Fusion protein of interleukin-3 derived fragment and purpose thereof
An interleukin and fusion protein technology, which is used in medical preparations with non-active ingredients, DNA/RNA fragments, and cells modified by introducing foreign genetic material, etc. It can solve shock and cell killing without tumor cell specificity. , prone to recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
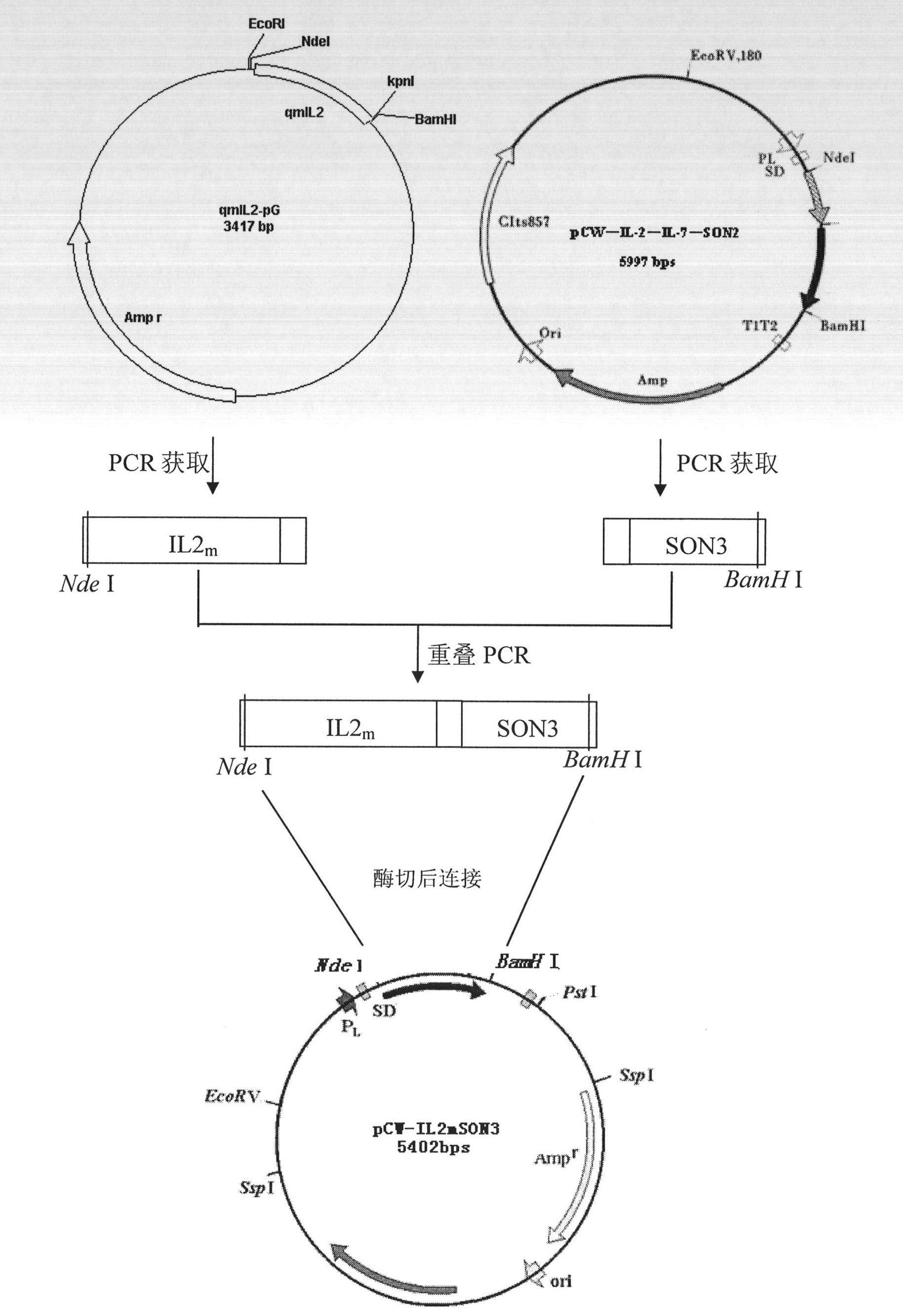
[0143] Example 1 IL2 encoding fusion protein non-viral vector m Acquisition of gene fragments
[0144] The present invention uses the qmIL2-pG vector (described in detail in the description of the patent application number 200610112334.3) as a template to design external primers (primer sequence as shown in SEQ ID NO.17) and upstream and downstream primers (primer sequence as shown in SEQ ID NO. .18 and 19) introduce Nde I restriction site sequence CATATG and initiation codon AUG at the 5' end, and introduce fusion protein linker (G 4 SG 4 T) sequence GGTGGCGGAGGTTCTGGTGGC GGTGGAACC, the IL2 gene coding that has mutated by overlapping PCR amplification obtains fragment NdeI-(IL2 m +linker)-, the size is 368bps. The total volume of the overlapping PCR reaction system is 60 μl, and the composition is: System I: 0.5-1 μg of DNA template, 4 μl of dNTPs (2mM), 0.25 μl of Pfu DNA polymerase (2U / μl), 10×PCR Buffer (20mMMg 2+ ) 5 μl, upstream primer (10 μM) 2 μl, downstream ...
Embodiment 2
[0145] The acquisition of the SON3 gene segment of embodiment 2 coding fusion protein non-viral vector
[0146] The present invention uses the NCBI accession number as the SON protein coding nucleotide of NM_032195 as a template to design external primers (primer sequences shown in SEQ ID NO.20) and upstream and downstream primers (primer sequences shown in SEQ ID NO.21 and 22) ) into the fusion protein linker (G at the 5' end 4 TG 4 T) The coding sequence GGTGGCGGCGGTACCGGTGGTGGCGGAACT and the basic amino acid KRKRS coding sequence AAACGCAAGCGTAGC, the BamH I restriction site sequence GGATCC was introduced at the 3' end, and the fragment (linker-SON3)-BamH I- was obtained by OVERLAP-PCR, the size was 237bps, agarose Gel electrophoresis confirmed that the obtained fragment sizes were in line with expectations.
Embodiment 3
[0147] Example 3 Prokaryotic expression of recombinant pCW-IL2 m Construction of SON3 expression recombinant
[0148] The present invention uses the IL2 obtained in Example 1 m With the SON3 obtained in Example 2 as a template, design external primers (primer sequence as shown in SEQ ID NO.23) and upstream and downstream primers (primer sequence as shown in SEQ ID NO.24 and 25) to carry out overlapping PCR to connect, Amplify DNA fragments. After recovery and purification, double digestion with BamH I and Nde I, the same double digestion of the pCW111 prokaryotic expression vector, ligation of the vector fragments according to the enzyme connection ratio, construction of pCW-IL2 m SON3 expression recombinant. For the recombinant construction process, see figure 1 , agarose gel electrophoresis confirmed that the obtained fragment sizes were in line with expectations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com