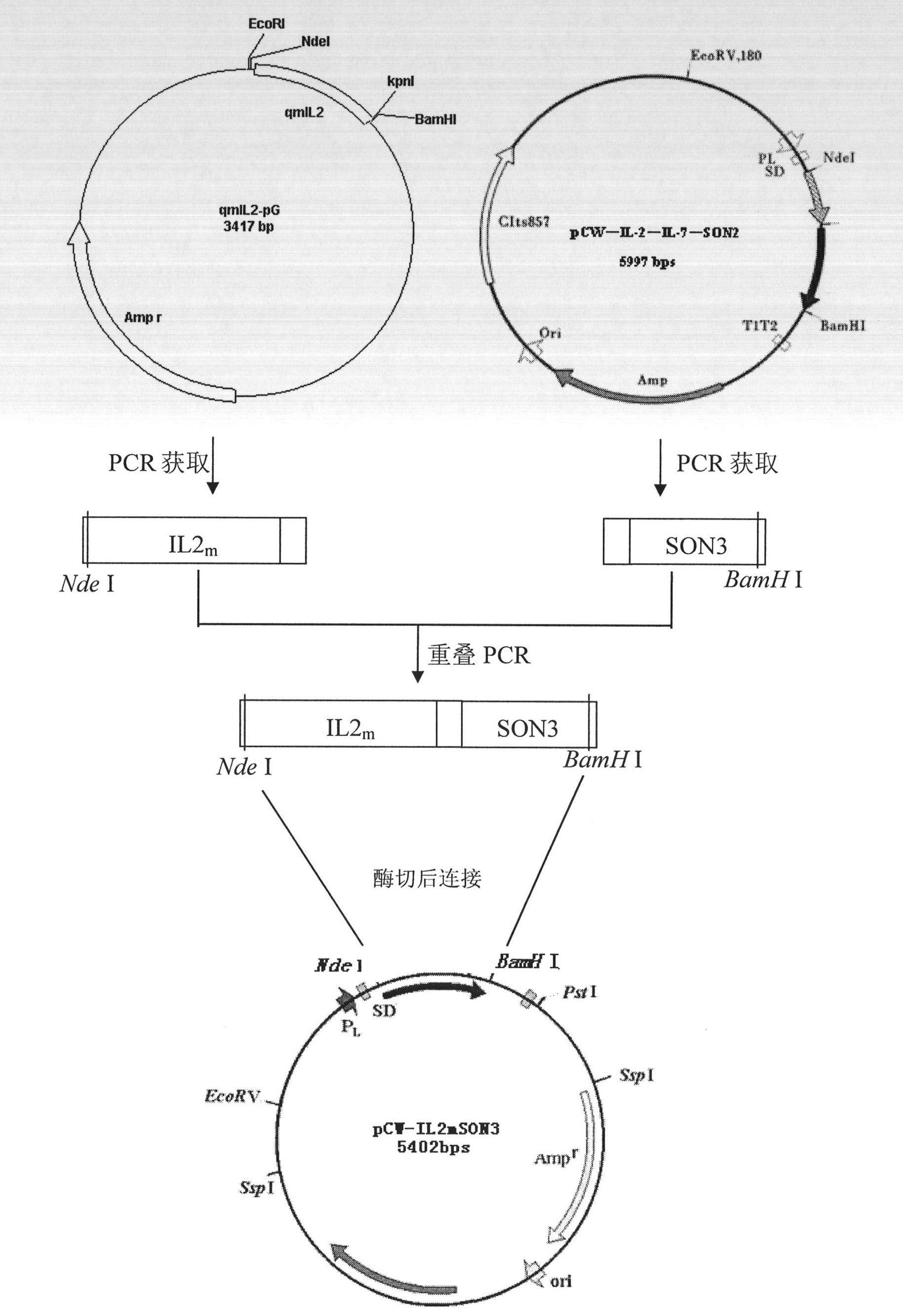
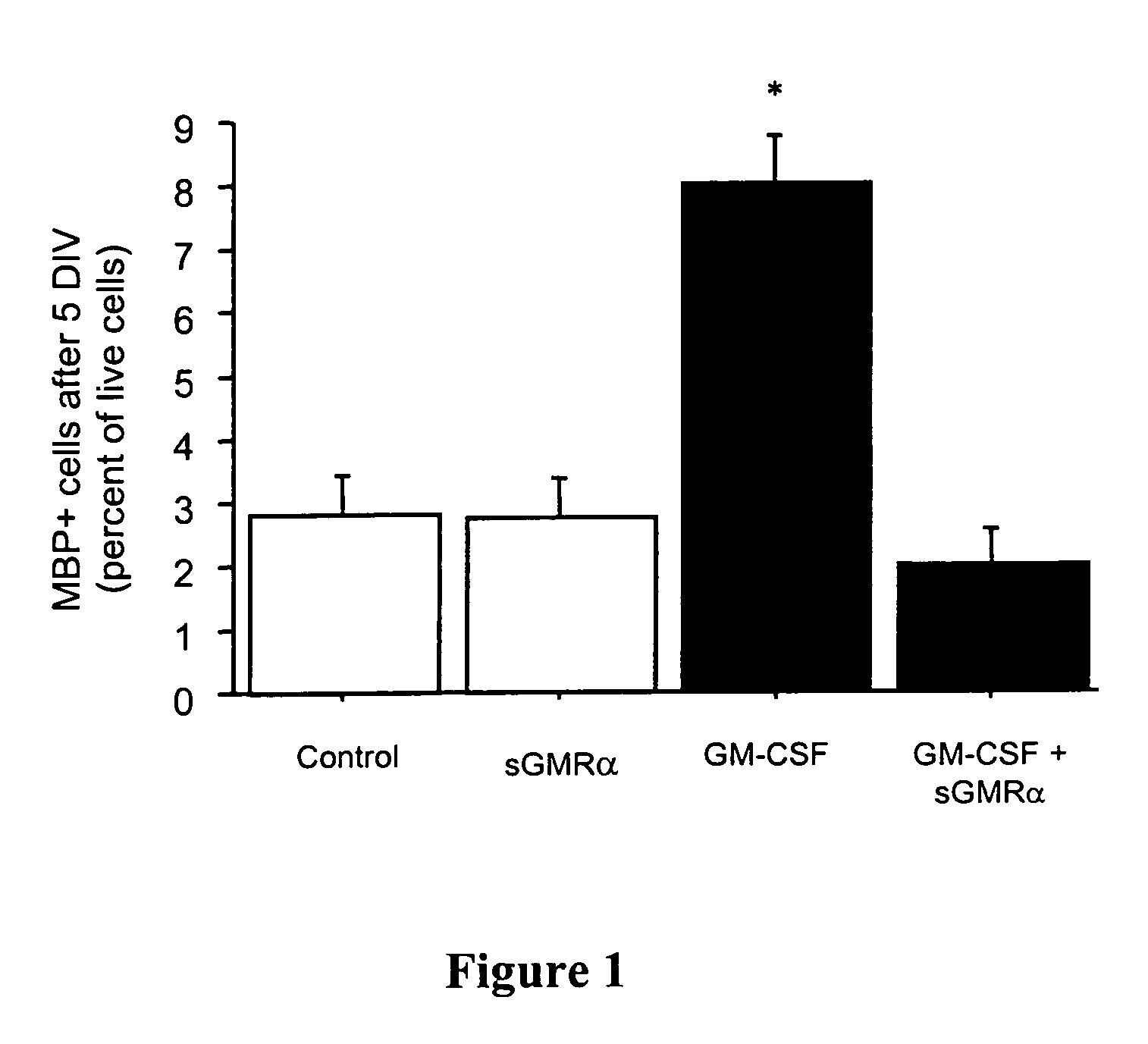
Patents
Literature
48 results about "Interleukin 3" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
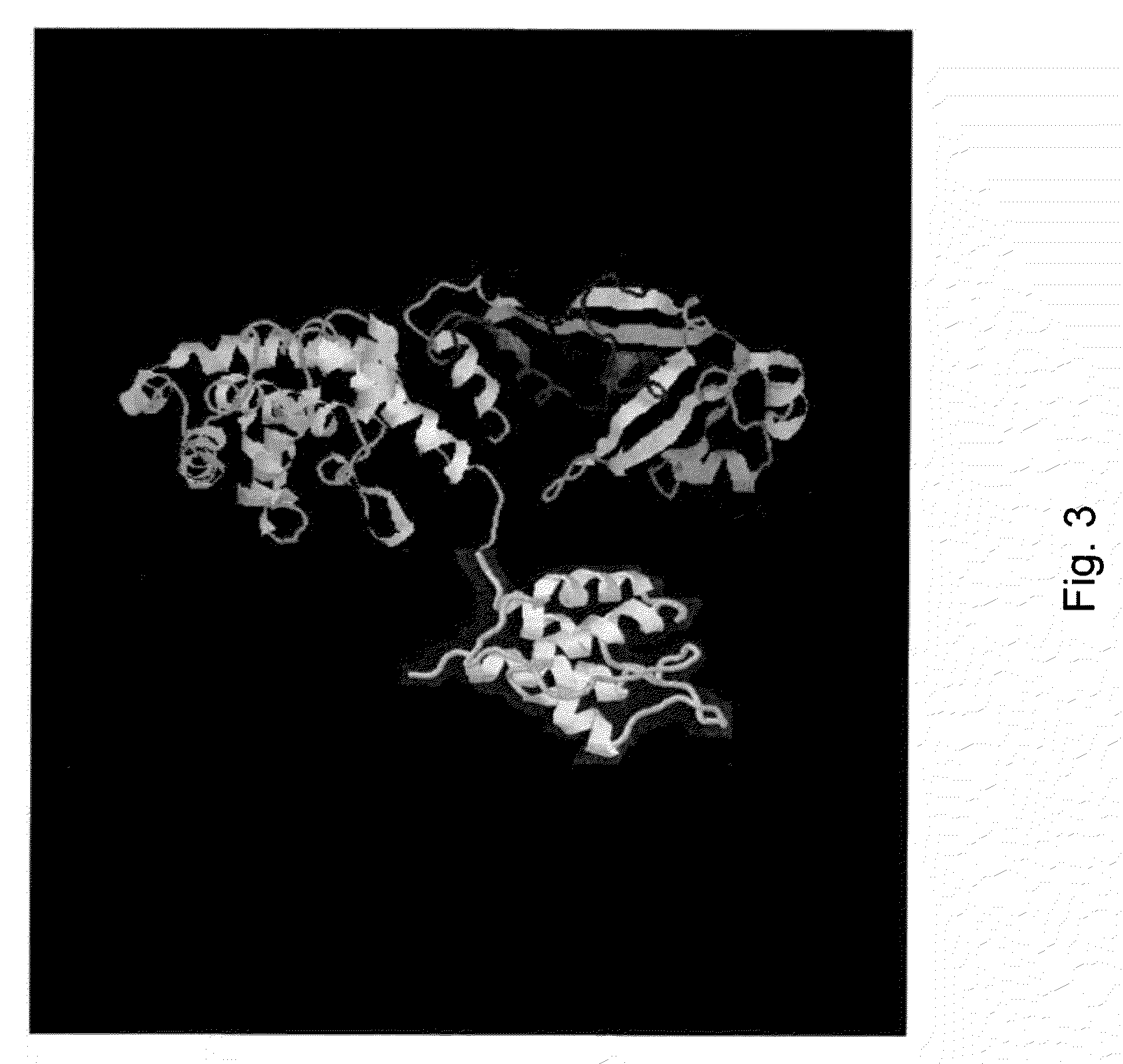
Interleukin 3 (IL-3) is a protein that in humans is encoded by the IL3 gene localised on chromosome 5q31.1. Synonyms: colony-stimulating factor; mast cell growth factor, MULTI-CSF, MCGF; MGC79398, MGC79399.
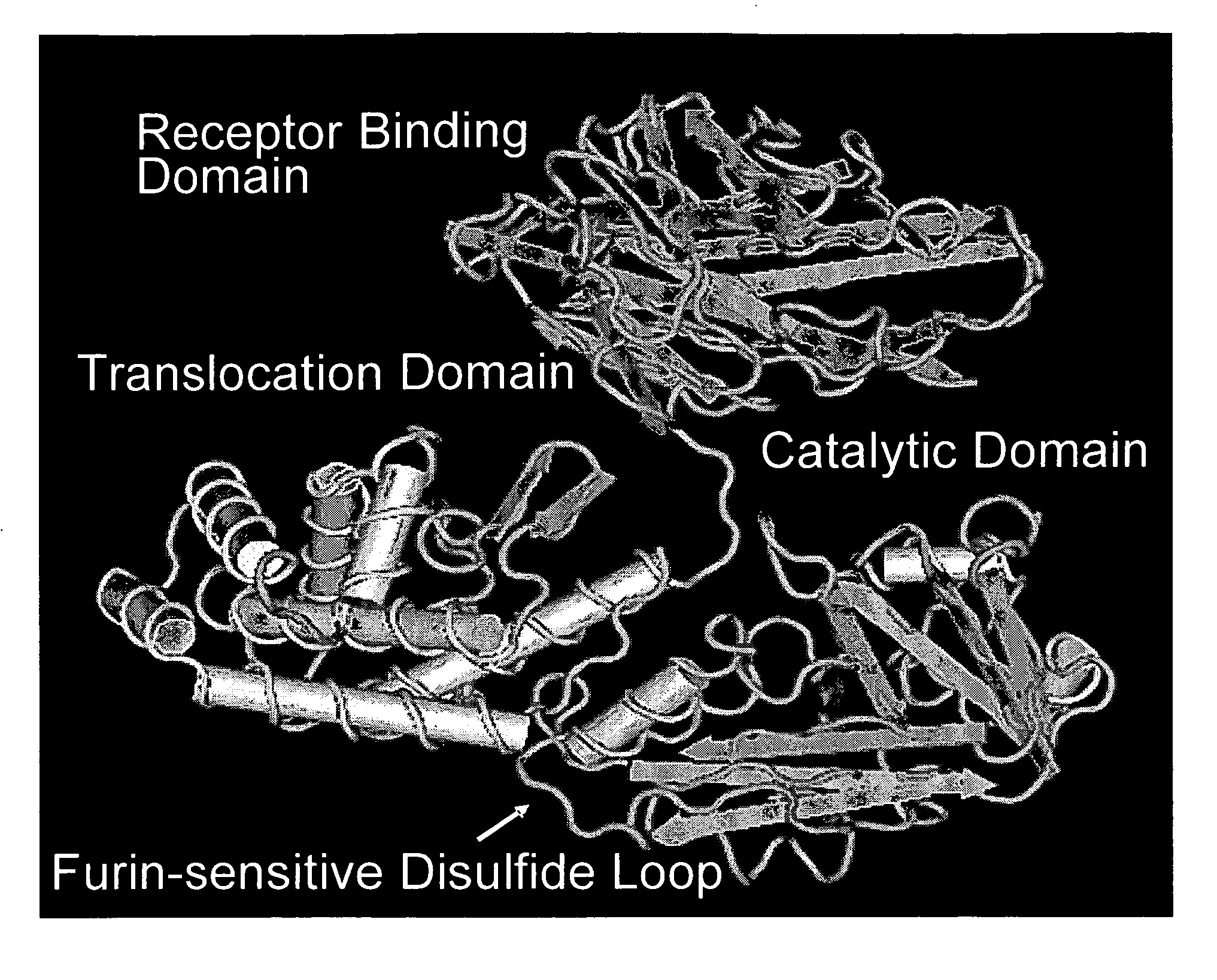
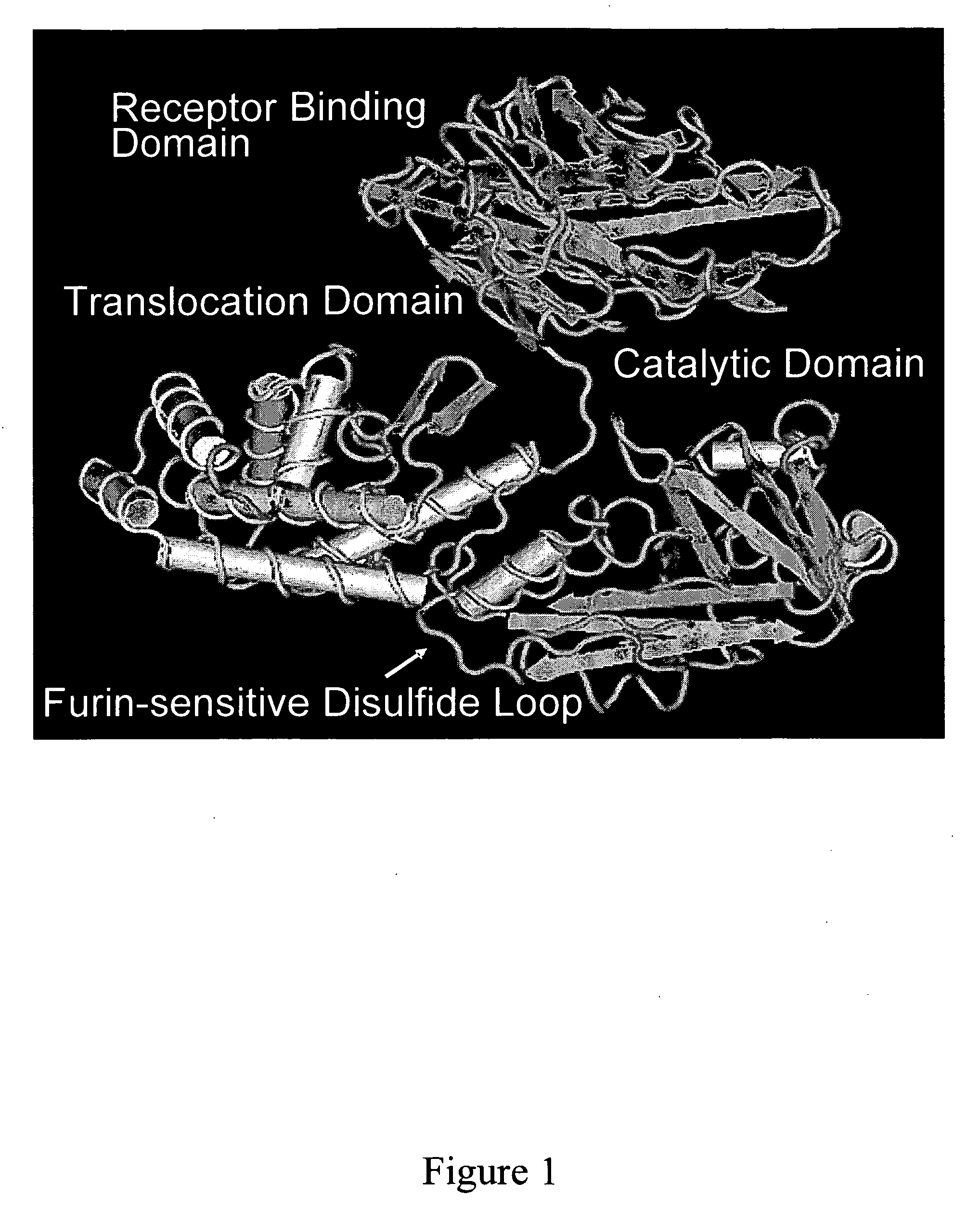
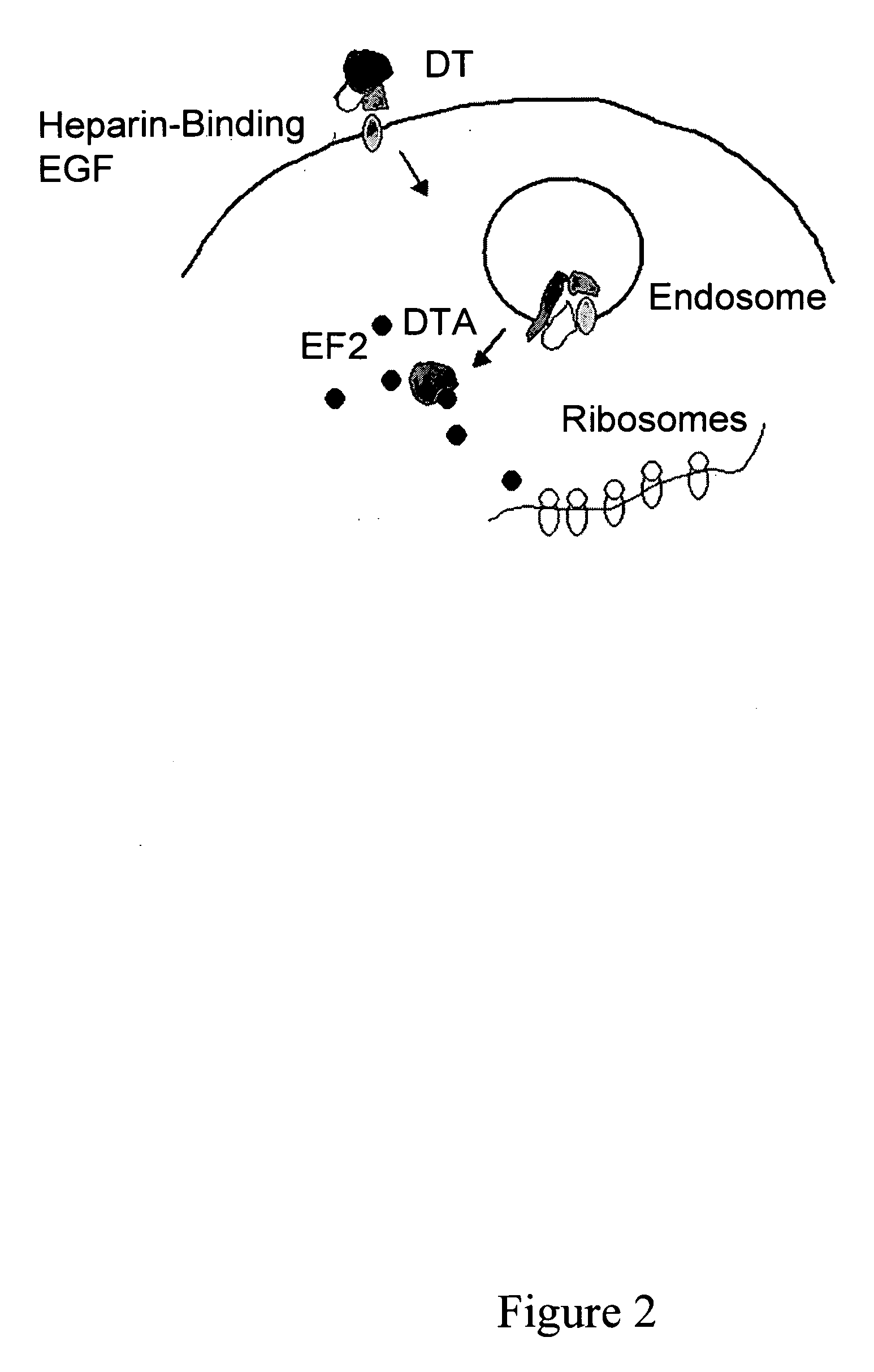
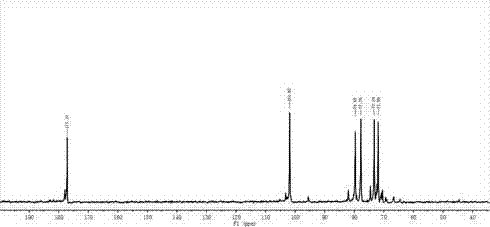
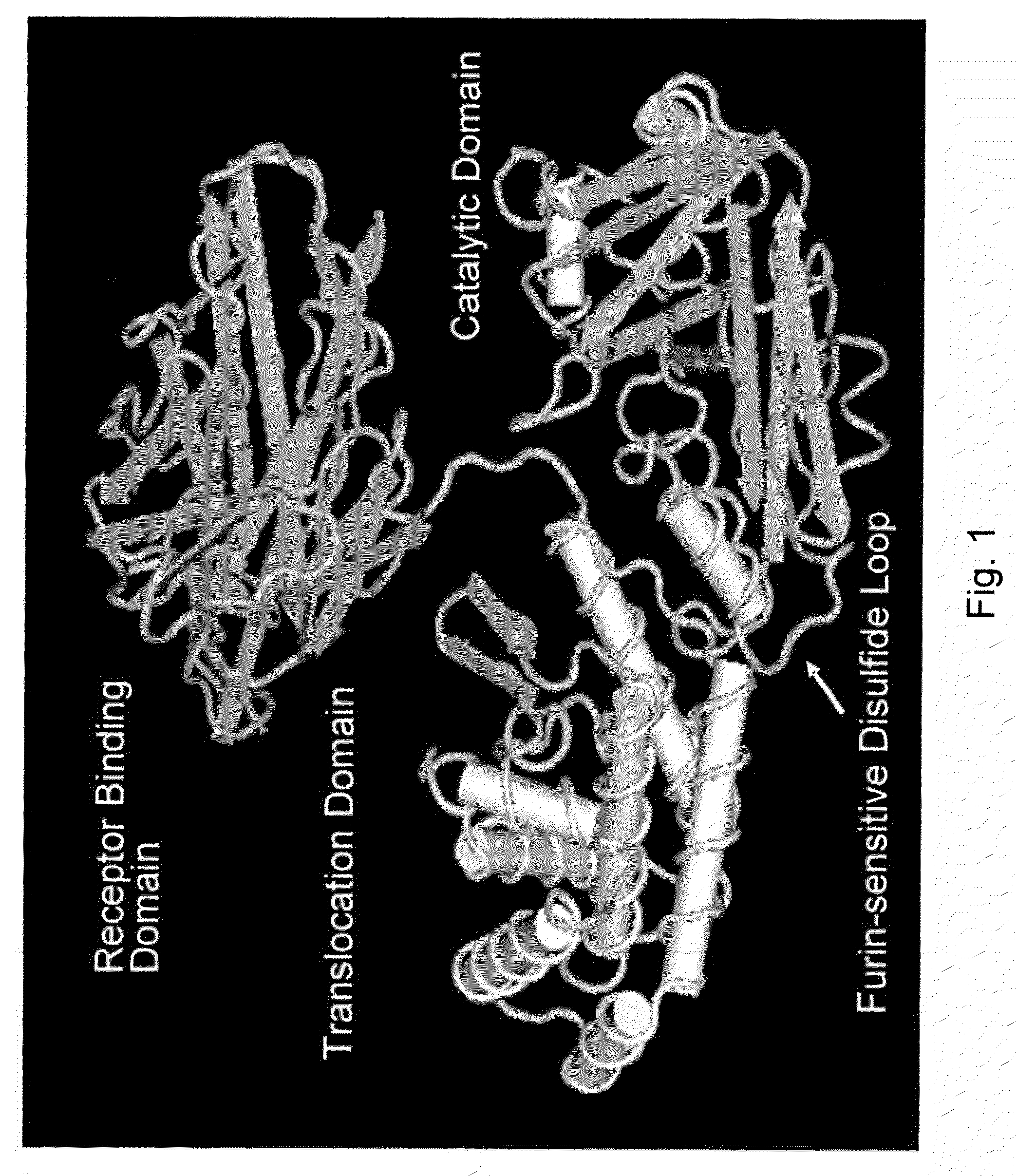
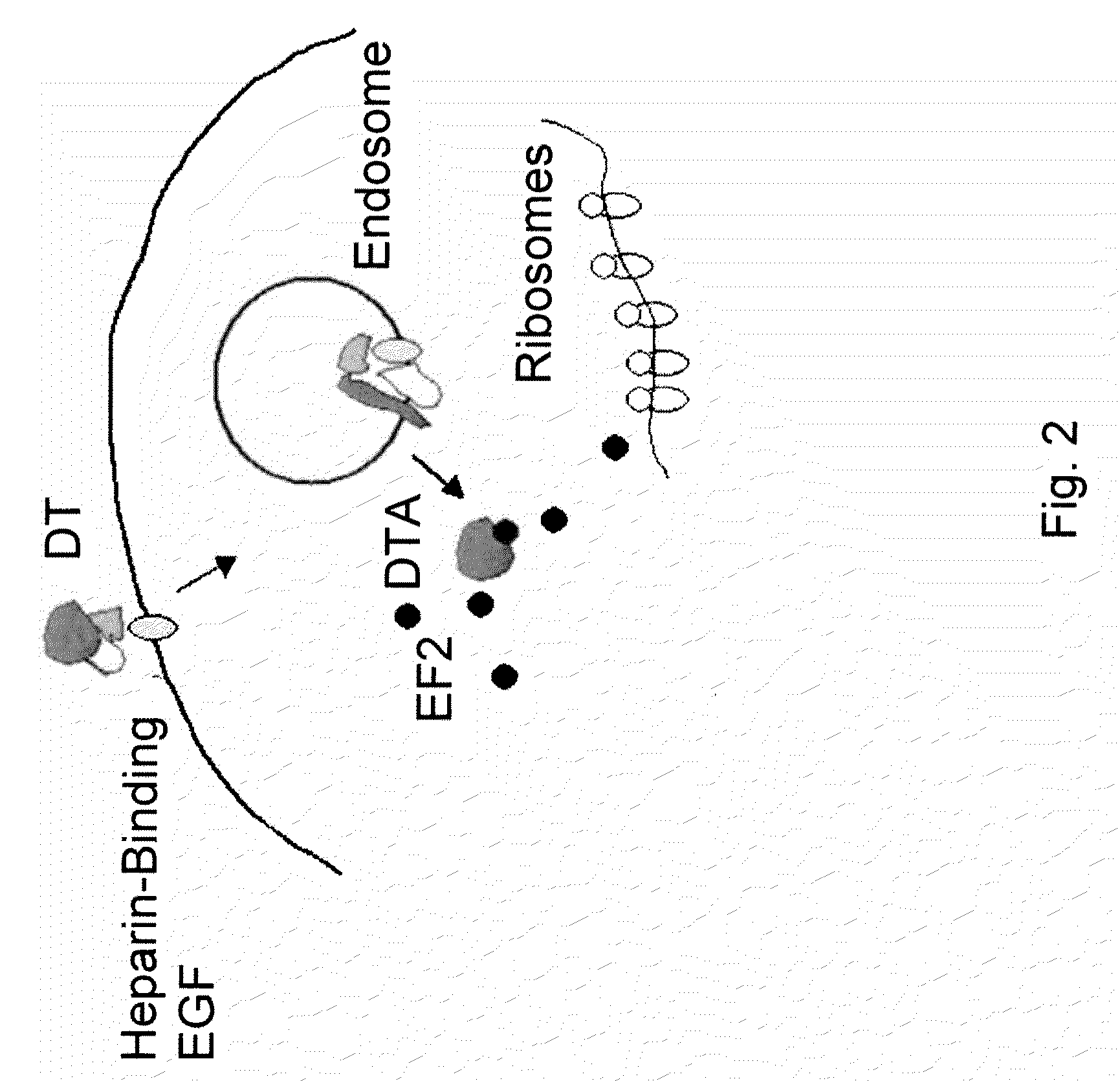
Methods and compositions based on diphtheria toxin-interleukin-3 conjugates
ActiveUS20080138313A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationBiocidePeptide/protein ingredientsCancer cellWhite blood cell
The present invention provides methods for inhibiting interleukin-3 receptor-expressing cells, and, in particular, inhibiting the growth of such cells by using a diphtheria toxin-human interleukin-3 conjugate (DT-IL3) that is toxic to cells expressing the interleukin-3 receptor. In preferred embodiments, the DT-IL3 conjugate is a fusion protein comprising amino acids 1-388 of diphtheria toxin fused via a peptide linker to full-length, human interleukin-3. In certain embodiments, the methods of the present invention relate to the administration of a DT-IL3 conjugate to inhibit the growth of cancer cells and / or cancer stem cells in humans, which cells express one or more subunits of the interleukin-3 receptor. Exemplary cells include myeloid leukemia cancer stem cells. In other embodiments, the methods of the present invention relate to ex vivo purging of bone marrow or peripheral blood to remove cells that express one or more subunits of the interleukin-3 receptor such that the purged bone marrow or peripheral blood is suitable, e.g., for autologous stem cell transplantation to restore hematopoietic function.
Owner:SCOTT & WHITE MEMORIAL HOSPITAL
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveUS20070092967A1Increase success rateArtificial cell constructsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an αMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the αMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:HAN HOON
Method of prodcing megacaryocyle using umbilical blood CD 344+cell in vitro induction method
InactiveCN1556197AIncrease multipleImprove production efficiencyTissue cultureHuman plateletWhite blood cell
A process for generating megacaryocyte by in vitro induction to umbilical blood cell CD34+ includes in vitro amplifying the CD34+ in the culture medium containing fetal calf serum, human SCF, human TPO and / or ligand flt-3 and inducing the generation of megacaryocytes in the non-serum culture medium containing human TPO, human interleukin 3(IL-3) and / or human GM-CSF. Its advantage is high inducing efficiency.
Owner:上海伯瑞生物技术发展有限公司 +1
CD123+ dendritic cells in blood and lymphoid tissues
InactiveUS6294381B1Artificial cell constructsImmunoglobulins against cell receptors/antigens/surface-determinantsCell lineageDendritic cell
Dendritic cells (DCs) are the primary antigen presenting cells during the initiation of T cell-dependent immune responses. The cells originate from the bone marrow and have been suggested to represent a distinct cell lineage. However, distinct DC precursors have not been identified in bone marrow, and mature monocytes can also give rise to DCs. The instant invention presents a distinct DC precursor among bone marrow CD34+ cells. The cells express high levels of the interleukin-3 receptor alpha chain and CD4 and can be uniquely identified also in blood and lymphoid tissues by this phenotype.
Owner:OLWEUS JOHANNA +1
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS8778615B2Easy to adaptMicrobiological testing/measurementAntibody ingredientsInterleukin 10Soluble P-Selectin
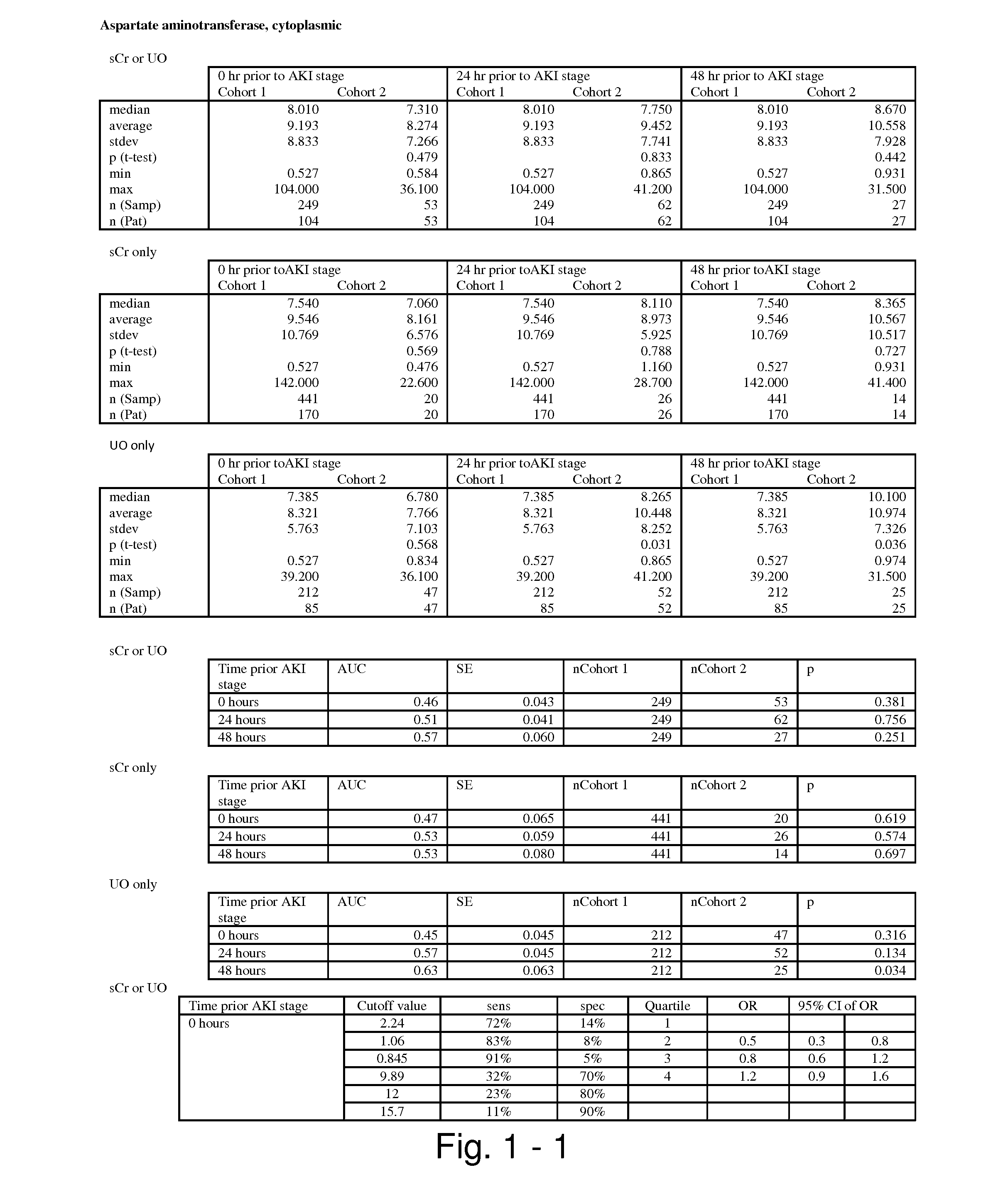
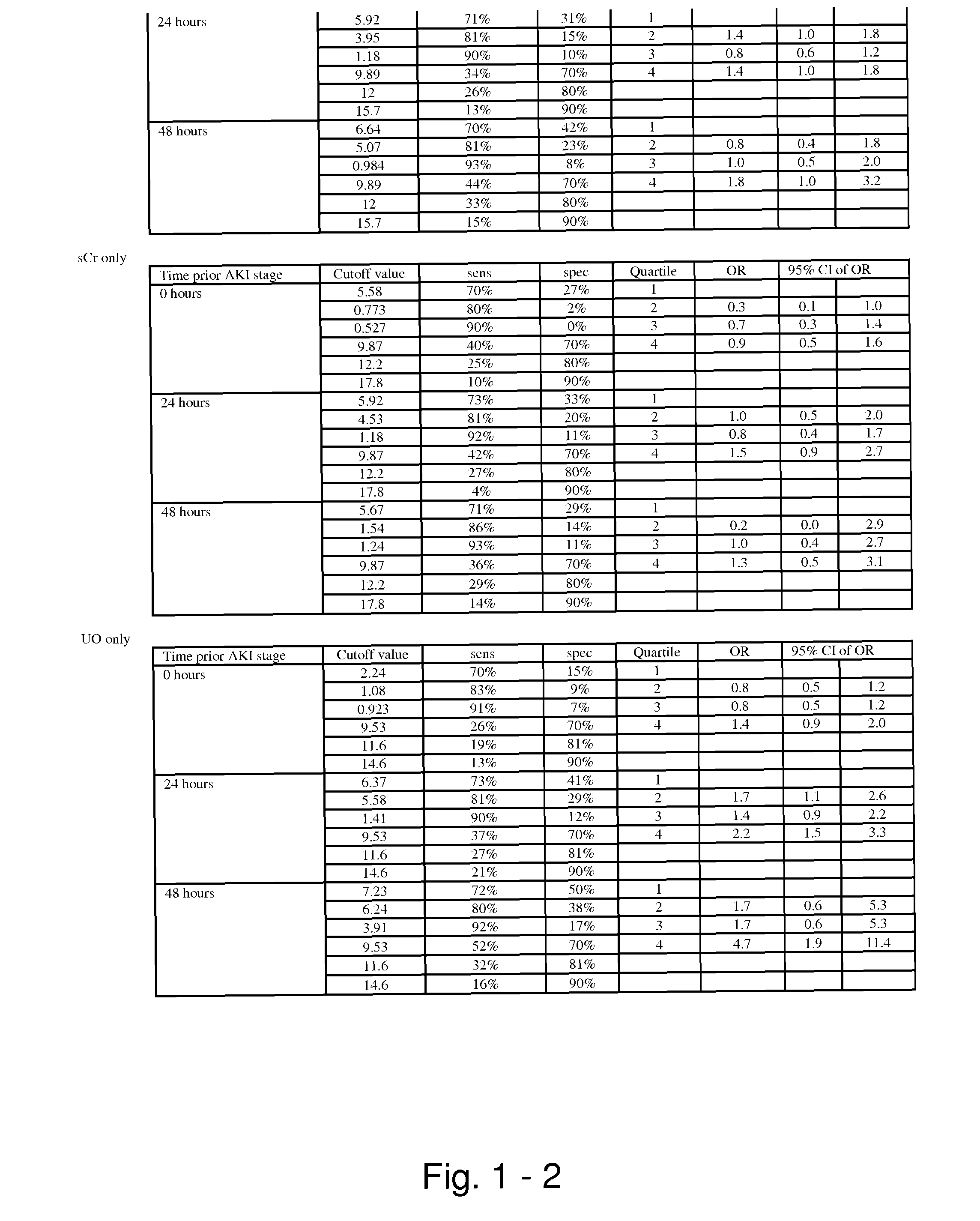
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
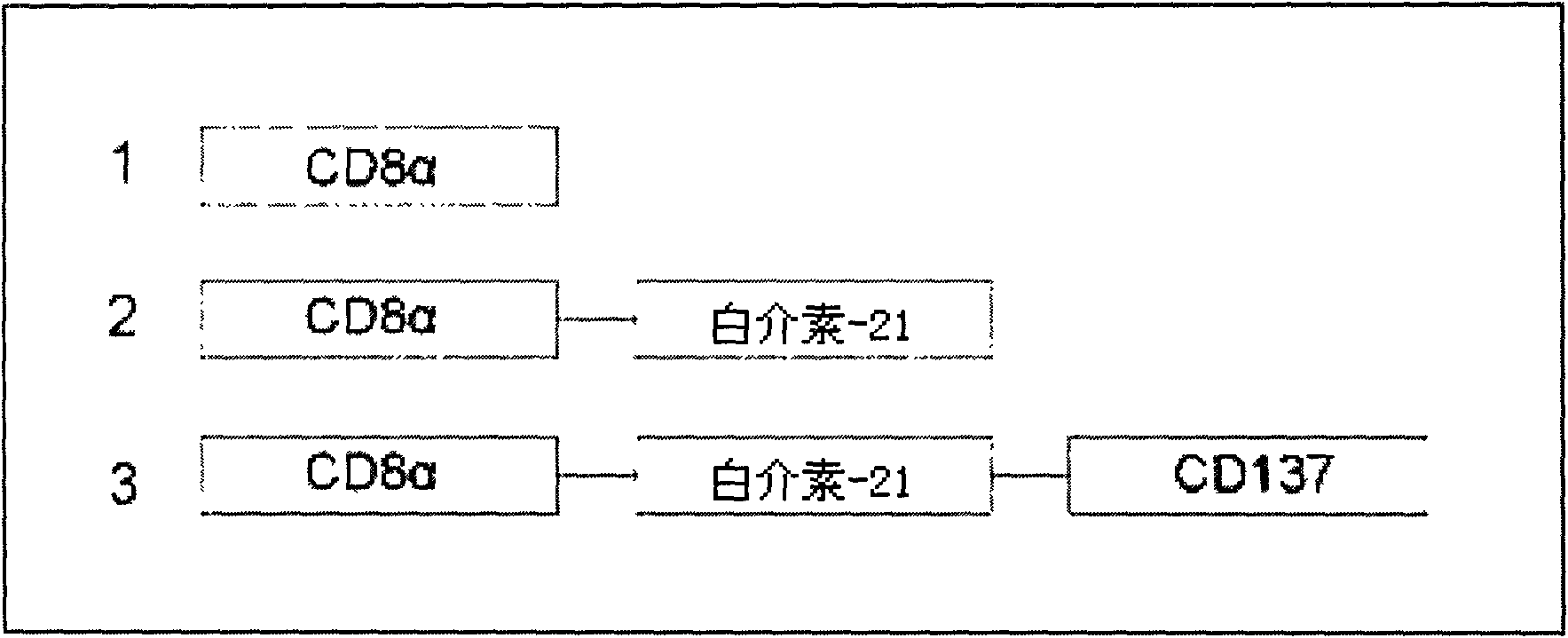
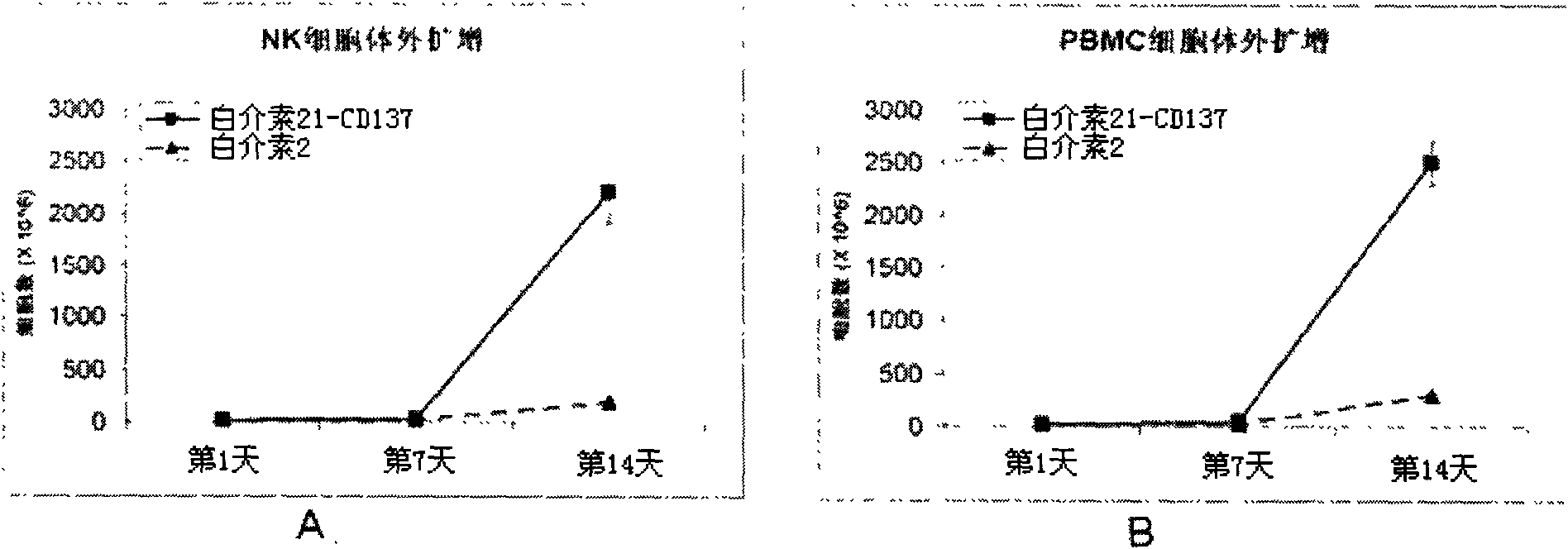
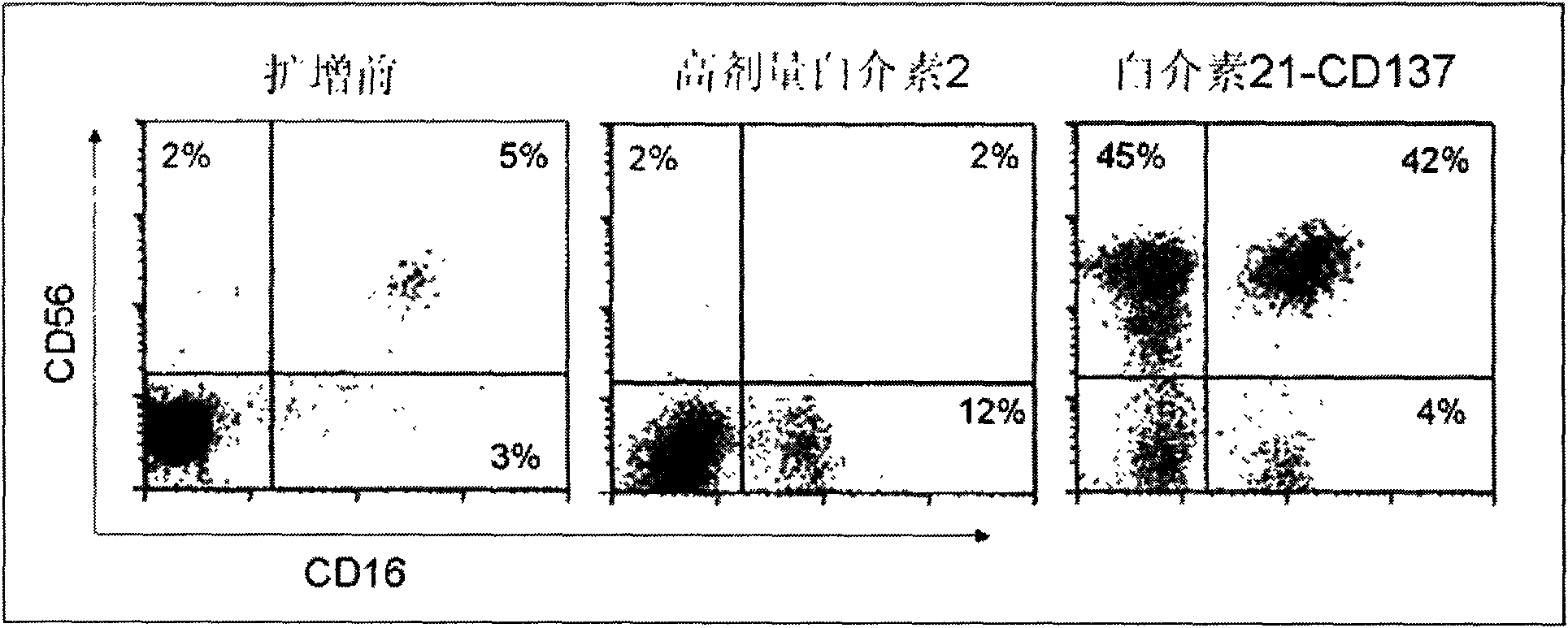
Method for amplifying and activating lymphocyte by using CD8 alpha-interleukin 21-CD137 compound
The invention relates to the field of immunology, in particular to a method for amplifying and activating a natural killer (NK) cell into a lymphokine-activated killer (LAK) cell by using a CD8 alpha-interleukin 21-CD137 compound. The method disclosed by the invention comprises the following steps of: forming the CD8 alpha-interleukin 21-CD137 compound by using CD8 alpha, interleukin 21 and a CD137 functional polypeptide, making an exogenous expression vector enter a host K562 cell, then activating a promoter and culturing a cell to obtain a cell for expressing a transmembrane interleukin 21-CD137 compound; and purifying the compound in the conventional way, and amplifying and activating a lymphocyte by using the purified compound to generate the LAK cell. The method has the advantage that: the LAK cell cultured and amplified by using the transmembrane interleukin 21-CD137 compound and a small dose of interleukin 2 is used for enhancing the immunity of a patient to help the patient resist tumors, viruses and bacteria. The method has a wide clinical using prospect.
Owner:杭州中赢生物医疗科技有限公司
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Method and kit for the measurement of the activation of basophils induced by allergen to determine hypersensitivity to some substances
InactiveUS20040265925A1Lower IgE levelsOvercome disadvantagesBiological material analysisAllergen ingredientsStainingCD63
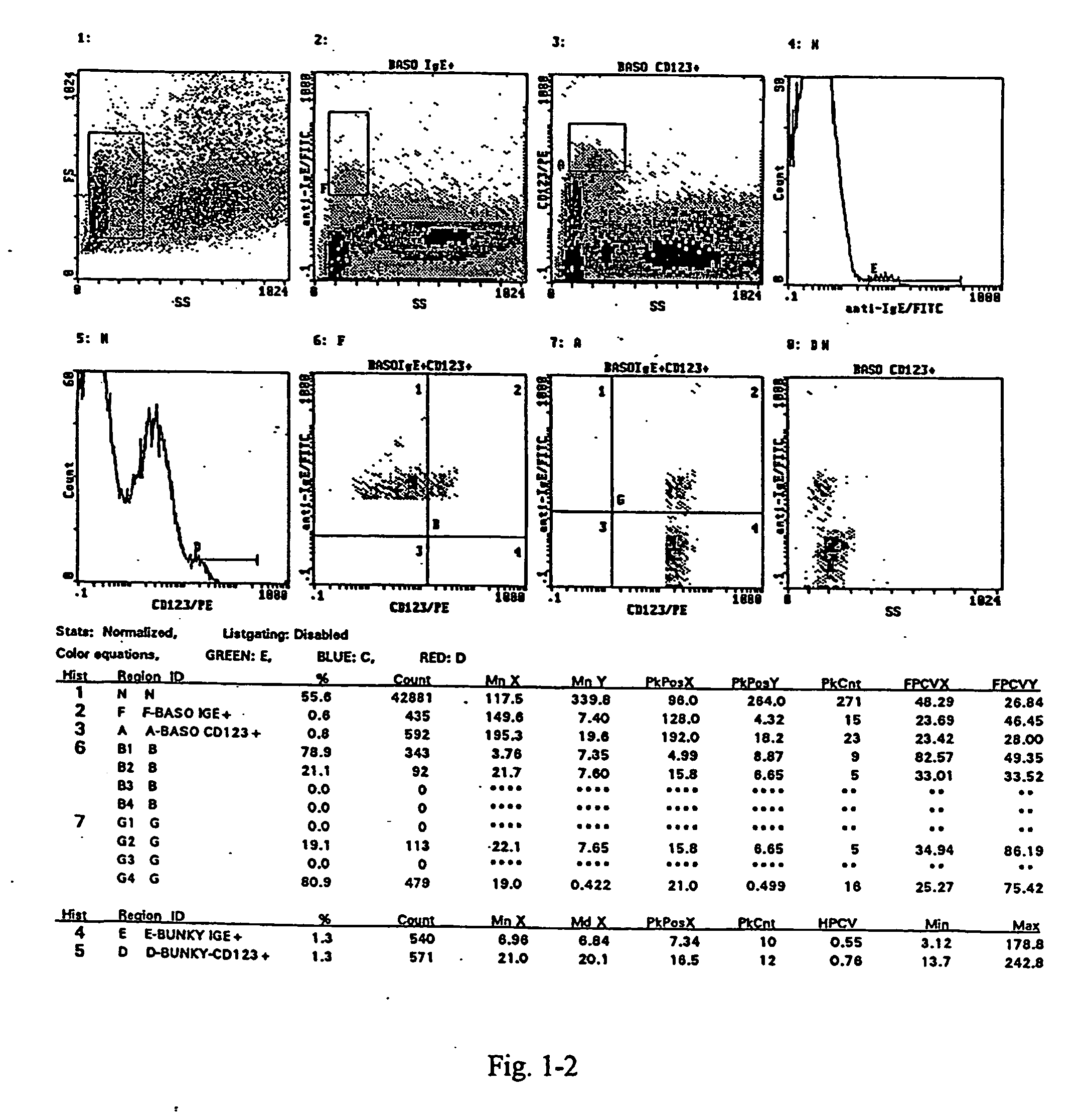
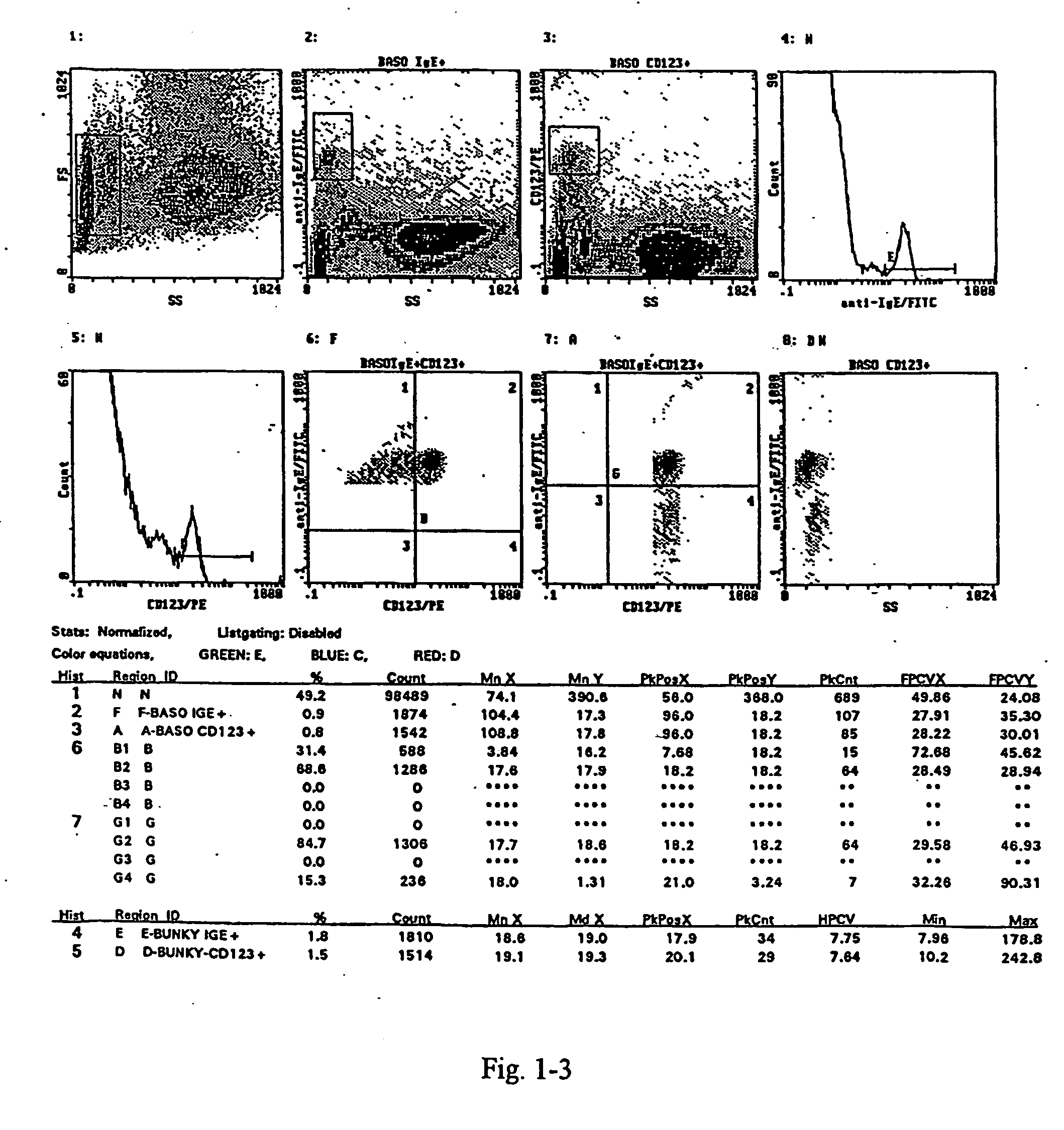
A method for the measurement of the activation of the basophils following stimulation with an allergen to determine hypersensitivity to some substances, in which a blood sample with an addition of interleukin-3 in a quantity of 0.05 to 50 ng / ml based on the volume of the sample, and of appropriately diluted allergen in a quantity of 0.5 to 100 units / ml is incubated at the temperature corresponding to the physiological environment for 15 to 45 minutes, whereafter a staining with anti-CD63.antibody in an amount of 3 to 30 .mu.l / 100 .mu.l of blood and with the antibody against a receptor of the basophil in an amount of 3 to 30 .mu.l / 100 .mu.l of blood are added at a temperature of 0 to +10.degree. C. and the sample, after vortexing, is then incubated in an ice bath for 15 to 30 minutes, and then is lysed and subjected to flow cytometry.
Owner:HAVRANOVA MARIE
Culture method of adipose tissue-derived stromal cells
InactiveCN106520686AImprove scalabilityImprove migration abilitySkeletal/connective tissue cellsCell culture active agentsStromal cellBrown adipose tissue
The invention provides a culture method of adipose tissue-derived stromal cells. The culture method of the adipose tissue-derived stromal cells comprises the following culture step: inoculating acquired primary-generation adipose tissue-derived stromal cells into a stem cell culture medium, wherein the stem cell culture medium contains stem cell factors and interleukin-3 factors, and the content ratio of the stem cell factors to the interleukin-3 factors is (5 to 20 [mu]mol / L) to (5 to 20 [mu]mol / L). The culture method can improve the multiplication ability of the adipose tissue-derived stromal cells and achieve relatively high transfer ability.
Owner:海南博鳌一龄迈迪精准医学研究院有限公司
Fusion protein of anti-cluster of differetiation (CD3) antibody Fv segment and interleukin 3, method for preparing fusion protein and application of fusion protein
InactiveCN102796198APreserve associativityEnhance killing activityFungiBacteriaGenetic engineeringBiology
The invention discloses a fusion protein of an anti-cluster of differetiation (CD3) antibody Fv segment and an interleukin 3, a method for preparing the fusion protein and an application of the fusion protein, relates to the fusion proteins of anti-CD3 star-delta IL3 and anti-CD3-delta IL3 and encoded genes thereof, and further relates to a genetic engineering construction method and an application of the strengthened fusion protein.
Owner:中国医学科学院血液病医院
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveCN1878860AImprove the success rate of cultivationMicroorganismsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an alphaMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the alphaMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:韩薰
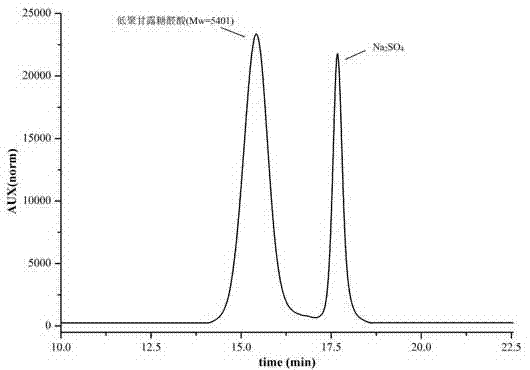
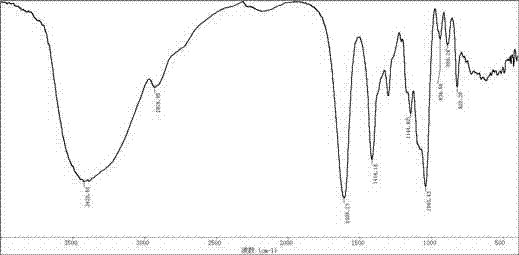
Oligomerization mannuronic acid and application of medicinal salts of oligomerization mannuronic acid in leucopenia prevention medicines
ActiveCN102920729AIncrease the number ofPromote formationOrganic active ingredientsBlood disorderPolymannuronic acidBone Marrow Stromal Cell
The invention provides oligomerization mannuronic acid and application of medicinal salts of oligomerization mannuronic acid in leucopenia prevention medicine. In in-vitro cell experiments, oligomerization mannuronic acid can obviously promote forming of colony-forming unit-granulocyte macrophage (CFU-GM), simultaneously can promote marrow stroma cells to breed and secrete CFU-GM and promote splenic lymphocytes to secrete interleukin-3. In vivo experiments further indicates that oligomerization mannuronic acid can obviously restrain reduction of white blood cells in mice caused by cyclophosphamide, increase the number marrow nucleated cell of the mice and promote forming of CFU-GM. Experiment results indicate that oligomerization mannuronic acid can be developed to be a clinical medicine capable of effectively preventing leucopenia.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD
Method for in vitro preparation of hepatocyte induced by umbilical cord blood hematopoietic stem cell
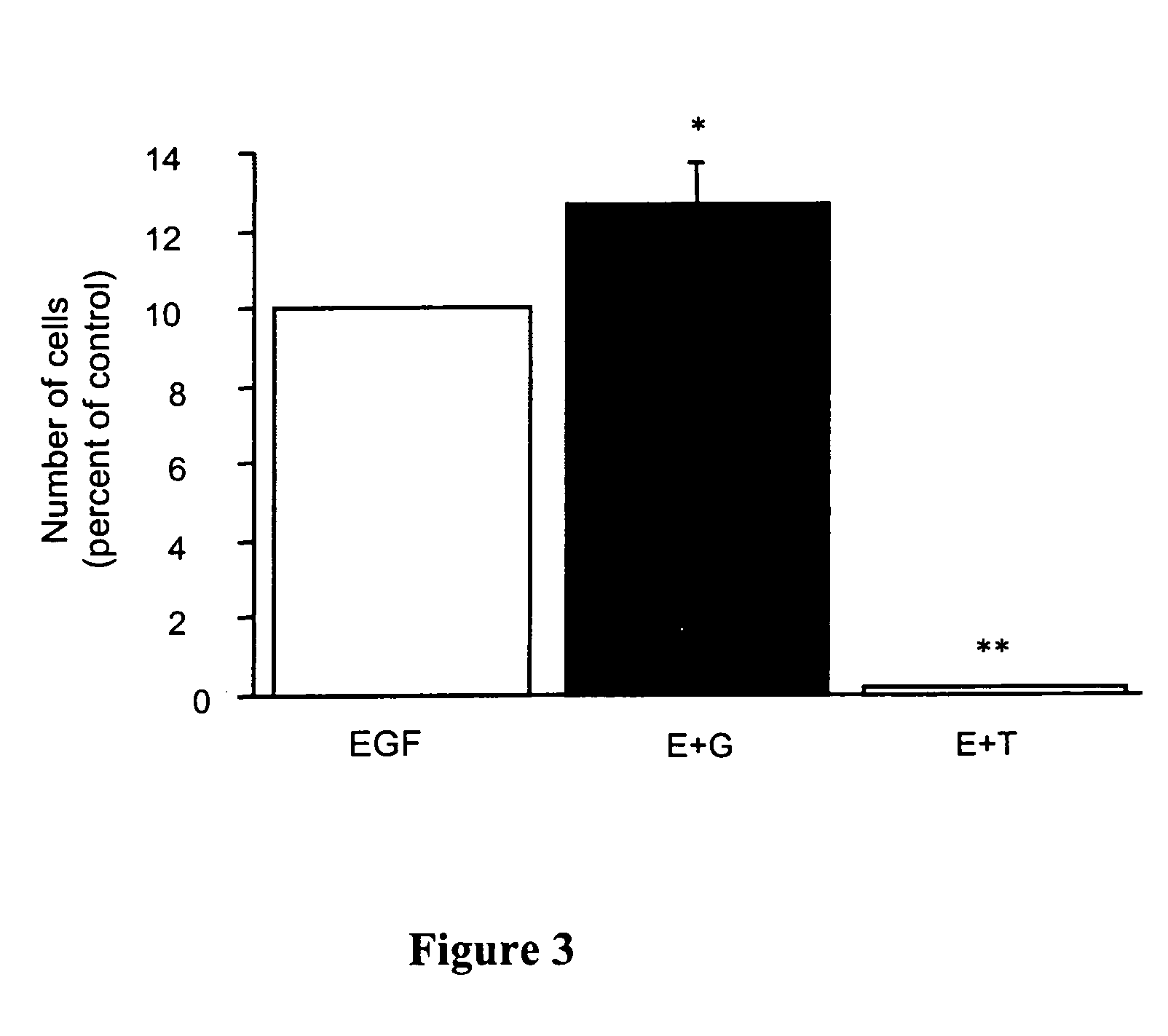
This invention discloses a method for producing liver cells by inducing haemopoietic stem cells in vitro, comprising: inoculating the haemopoietic stem cells or CD34+ cells into the culture media containing embryonic bovine serum, human stem cell factor, human interleukins 3(IL-3), interleukins 6(IL-6), human liver cell growth factor, human epidermal growth factor(EGF), human acdic fibroblast growth factor, human basic fibroblast growth factor(bFGF), human leukemia inhibitory factor(LIF) and oncostatin M(OSM), and inducing the producing of liver cells in vitro. By applying the method of this invention to induce liver cells from the haemopoietic stem cells, it can acquire a high proportion of liver cells in the culture with good effects.
Owner:EAST CHINA UNIV OF SCI & TECH
Methods and compositions based on diphtheria toxin-interleukin-3 conjugates
ActiveUS20090232767A1Enhance or improve the prophylactic effect(s) of another therapyShorten the durationPeptide/protein ingredientsMammal material medical ingredientsCancer cellWhite blood cell
The present invention provides methods for inhibiting interleukin-3 receptor-expressing cells, and, in particular, inhibiting the growth of such cells by using a diphtheria toxin-human interleukin-3 conjugate (DT-IL3) that is toxic to cells expressing the interleukin-3 receptor. In preferred embodiments, the DT-IL3 conjugate is a fusion protein comprising amino acids 1-388 of diphtheria toxin fused via a peptide linker to full-length, human interleukin-3. In certain embodiments, the methods of the present invention relate to the administration of a DT-IL3 conjugate to inhibit the growth of cancer cells and / or cancer stem cells in humans, which cells express one or more subunits of the interleukin-3 receptor. Exemplary cells include myeloid leukemia cancer stem cells. In other embodiments, the methods of the present invention relate to ex vivo purging of bone marrow or peripheral blood to remove cells that express one or more subunits of the interleukin-3 receptor such that the purged bone marrow or peripheral blood is suitable, e.g., for autologous stem cell transplantation to restore hematopoietic function.
Owner:SCOTT & WHITE MEMORIAL HOSPITAL
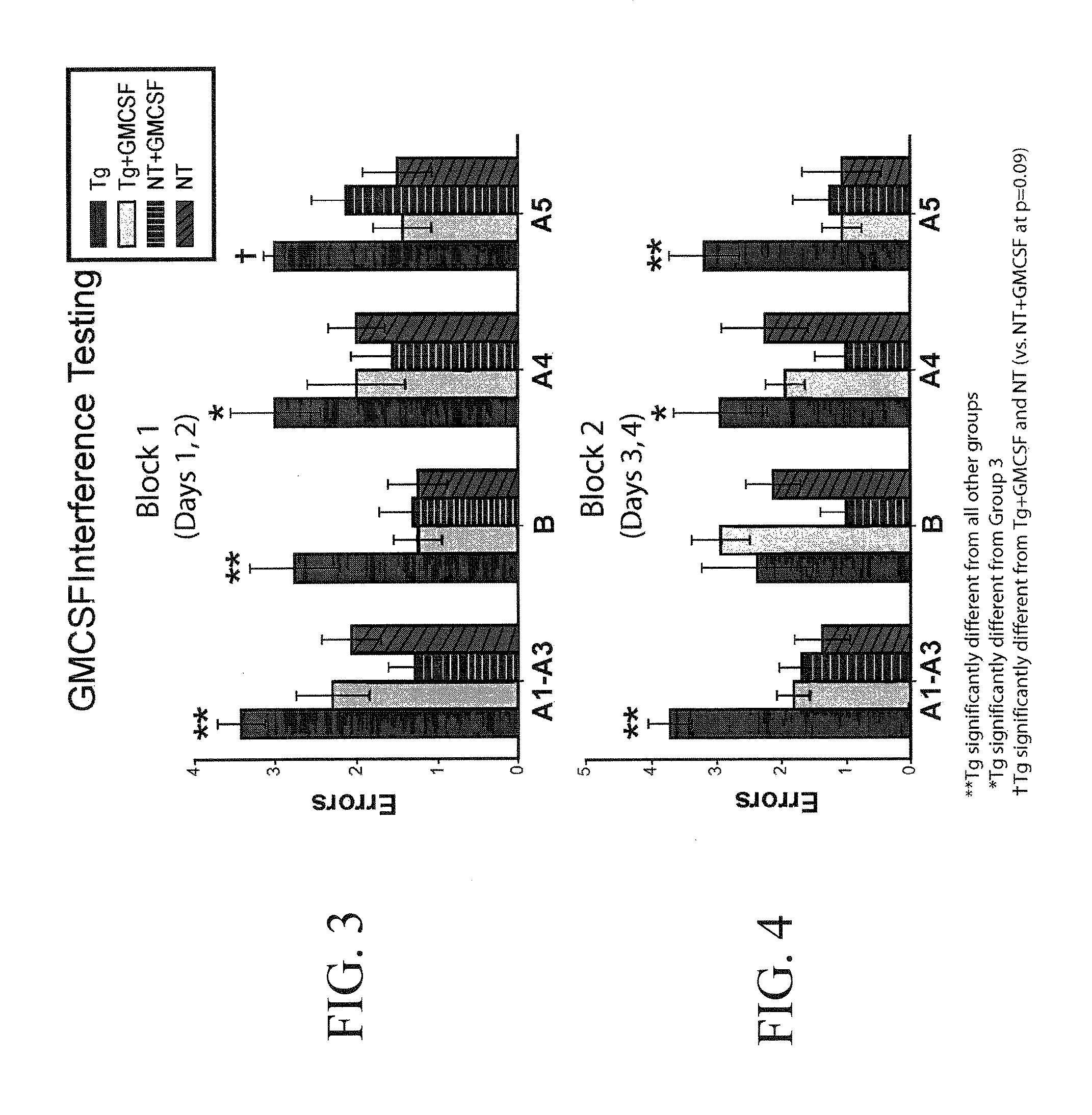
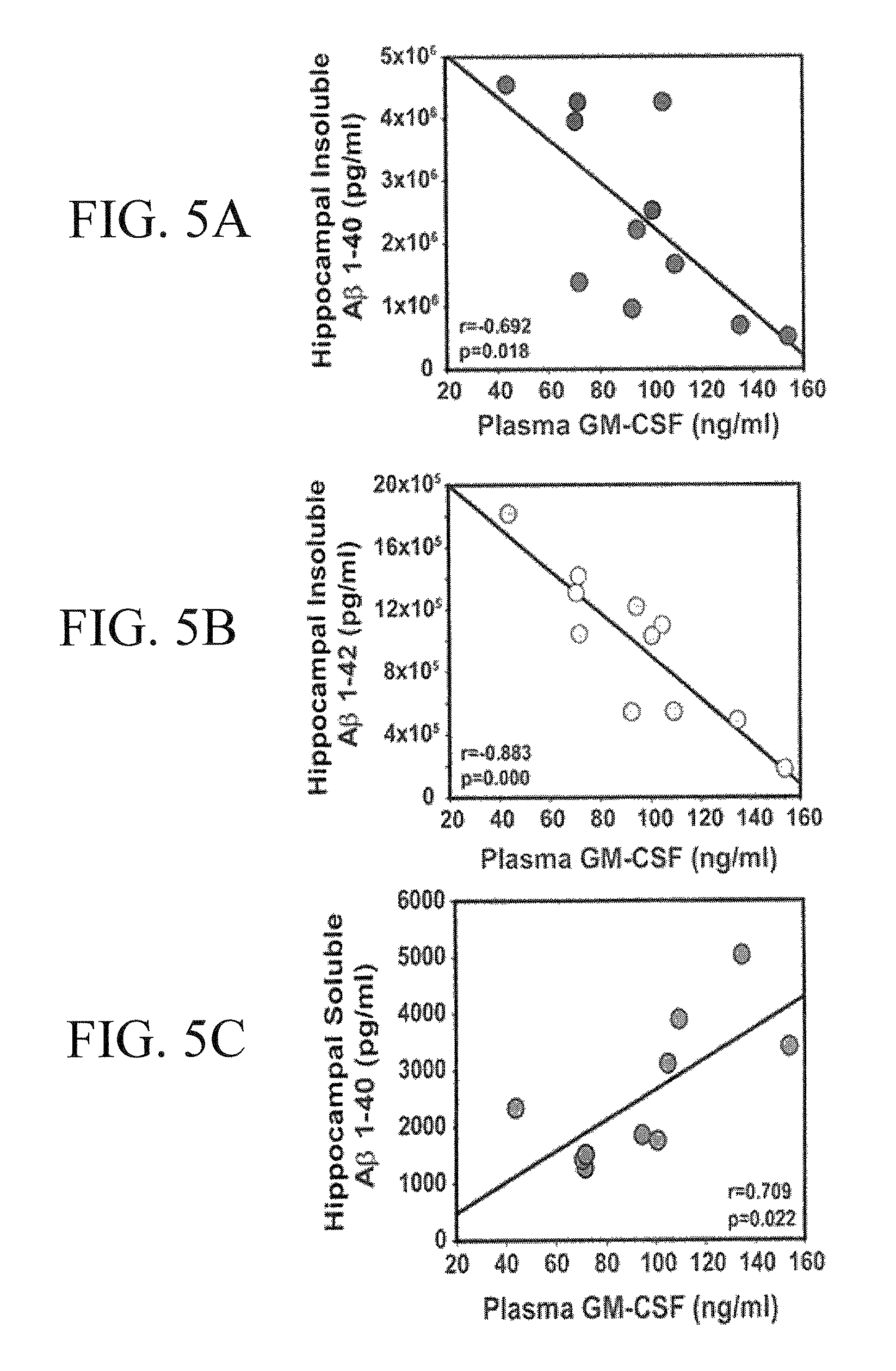
Methods of treating cognitive impairment
InactiveUS20110142795A1Decreasing and inhibiting progressionOrganic active ingredientsNervous disorderInsulin-like growth factorInterleukin 6
The subject invention concerns materials and methods for treating a person or animal having cognitive impairment. In one embodiment, the method comprises administering an effective amount of one or more inflammatory mediator(s), for example, fms-related tyrosine kinase 3 (Flt3) ligand, interleukin-6 (IL-6), macrophage migration inhibitory factor (MIF), interleukin-1 (IL-1), interleukin-3 (IL-3), erythropoietin (EPO), vascular endothelial growth factor A (VEGF-A), hypoxia-inducible transcription factor (HIF-1alpha), insulin like growth factor-1 (IGF-1), tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF), granulocyte / macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), Stem Cell Factor (SCF), Darbepoetin (ARANESP), and metalloproteinases, to an animal or person in need of treatment.
Owner:UNIV OF SOUTH FLORIDA
Method for preparing autologous hematopoietic stem cells, kit, the stem cells and application
ActiveCN104419660AYield advantagePurity advantageAntinoxious agentsDead animal preservationInterleukin 6Culture fluid
Owner:深圳百年干细胞技术研究院有限公司
Reagent kit for primary culture on umbilical cord mesenchymal stem cells
InactiveCN107034184APromote rapid proliferationNot easy to ageSkeletal/connective tissue cellsPhosphateCuticle
The invention discloses a reagent kit for primary culture on umbilical cord mesenchymal stem cells. The reagent kit comprises umbilical cord cleaning solution, envelope solution and a cell primary culture medium. The umbilical cord cleaning solution is normal saline with penicillin and streptomycin or PBS (phosphate buffer solution) with the pH (potential of hydrogen) of 7.4; the envelope solution is normal saline solution with fibronectin or normal saline solution with type IV collagen; an alpha-MEM culture medium with L-glutamine is used as base solution, and human insulin, transferrin, human serum albumin, vitamin C, trehalose, epidermal growth factors, vascular endothelial growth factors, recombinant human interleukin-3 are added into the base solution to obtain the cell primary culture medium which is culture solution. The reagent kit has the advantages that a series of procedures from umbilical cord pretreatment to separation, inoculation and culture can be completely carried out by the aid of the reagent kit, and the reagent kit is convenient to operate and low in cost and is safe and controllable; the cultured umbilical cord MSCs (mesenchymal stem cells) are high in proliferation speed and difficult to age and have broad clinical application prospects.
Owner:济南赛尔生物科技股份有限公司
Methods of treating cognitive impairment
The subject invention concerns materials and methods for treating a person or animal having cognitive impairment. In one embodiment, the method comprises administering an effective amount of one or more inflammatory mediator(s), for example, fms-related tyrosine kinase 3 (Flt3) ligand, interleukin-6 (IL-6), macrophage migration inhibitory factor (MIF), interleukin-1 (IL-1), interleukin-3 (IL-3), erythropoietin (EPO), vascular endothelial growth factor A (VEGF-A), hypoxia-inducible transcription factor (HIF-1alpha), insulin like growth factor-1 (IGF-1), tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF), granulocyte / macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), Stem Cell Factor (SCF), Darbepoetin (ARANESP), and metalloproteinases, to an animal or person in need of treatment.
Owner:UNIV OF SOUTH FLORIDA +1
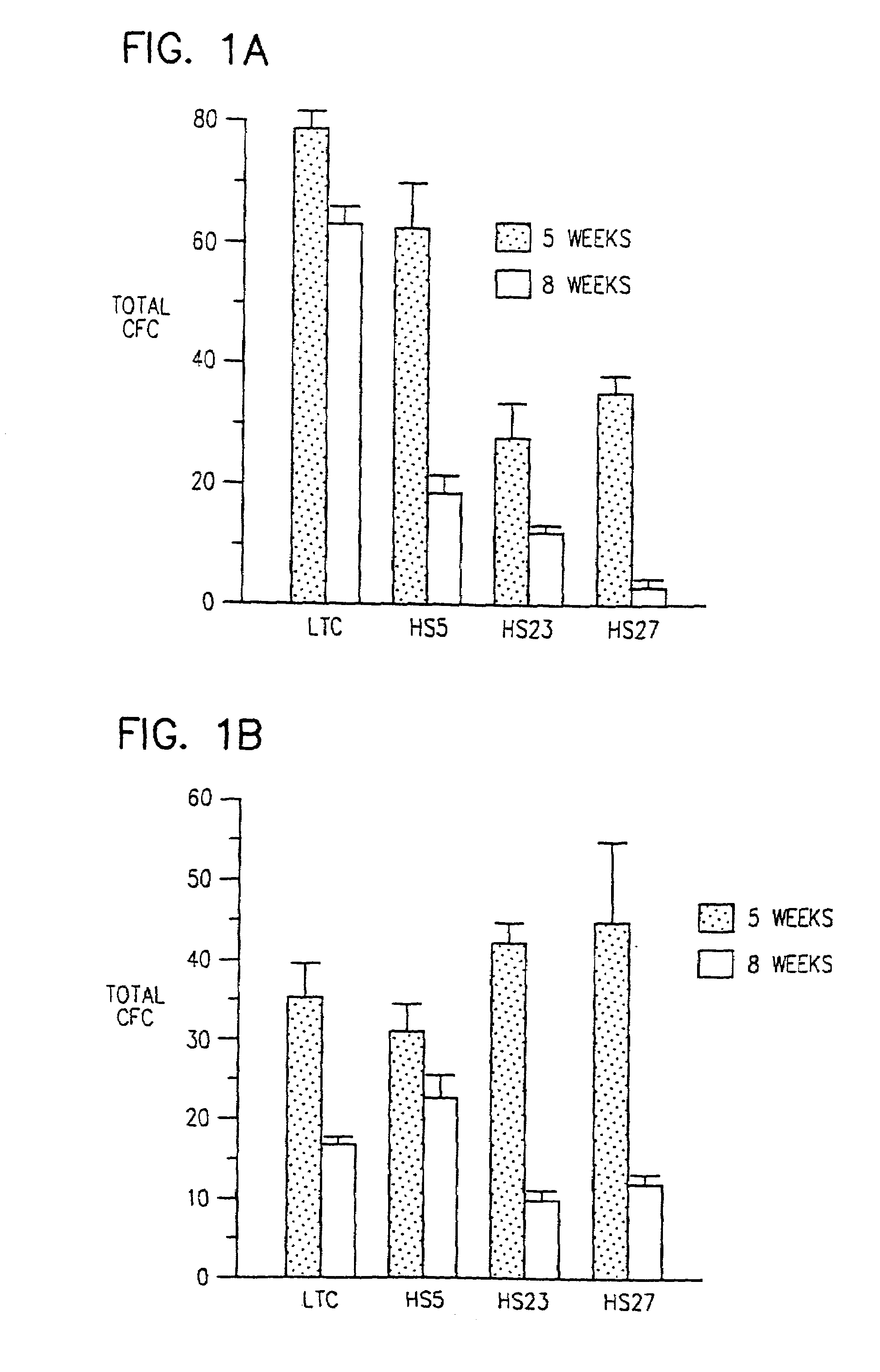
Human marrow stromal cell lines which sustain hematopoiesis
InactiveUS7129086B2Genetically modified cellsArtificial cell constructsWhole blood productBone Marrow Stromal Cell
Immortalized human stromal cell lines sustain and expand human hematopoietic precursor cells. The precursor cells are obtained from a blood product and inoculated into a culture medium conditioned by exposure to a human stromal cell line. Preferred human stromal cell lines secrete SCF, LIF, MIP1α, and IL-6, as exemplified by a human stromal cell line designated HS-1. The conditioned culture medium may be supplemented with additional growth factors, such as interleukin-3. After expansion the human hematopoietic precursor cells are harvested and returned to a patient or frozen and stored. The immortalized human stromal cell lines can also be used as feeder layers in ex vivo bone marrow cultures or in colony forming assays.
Owner:FRED HUTCHINSON CANCER RES CENT
Oligodendrocyte production from multipotent neural stem cells
InactiveUS20050244965A1Increase percentageMaximize productionOrganic active ingredientsNervous disorderOligodendrocyteInterleukin 5
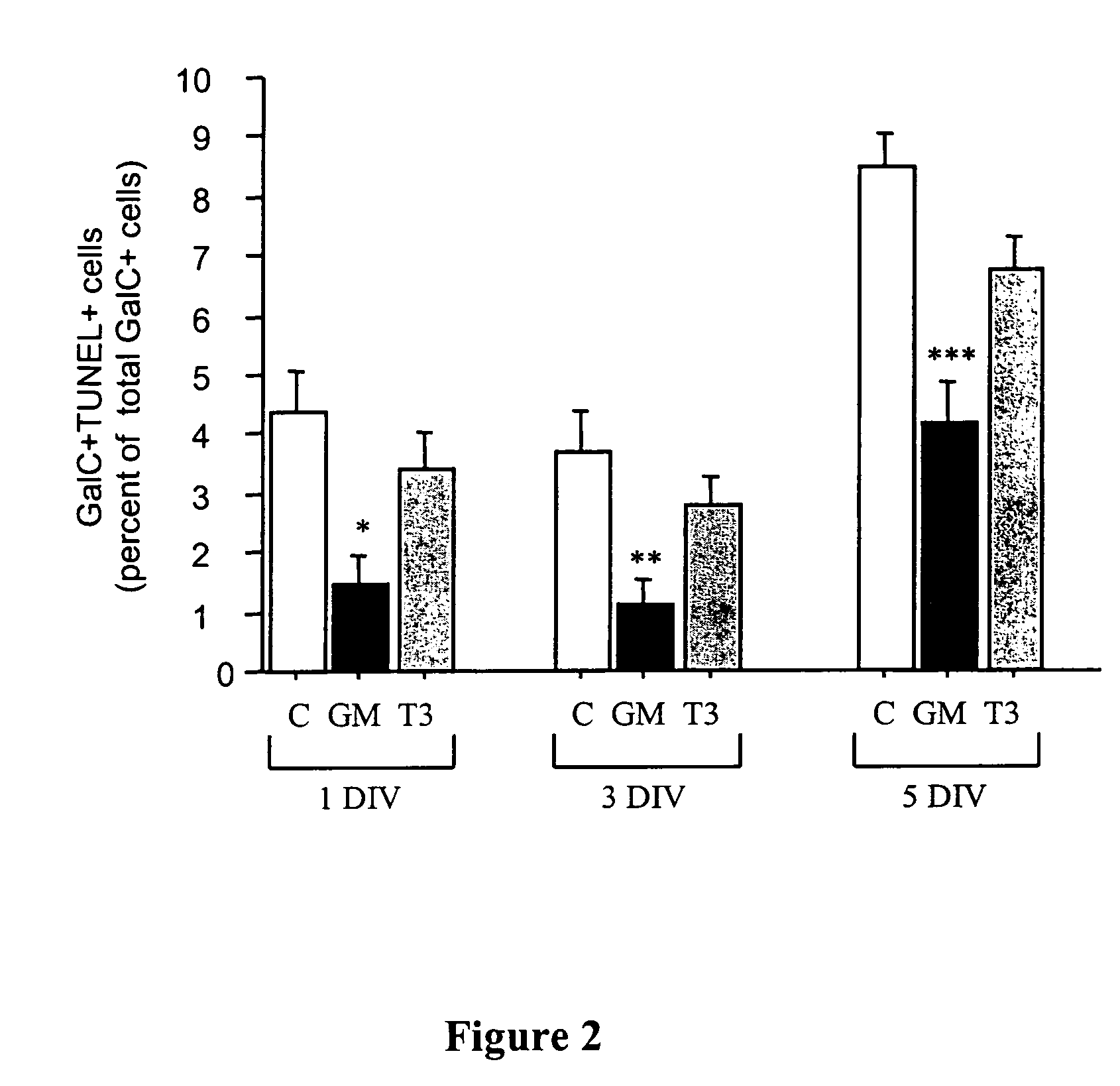
This invention relates to methods of producing oligodendrocytes from multipotent neural stem cells by using at least one oligodendrocyte promoting factor, particularly granulocyte-macrophage colony stimulating factor, granulocyte colony stimulating factor, interleukin 3 or interleukin 5. The neural stem cells may optionally be expanded prior to being subjected to the oligodendrocyte promoting factor.
Owner:STEM CELL THERAPEUTICS
Method of isolating and culturing mesenchymal stem cell derived from cryopreserved umbilical cord blood
InactiveUS7582477B2Increase success rateSkeletal/connective tissue cellsBlood/immune system cellsInterleukin 6G-csf therapy
Owner:HOON HAN
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveUS7704739B2Increase success rateArtificial cell constructsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an αMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the αMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:HAN HOON
Methods of using retro-inverso peptides derived from interleukin-3
The invention provides methods of treatment using retro-inverso peptides derived from interleukin-3 (IL-3) having between 12 and about 40 amino acids and including the sequence that is retro-inverso with respect to SEQ ID NO: 1. These peptides of the invention have the same activity as native IL-3 and also have neurotrophic activity. The peptides of the invention are also less susceptible to proteolytic degradation in vivo because of their D-amino acid linkage.
Owner:WRIGHT DAVID E +1
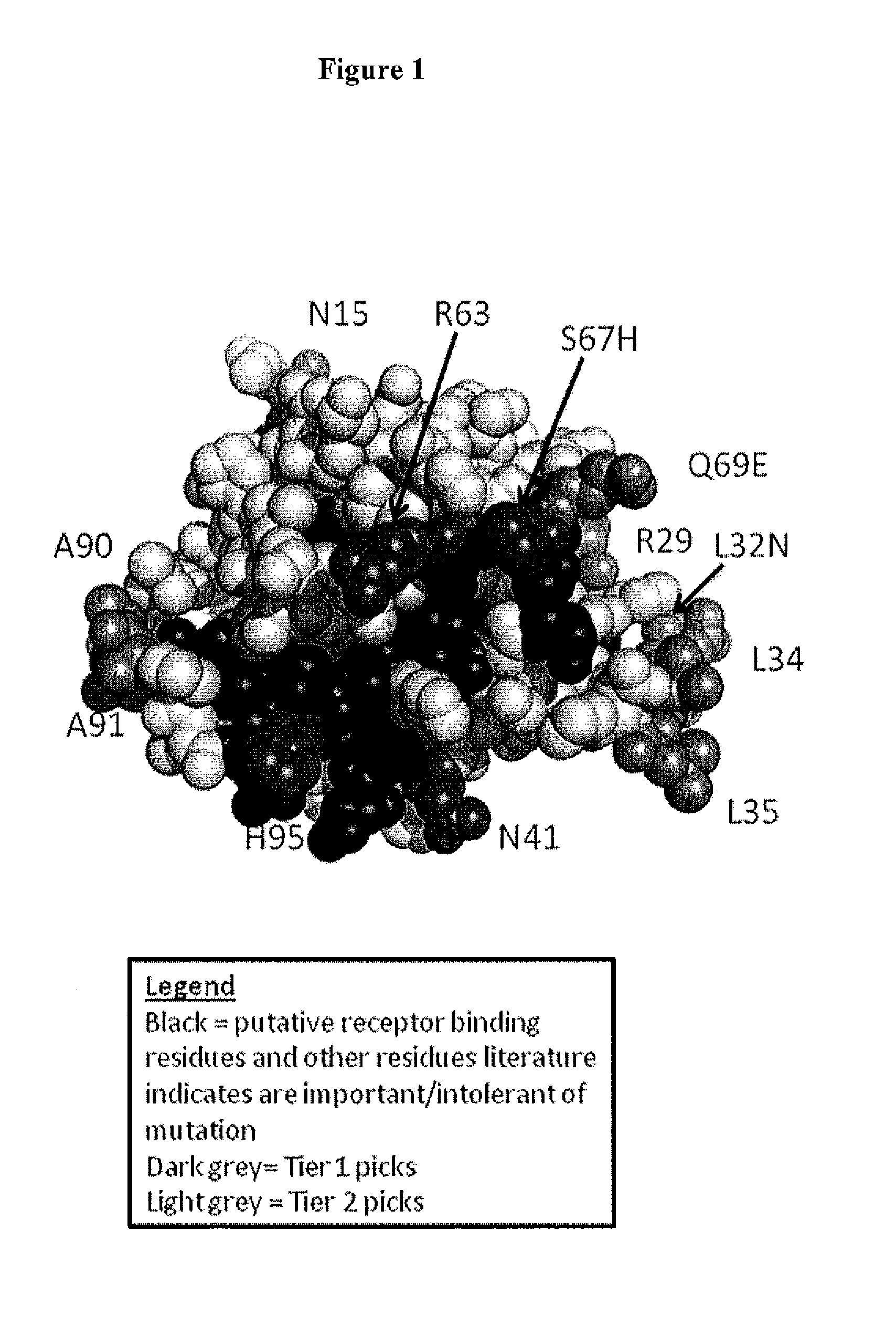
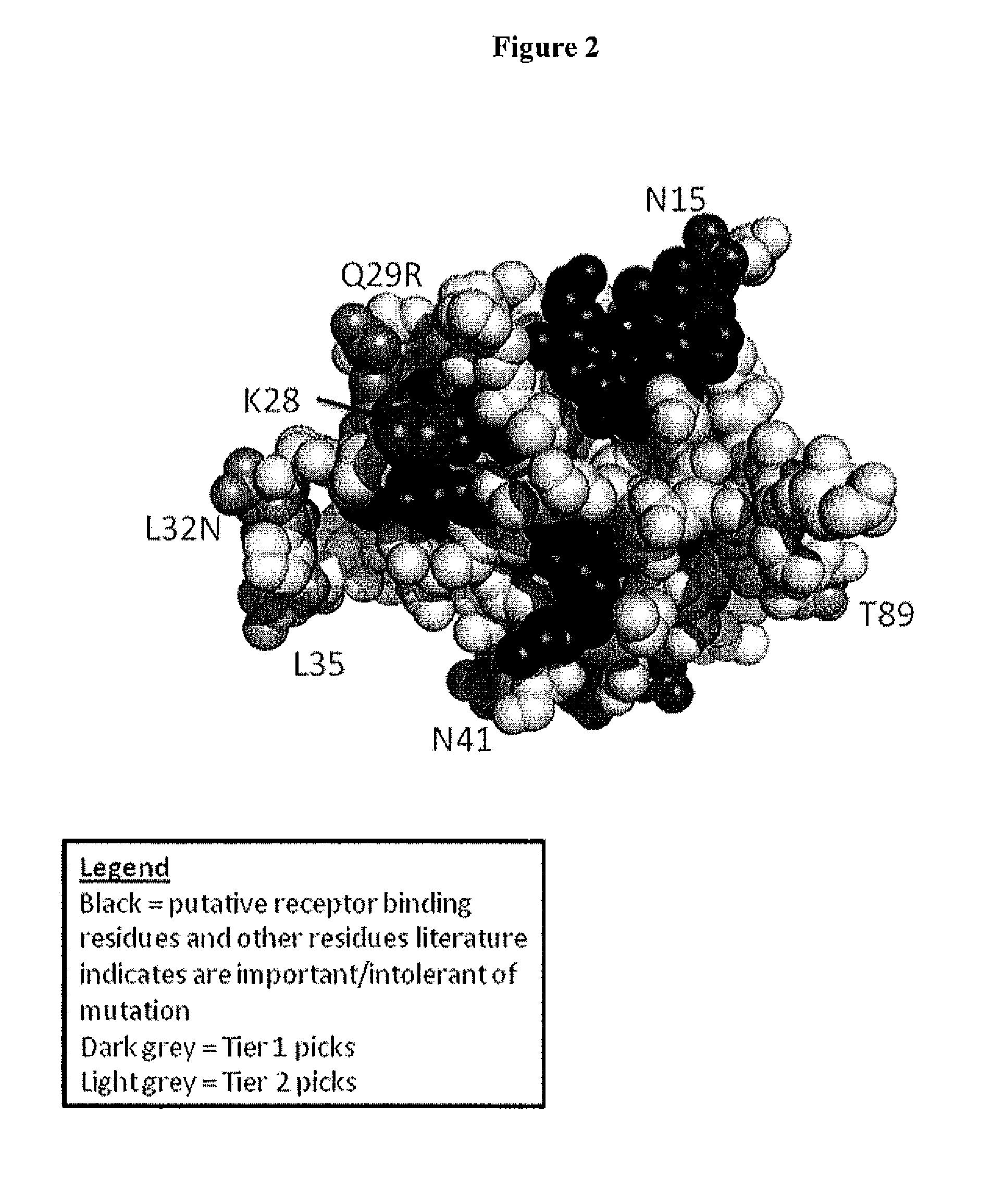
Novel interleukin-3 and uses thereof
The present invention provides novel nucleic acids isolated from a cDNA library for fetal liver-spleen tissue, and the novel polypeptide sequences encoded by these nucleic acids. These novel polynucleotide and polypeptide sequences were determined to be a novel Interleukin-3.
Owner:HYSEQ
Methods of treating cognitive impairment
ActiveUS20110311473A1Increase cognitive responseDecreasing and inhibiting progressionNervous disorderPeptide/protein ingredientsInsulin-like growth factorInterleukin 6
The subject invention concerns materials and methods for treating a person or animal having cognitive impairment. In one embodiment, the method comprises administering an effective amount of one or more inflammatory mediator(s), for example, fms-related tyrosine kinase 3 (Flt3) ligand, interleukin-6 (IL-6), macrophage migration inhibitory factor (MIF), interleukin-1 (IL-1), interleukin-3 (IL-3), erythropoietin (EPO), vascular endothelial growth factor A (VEGF-A), hypoxia-inducible transcription factor (HIF-1alpha), insulin like growth factor-1 (IGF-1), tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF), granulocyte / macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), Stem Cell Factor (SCF), Darbepoetin (ARANESP), and metalloproteinases, to an animal or person in need of treatment.
Owner:UNIV OF SOUTH FLORIDA +1
Fusion protein of interleukin-3 derived fragment and purpose thereof
The invention relates to fusion protein of an interleukin-3 derived fragment and a purpose thereof. Particularly, the invention relates to fusion protein containing mutated interleukin-3 derived fragment, which is selected from: (1) IL2mIL3m2SON3, (2) IL2mIL3m3SON3 or (3) IL2mIL3m1SON3, wherein IL2m is an IL2-mutated derived fragment, IL3m2 is an IL3-mutated derived fragment 2, IL3m3 is an IL3-mutated derived fragment 3, and IL3m1 is an IL3-mutated derived fragment 1, and SON3 is a DNA-binding protein SON derivative fragment and is rich in basic amino acids. The invention also related to the purpose of the fusion protein, an encoding nucleotide sequence, a recombinant containing the encoding nucleotide sequence, an engineering cell or engineering bacteria containing the recombinant, a method for production of the fusion protein, a composition of the fusion protein, a compound of the fusion protein, and a purpose of the compound. The invention provides a drug with a fully new mechanism for killing and / or eliminating ordinary leukemia cells and leukemia stem cells.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Oligodendrocyte production from multipotent neural stem cells
InactiveUS7704737B2Increase percentageProliferating neural stem cells were decreasedOrganic active ingredientsNervous disorderOligodendrocyteInterleukin 5
This invention relates to methods of producing oligodendrocytes from multipotent neural stem cells by using at least one oligodendrocyte promoting factor, particularly granulocyte-macrophage colony stimulating factor, granulocyte colony stimulating factor, interleukin 3 or interleukin 5. The neural stem cells may optionally be expanded prior to being subjected to the oligodendrocyte promoting factor.
Owner:STEM CELL THERAPEUTICS
Interleukin-3 Polypeptide Conjugates and Their Uses
InactiveUS20150038679A1Lower Level RequirementsReduce in quantityPeptide/protein ingredientsDepsipeptidesToxinAmino acid
The disclosure provides methods for targeting interleukin-3 receptor-expressing cells, particularly inhibiting the growth of such cells by using an interleukin-3 (IL-3) variant conjugated to a toxin that will affect cells expressing the interleukin-3 receptor. Further disclosed are interleukin-3 (IL-3) variants comprising one or more non-naturally encoded amino acids, and the structures of non-naturally encoded amino acids.
Owner:AMBRX
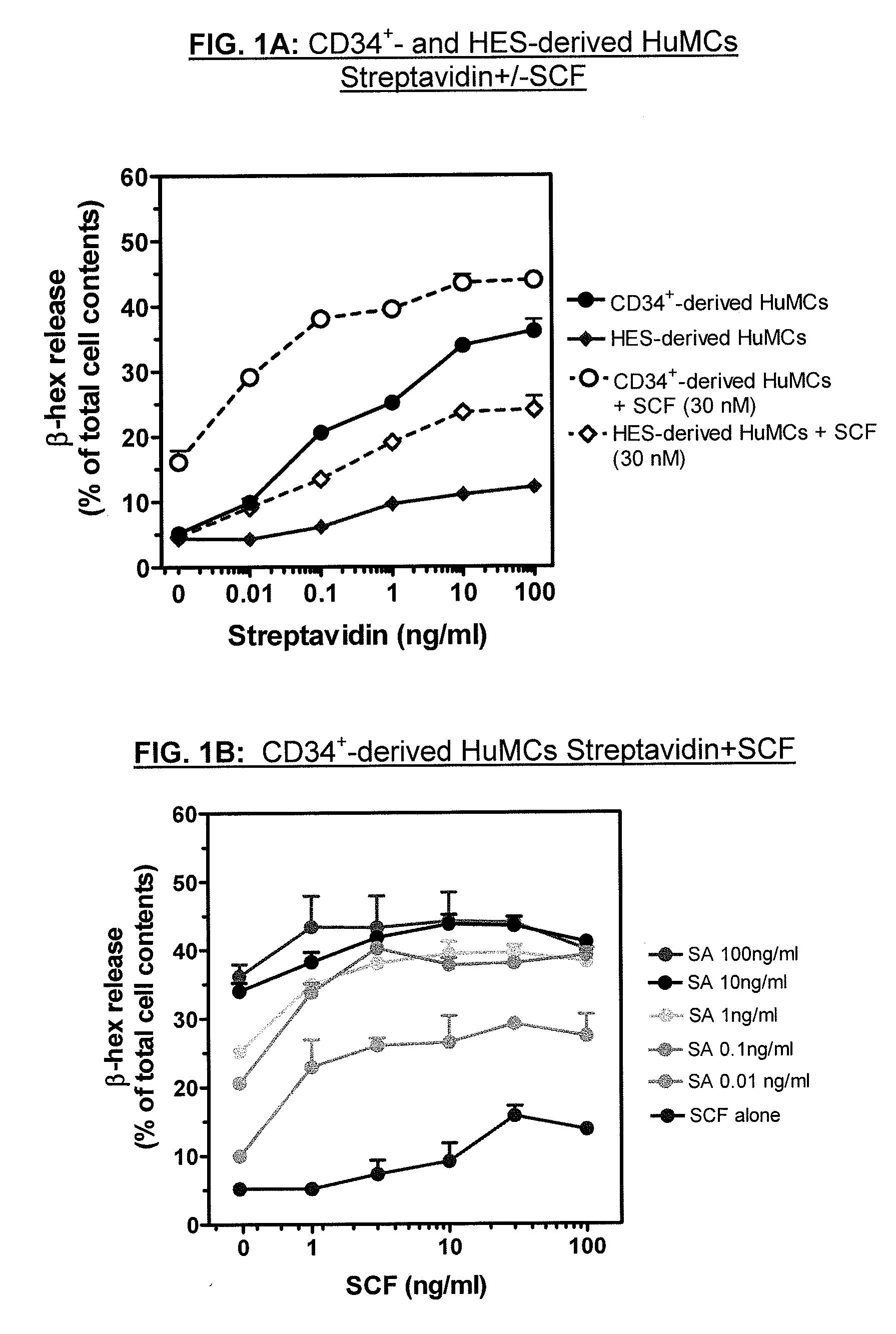
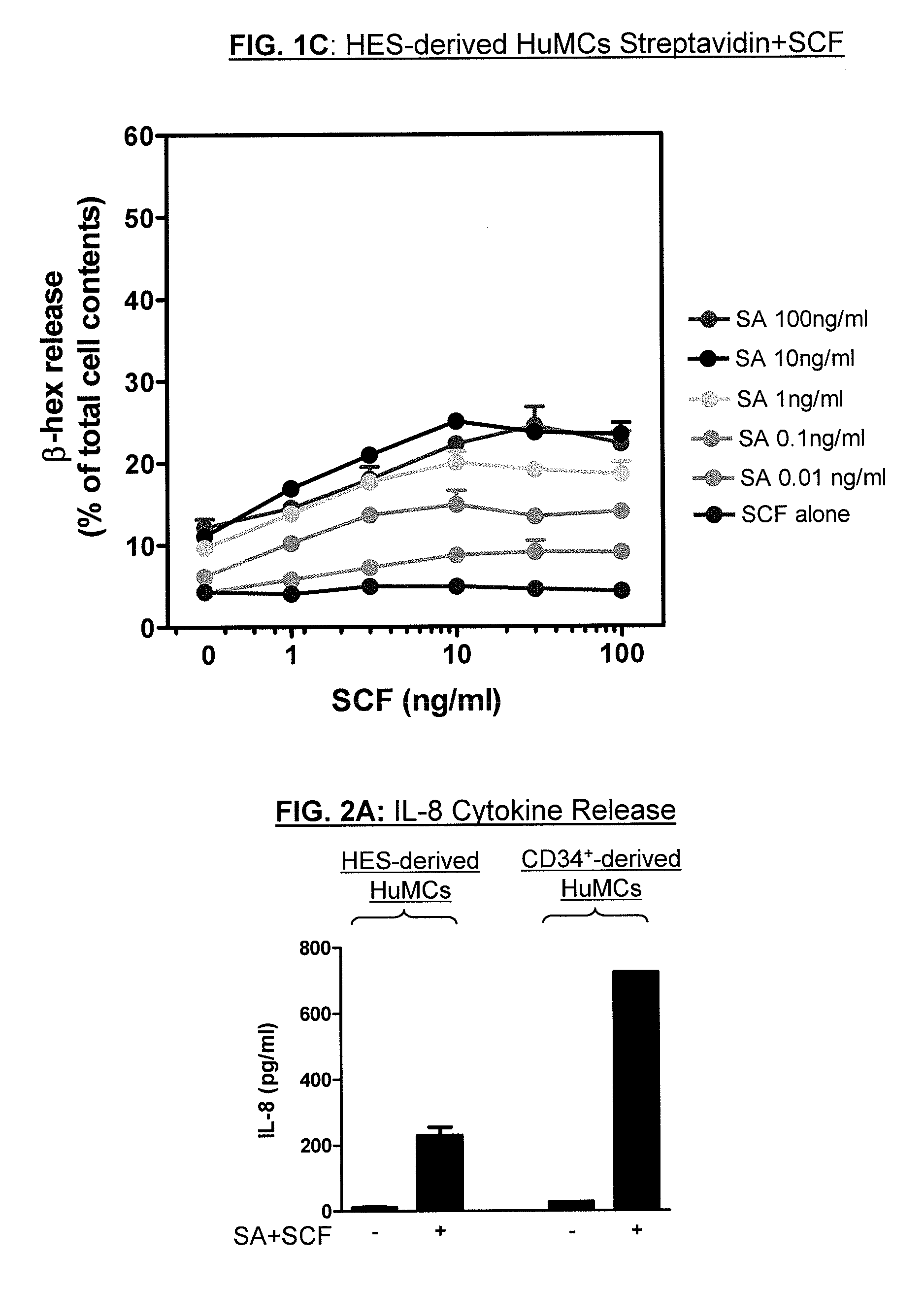
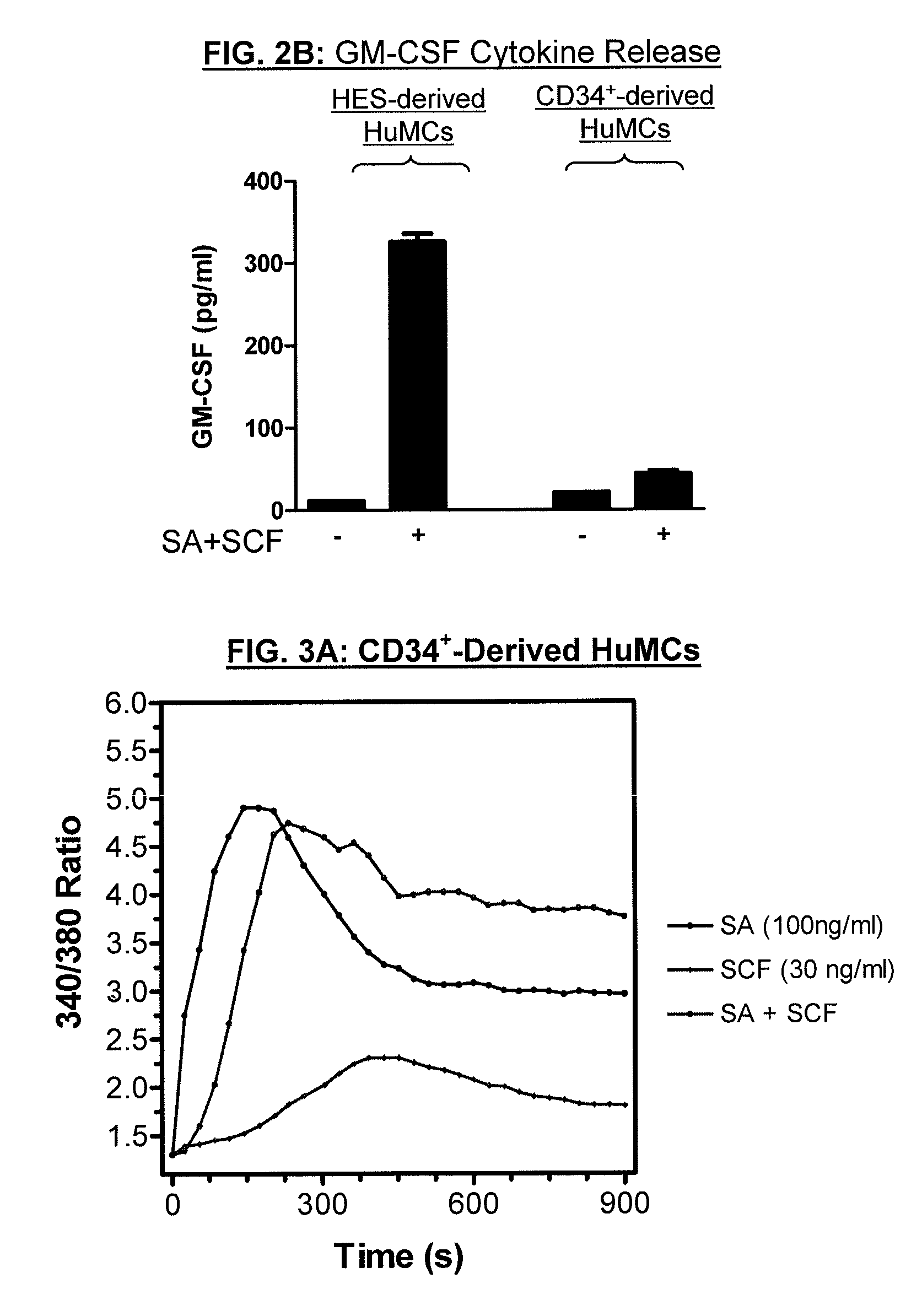
Method for production of mast cells from stem cells
ActiveUS8513012B2Overcome limitationsCell dissociation methodsArtificial cell constructsInterleukin 6Mast cell
Provided are methods for generating mast cells from pluripotent stem cells in vitro. Methods are disclosed for the differentiation of pluripotent cells, such as iPS cells and / or human embryonic stem cells, into mast cells. The resulting mast cells may be used for various purposes including screening cells for drug development and research. Growth factors which may be included in culture media according to the present invention include stem cell factor (SCF), FLT-3 ligand, thrombopoietin (TPO), interleukin-3 (IL-3), and / or interleukin-6 (IL-6).
Owner:FUJIFILM CELLULAR DYNAMICS INC
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810BAvoid instabilityClear natureBlood/immune system cellsSodium bicarbonateLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com