Methods of purification of native or mutant forms of diphtheria toxin
A technology of diphtheria toxin and mutants, which is applied in the field of purifying natural or mutant forms of diphtheria toxin, and can solve problems such as heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] Several chromatographic supports can be used in the preparation of HA columns, the most commonly used are type I and type II hydroxyapatite. Type I has a high protein binding capacity and better capacity for acidic proteins. Type II, however, has a lower protein binding capacity, but better resolution of nucleic acids and some proteins. The choice of a particular hydroxyapatite type can be determined by the skilled artisan.
[0084] A variety of hydroxyapatite chromatography resins are commercially available, and any available form of the material may be used in the practice of the present invention. In one embodiment of the invention the hydroxyapatite is in crystalline form. The hydroxyapatites used in the present invention may be those that are agglomerated to form particles and sintered at high temperature to form a stable porous ceramic body.
[0085] The particle size of hydroxyapatite can vary widely, but typical particle sizes range from 1 μm to 1000 μm in di...
Embodiment 1
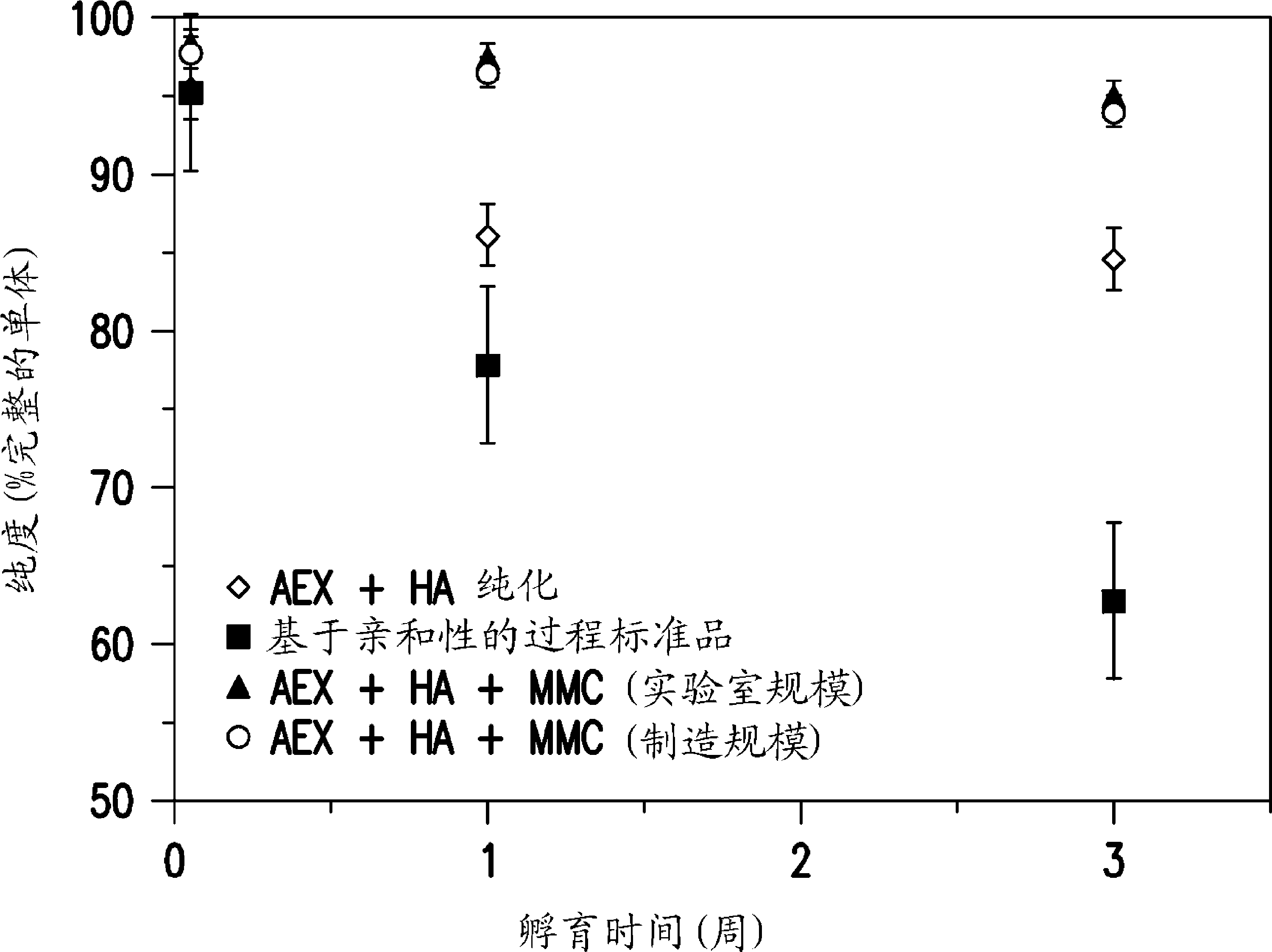
[0145] Example 1: Purification using hydroxyapatite chromatography
[0146] The suitability of hydroxyapatite chromatography was tested. A standard anion-exchange chromatography step was included prior to hydroxyapatite chromatography to increase protein purity to >90% and reduce endotoxins.
[0147] 200 L of fermentation broth was prepared as described above.
[0148] Cell Recovery and Harvesting
[0149] Recovery and concentration of Pseudomonas fluorescens was achieved using continuous centrifugation. The 200L fermentation batch was first cooled to 2 )) of Q / Σ. This step is run to harvest the cytoplasm while the centrate is directed to waste.
[0150] Temperature was controlled to maintain rotor (<8 °C) temperature during the harvest step. After each discharge, the harvested cell slurry was transferred to buckets.
[0151] Osmotic shock and flocculation
[0152] In this step, the CRM 197 Protein release from the periplasm of Pseudomonas fluorescens was achieved ...
Embodiment 2
[0164] Example 2: Commercial-scale purification using CAPTO-MMC™
[0165] Cell Recovery and Harvesting
[0166] Recovery and concentration of Pseudomonas fluorescens was achieved using continuous centrifugation. The 1300L fermentation batch was first cooled to 2 )) of Q / Σ. This step is run to harvest the cytoplasm while the centrate is directed to waste.
[0167] Temperature was controlled to maintain rotor (<8 °C) temperature during the harvest step. After each discharge, the harvested cell slurry was transferred to buckets.
[0168] Osmotic shock and flocculation
[0169] In this step, the CRM 197 Protein release from the periplasm of Pseudomonas fluorescens was achieved by osmotic shock, and flocculants were added to facilitate clarification. When adding resuspension buffer (50% w / v sucrose, 200 mM Tris, 100 mM EDTA, pH 7.5) to resuspend the harvested cell plasma, set agitation to generate vigorous mixing. The resuspended cells were then osmotically shocked by ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com