Method for preparing autologous hematopoietic stem cells, kit, the stem cells and application
A technology of hematopoietic stem cells and somatic cells, applied in the field of biomedicine, can solve the problems of low safety, less available quantity, insufficient sources of hematopoietic stem cells, etc., and achieve the effect of production speed and safety advantages.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 kit
[0060] It is operated in a safe operation bench with a cleanliness level of 10-100, and is prepared under a low temperature of 4-10 degrees. In 500ml of RPMI640 culture medium, add: 10μM Y-27632 (C14H21N3O 2HCl); 10ng / mL stem cell factor; 10ng / mL interleukin 3; 10ng / mL interleukin 6; 10ng / mL Interleukin 11; 10 ng / mL macrophage colony-stimulating factor (M-CSF); 10 ng / mL granulocyte colony-stimulating factor (G-CSF); 10 μg / mL fucoidan; 10 μg / mL sulfuric acid Dextran; 10 nM AMD3100 (1,1′-[1,4-phenylenebis(methylene)]-bis-1,4,8,11-tetraazacyclotetradecane); 10 μM M-Malendronate sodium trihydrate; Pamidronate disodium at 10 μM.
[0061] Thoroughly mix and dissolve, then filter and sterilize through a filter with a pore size of 0.22 microns, and prepare cell culture fluid.
[0062] The cell culture solution is placed in the kit, so as to prepare the kit for producing protein-induced autologous hematopoietic stem cells.
Embodiment 2
[0063] Example 2 Preparation of protein-induced autologous hematopoietic stem cells (M-PiHPS) in mice
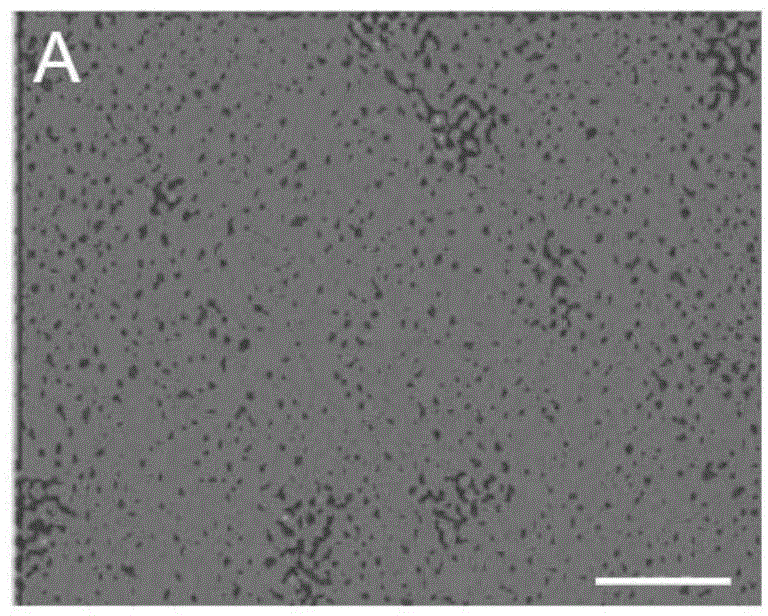
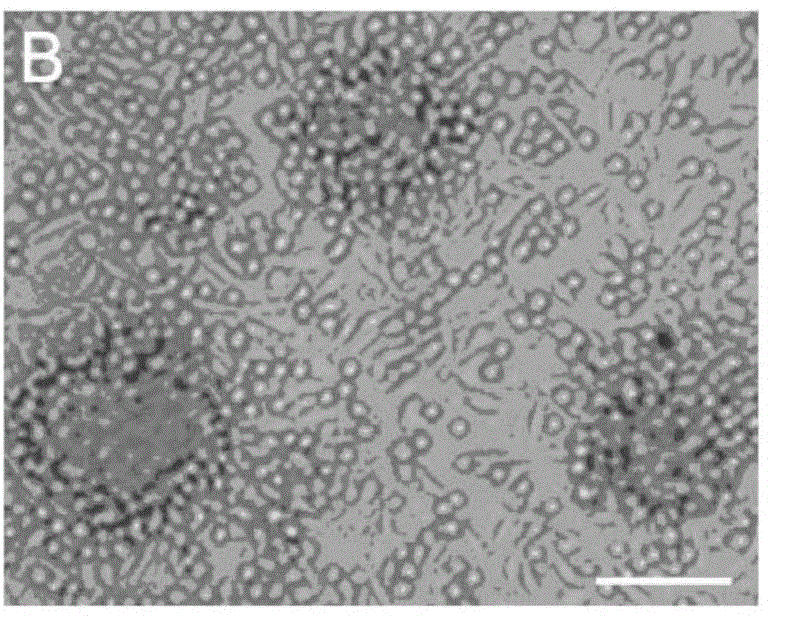
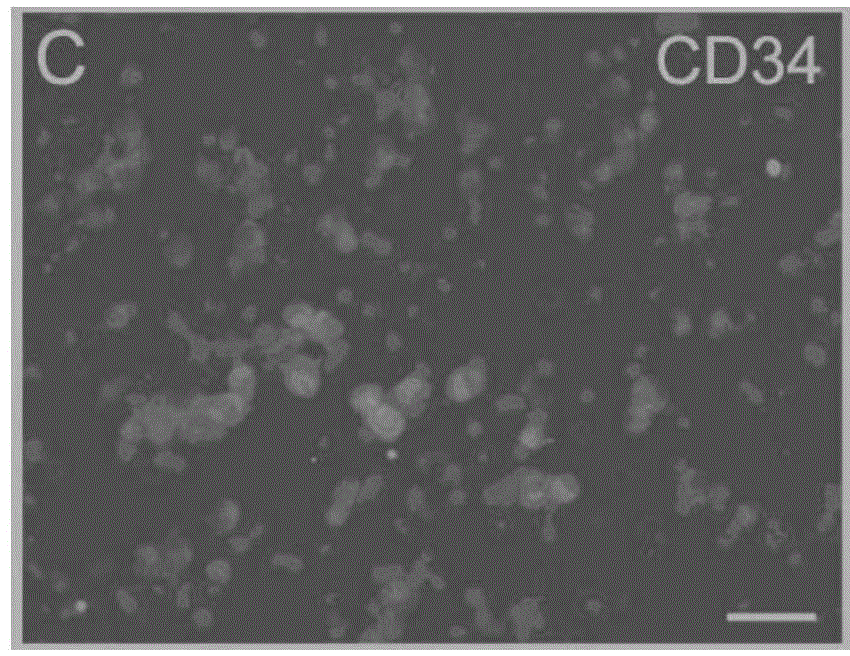
[0064] Select 3-5 month-old Balb / C male mice (purchased from the Chinese Academy of Medical Sciences School of Radiology (Tianjin), animal qualification certificate number: SCXK (Jin) 2010-0002), collect all their blood, and use conventional Ficoll centrifugation The technology centrifuges and separates blood to obtain blood mononuclear cells. The obtained mononuclear cells were divided into 5×10 6 Individual / ml density, inoculate in the cell culture dish, and add the cell culture solution prepared in embodiment 1 wherein, at 37 ℃ and 5% CO 2 Protein-induced autologous hematopoietic stem cells were obtained by culturing them in an incubator for 5 days. Gently pipette the cultured cells to suspend the cells completely, then collect the cells in a centrifuge tube, perform the centrifugation-saline suspension cell washing operation, repeat 3 times, suspend protein-induced aut...
Embodiment 3
[0065] Example 3 Preparation of human protein-induced autologous hematopoietic stem cells (M-PiHPS)
[0066] Collect 50ml of peripheral blood from the human body, and use conventional Ficoll centrifugal separation technology to centrifuge and separate the blood to obtain blood mononuclear cells. The obtained mononuclear cells were divided into 5×10 6 Individual / ml density, inoculate in the cell culture dish, and add the cell culture solution prepared in embodiment 1 wherein, at 37 ℃ and 5% CO 2 Protein-induced autologous hematopoietic stem cells were obtained by culturing them in an incubator for 5 days. Gently pipette the cultured cells to suspend the cells completely, then collect the cells in a centrifuge tube, perform the centrifugation-saline suspension cell washing operation, repeat 3 times, suspend protein-induced autologous hematopoietic stem cells with normal saline, and obtain protein-induced autologous hematopoietic stem cells Autologous hematopoietic stem cells. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com