Patents
Literature
74 results about "Diphtheria vaccination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
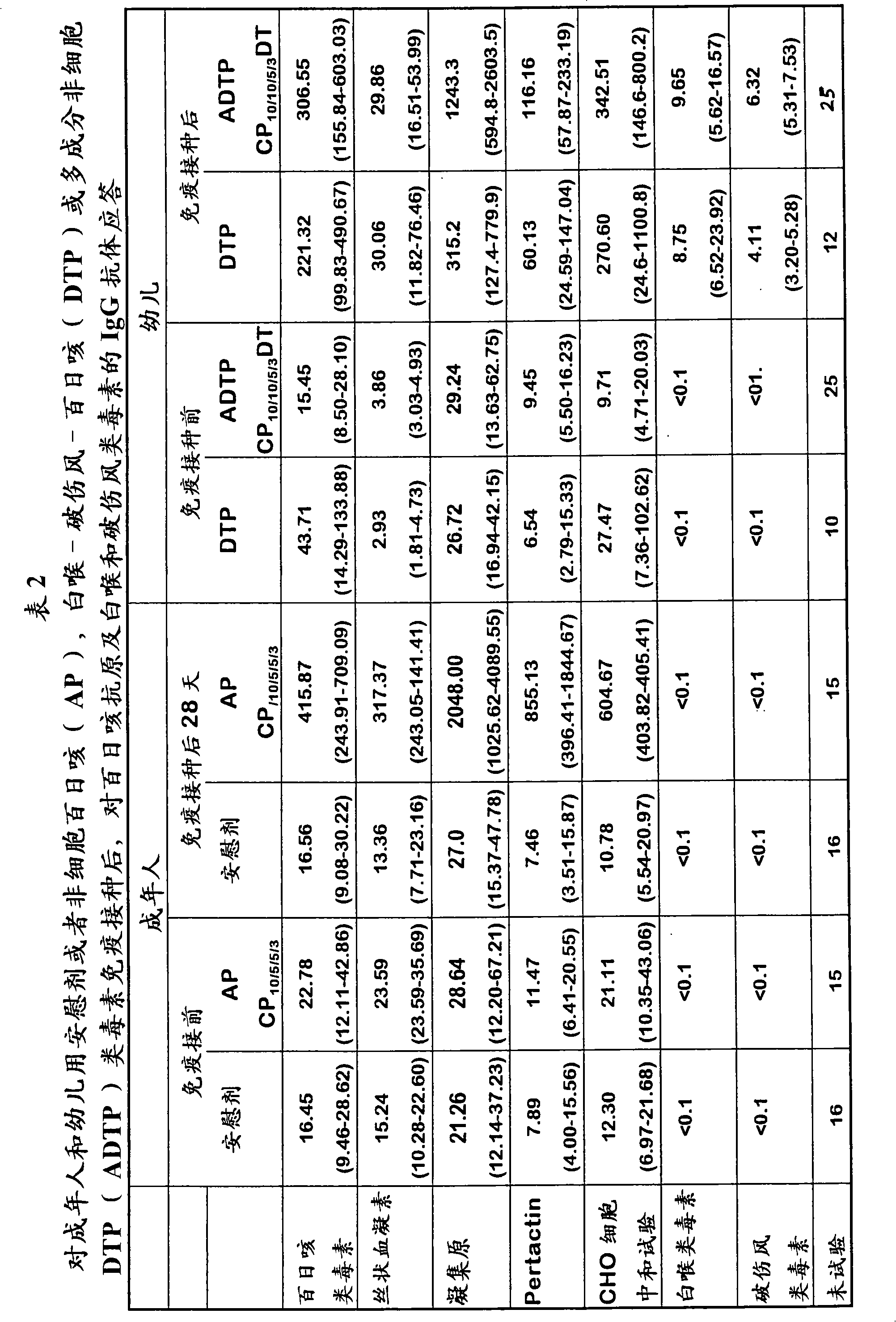
Quinvaxem is a widely administered pentavalent vaccine, which is a combination of five vaccines in one that protect babies from diphtheria, among other common childhood diseases. Diphtheria vaccine is usually combined at least with tetanus vaccine (Td) and often with pertussis (DTP, DTaP, TdaP) vaccines, as well.
RNA cancer vaccines
PendingUS20190351040A1Balanced immune responseOrganic active ingredientsAntibody ingredientsTetanusAdjuvant
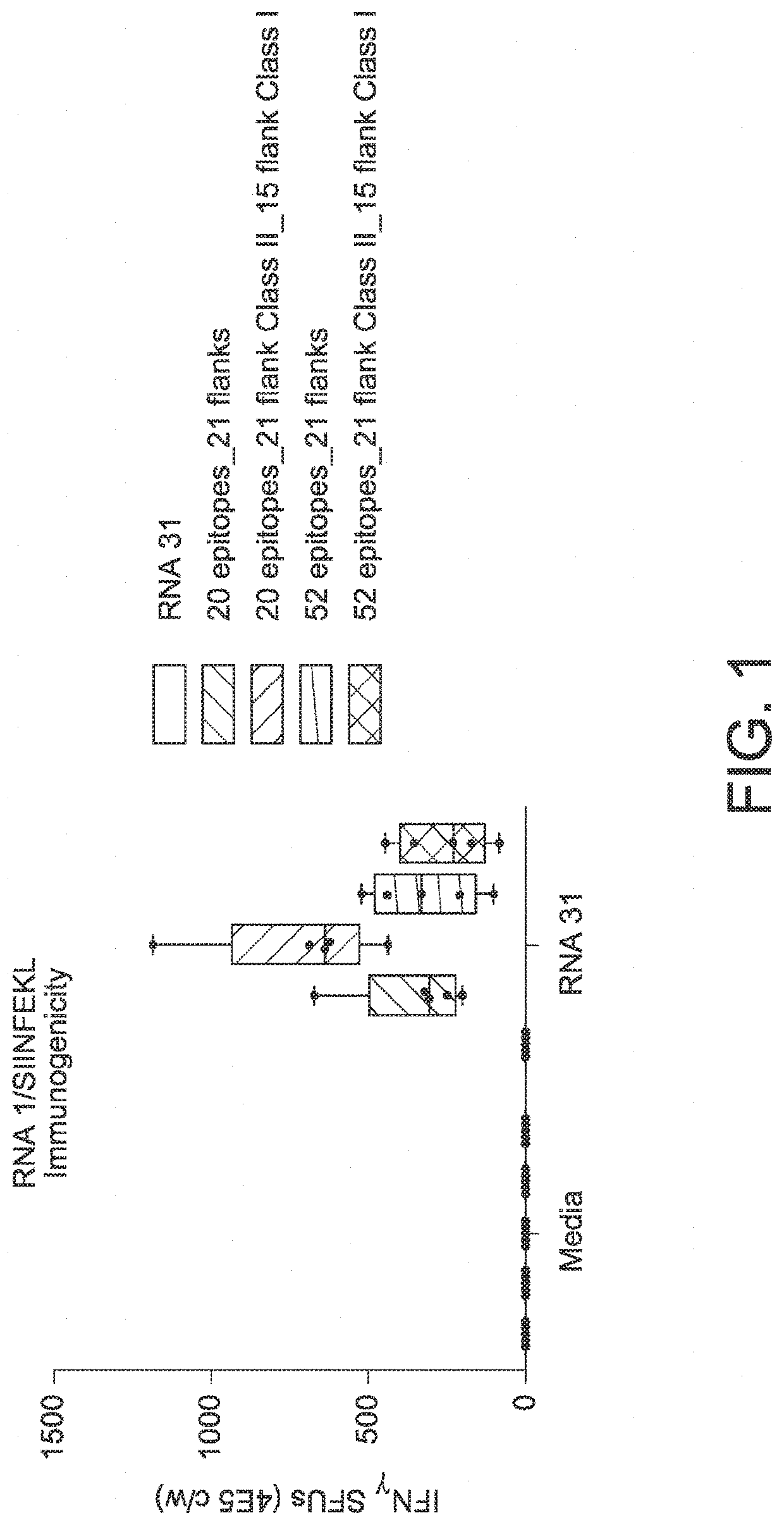
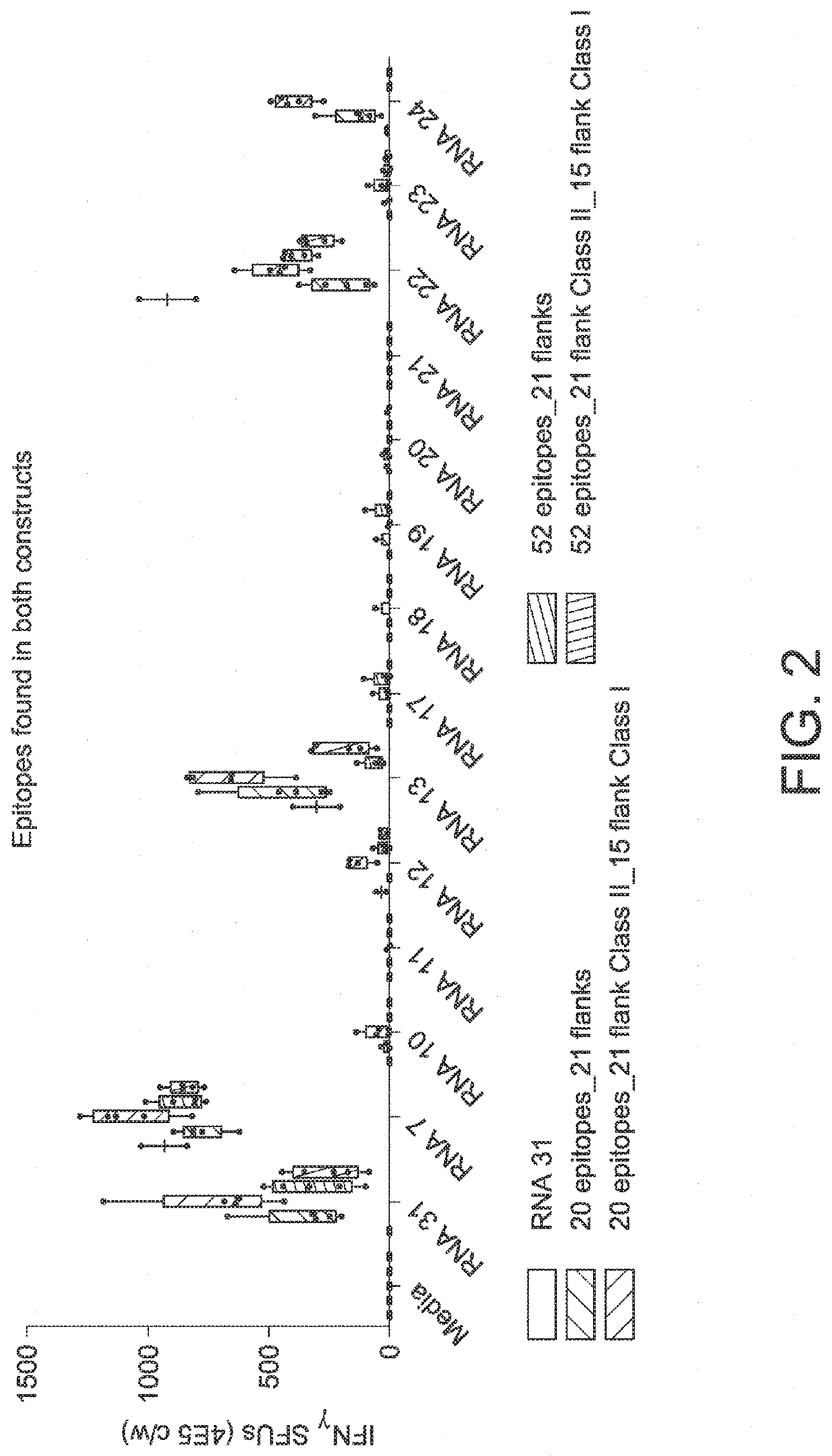
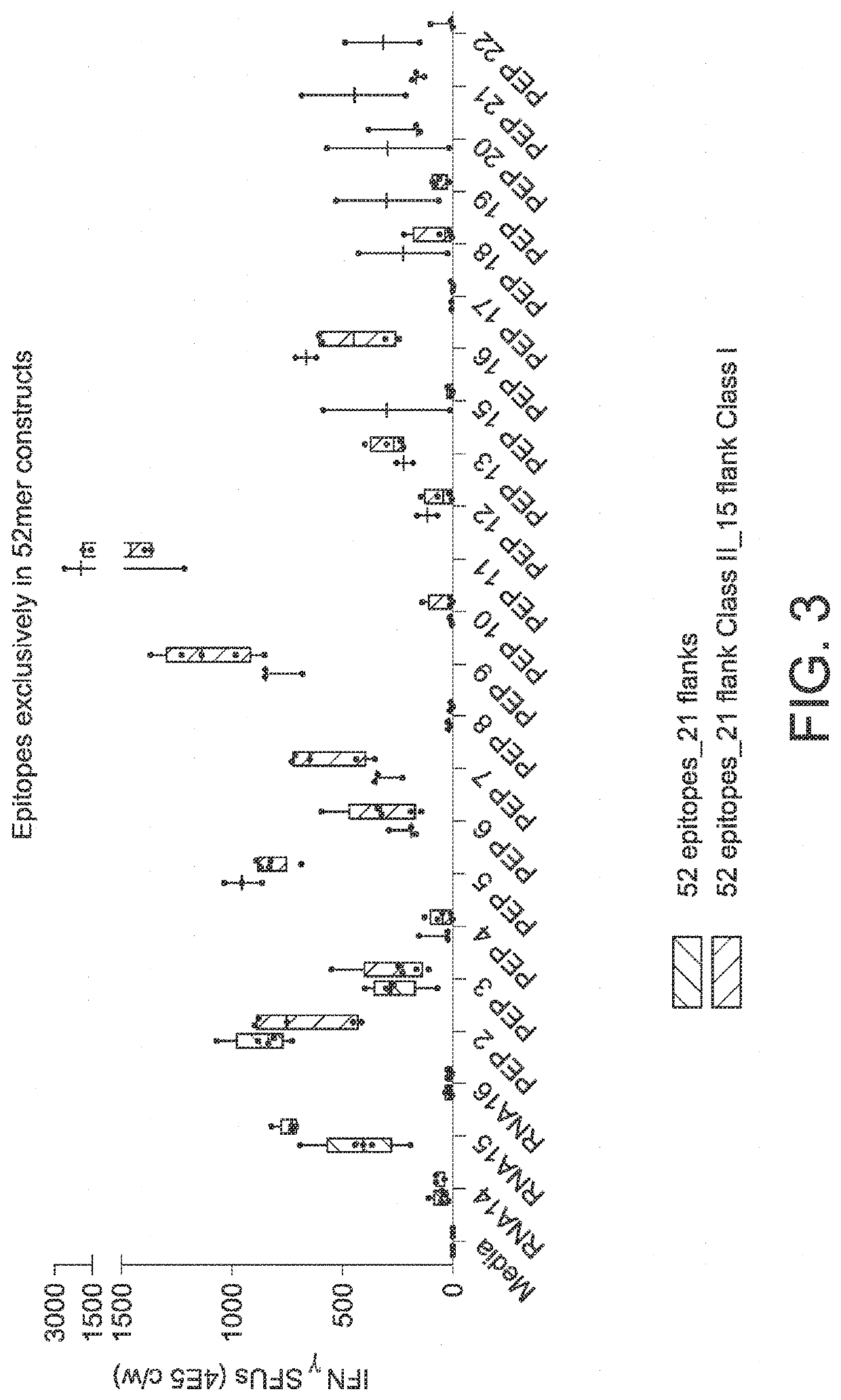
The disclosure relates to cancer ribonucleic acid (RNA) vaccines, as well as methods of using the vaccines and compositions comprising the vaccines. In particular, the disclosure relates to concatemeric mRNA cancer vaccines encoding several cancer epitopes on a single mRNA construct, i.e. poly-epitope mRNA constructs or poly-neo-epitope constructs. The disclosure further relates to p53 and KRAS mutations, as well as incorporation of immune enhancers such as STING, e.g. mRNA constructs further encoding an immune stimulator or adjuvant. The disclosure further relates to inclusion of universal T cell epitopes, such as tetanus or diphtheria toxins to elicit an enhanced immune response.
Owner:MODERNATX INC
Modified diphtheria toxins
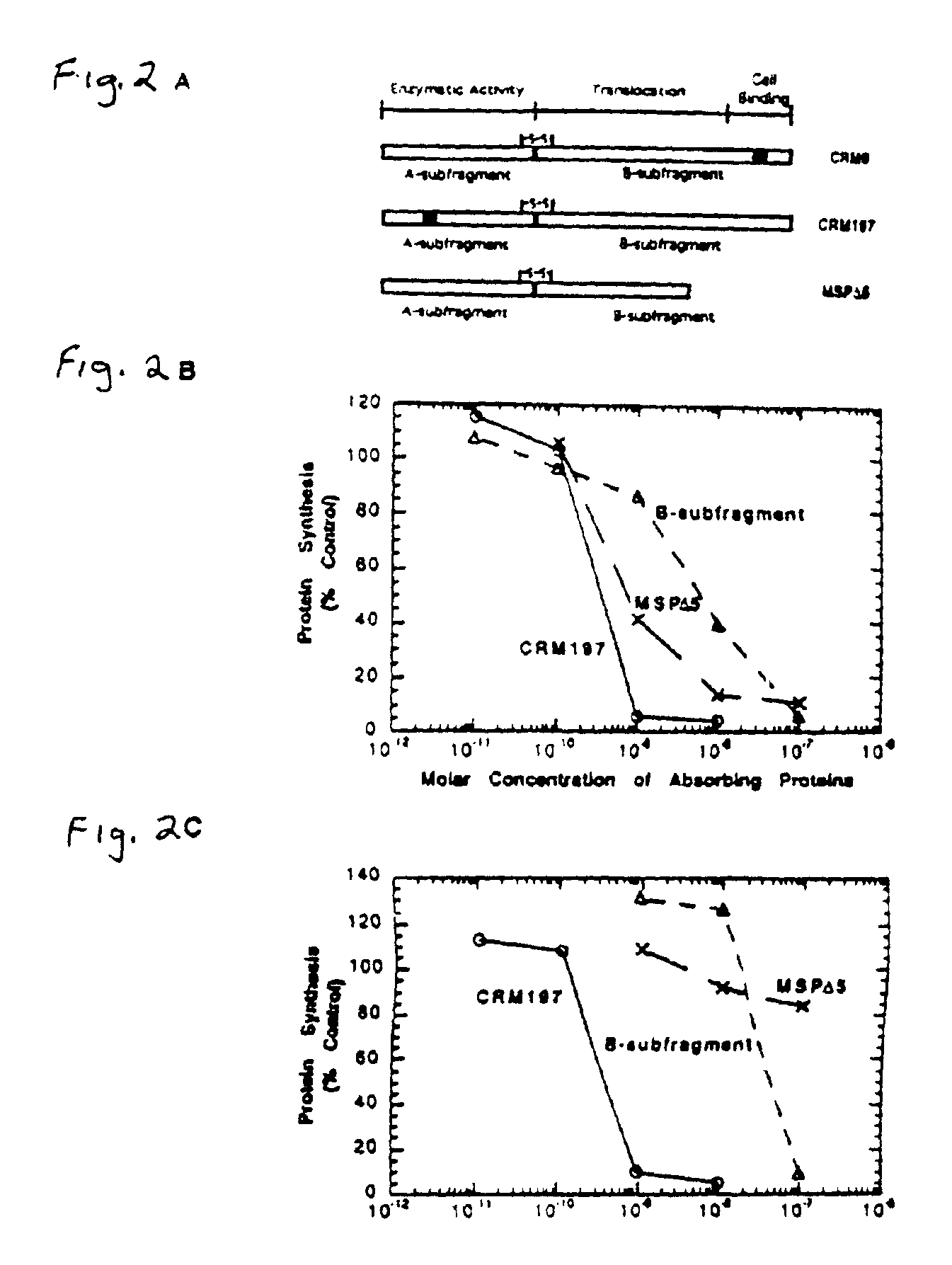
InactiveUS20090010966A1High anticancer activityReduced binding activityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseCell binding
The present application relates to compositions of modified diphtheria toxin and fusion proteins containing modified diphtheria toxin that reduce binding to vascular endothelium or vascular endothelial cells, and therefore, reduce the incidence of Vascular Leak Syndrome, as well as methods of making the compositions. The present application also relates to a polypeptide toxophore from a modified diphtheria toxin, where the modification is at least one amino acid residue at the amino acid residues 6-8, 28-30 or 289-291 of an unmodified native diphtheria toxin. Also described are fusion proteins which contain a modified diphtheria toxin and a non-diphtheria toxin fragment which contains a cell binding portion. The modified diphtheria toxins described can be used for the treatment of a malignant disease or a non-malignant disease.
Owner:ANGELICA THERAPEUTICS
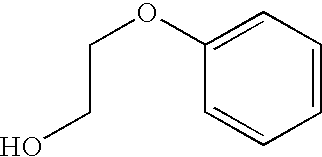
Combination vaccines with 1-hydroxy-2-phenoxyethane preservative
Processes for preparing combination vaccines that include diphtheria and tetanus toxoids, where these two toxoids are used in the processes as a single component containing both toxoids, and also containing 1-hydroxy-2-phenoxyethane.
Owner:NOVARTIS AG
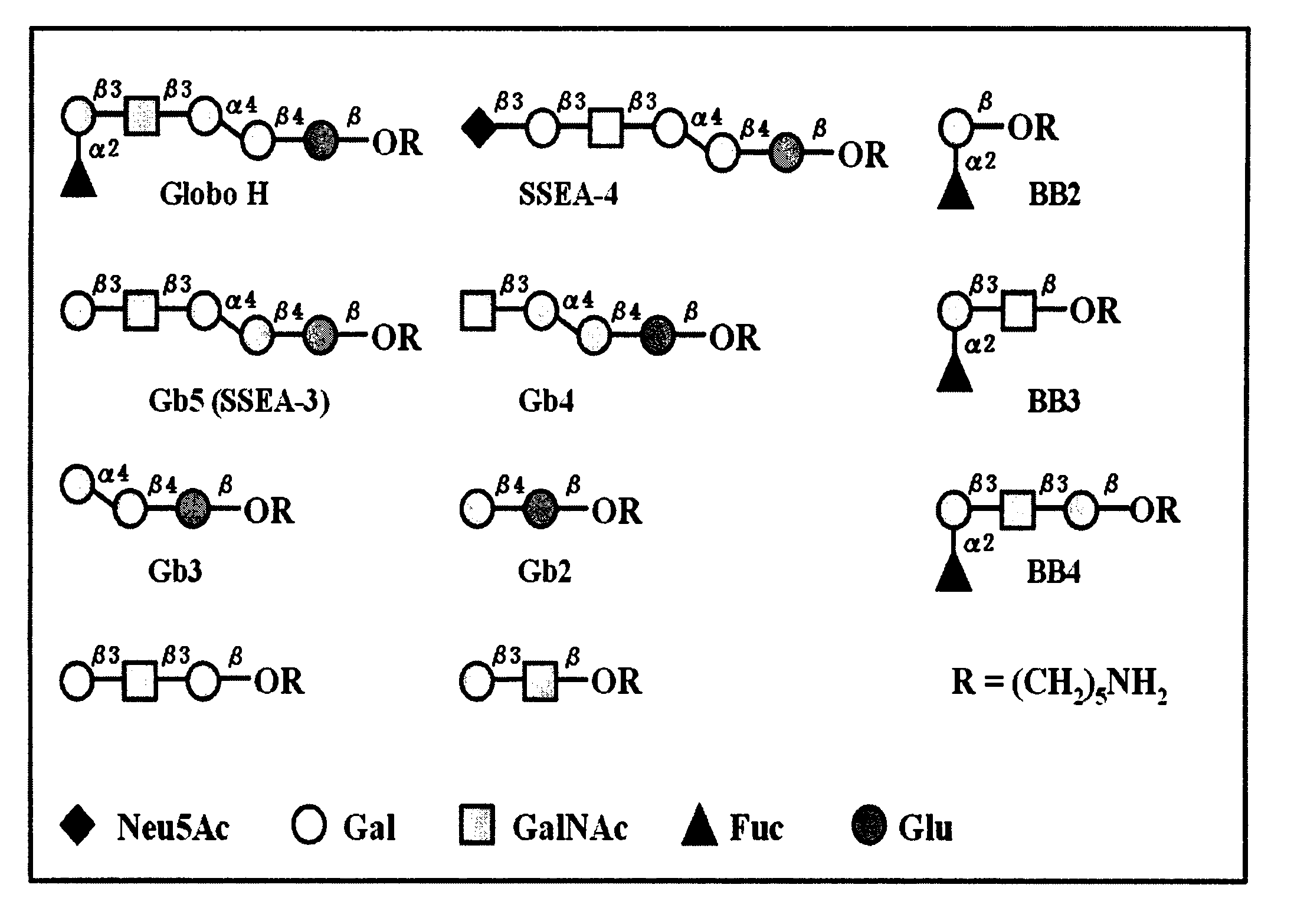
Globo h and related anti-cancer vaccines with novel glycolipid adjuvants
Immunogenic compositions, cancer vaccines and methods for treating cancer are provided. Compositions comprising: (a) a glycan such as Globo H or an immunogenic fragment thereof, wherein the glycan is conjugated with a carrier protein by a linker such as para-nitrophenyl; and (b) an adjuvant comprising glycolipid capable of binding CDId on a dendritic cell, such as an a-galactosyl-ceramide derivative, wherein the immunogenic composition induces an immune response that induces a higher relative level of IgG isotype antibodies as compared to IgM isotype antibodies, are provided. Immunogenic compositions comprising the carrier protein diphtheria toxin cross-reacting material 197 (DT-CRM 197) and the adjuvant C34 are provided. Antibodies generated by immunogenic compositions disclosed herein further neutralize at least one of the antigens Globo H, stage-specific embryonic antigen-3 (SSEA-3) and stage-specific embryonic antigen-4 (SSEA-4). Therapeutics against breast cancer stem cells comprising immunogenic compositions comprising Globo H, SSEA-3 or SSEA-4 conjugated with DT-CRM 197.
Owner:ACAD SINIC
Multivalent DTP-POLIO vaccines
InactiveCN101310769AEffective preventionSsRNA viruses negative-senseAntibacterial agentsTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
Interfusion protein between diphtheria toxin and GM CSF mutant, coded gene and application
This invention discloses fusion protein of diphtherin and GM-CSF mutant, its coding gene, and its application. The fusion protein is selected from: (a) the protein shown in SEQ ID No.2; (b) the protein derived from SEQ ID No.2 by substituting, deleting and / or adding one or more amino acid residues, which can kill acute myeloid leukemia cells. The fusion protein can kill target cells, and has a high expression level.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Integration of meningococcal conjugate vaccination
ActiveUS20090060945A1Easy to useAvoid carrier suppressionAntibacterial agentsCarrier-bound antigen/hapten ingredientsDiphtheria vaccinationCarrier protein
Conjugated meningococcal capsular saccharides will be introduced into immunisation schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunising a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunised with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine
InactiveUS20100040647A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseDiseaseTetanus
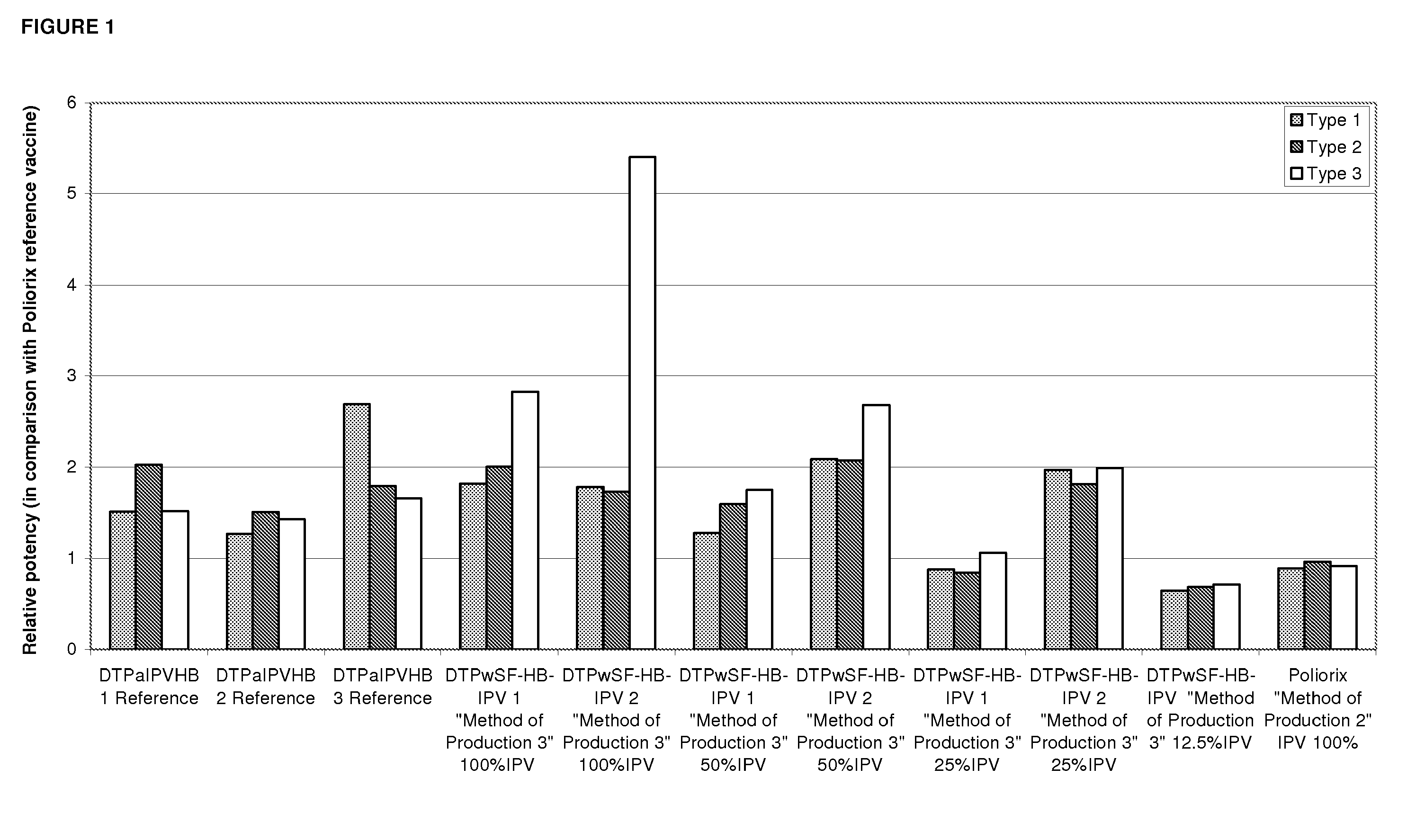
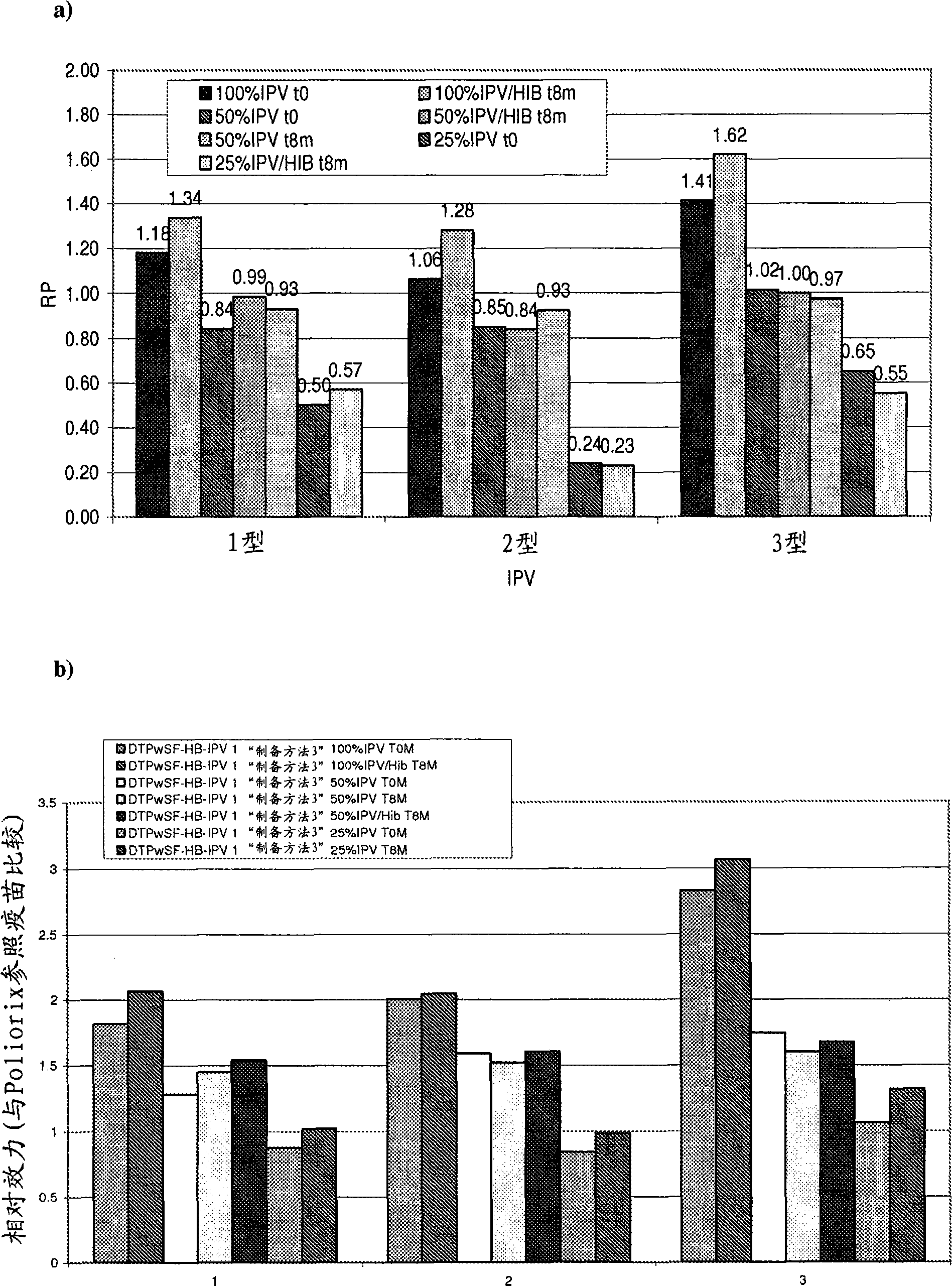
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus are provided
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
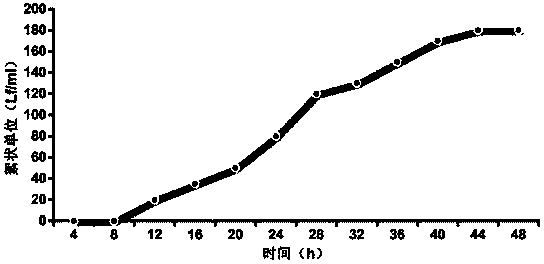
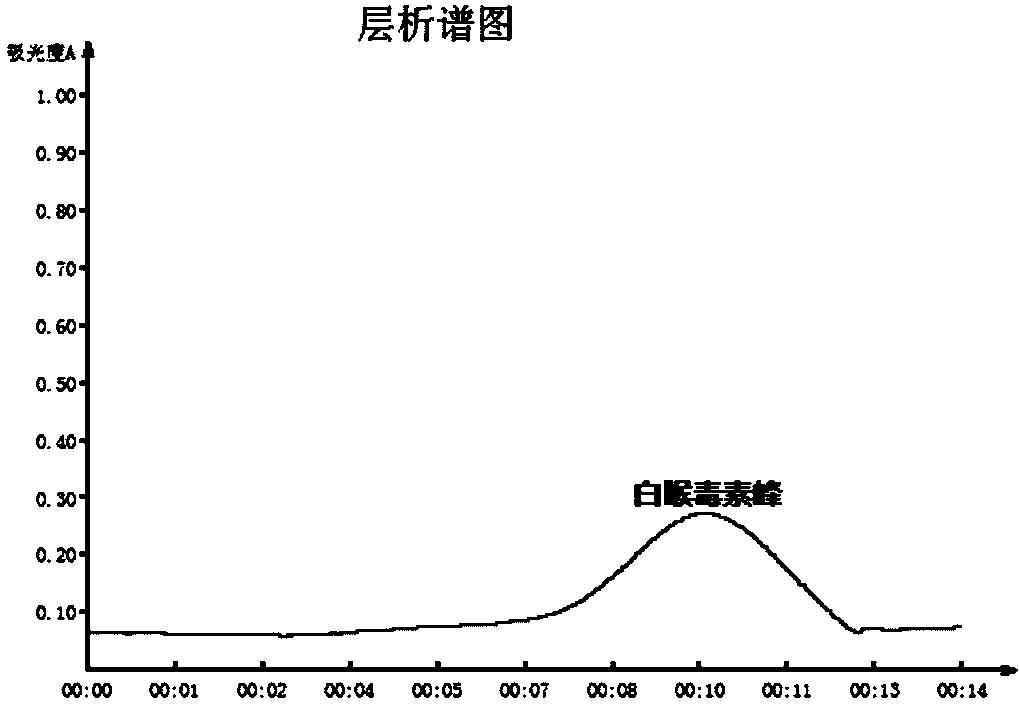
Technique for improving purity of diphtheria toxoid
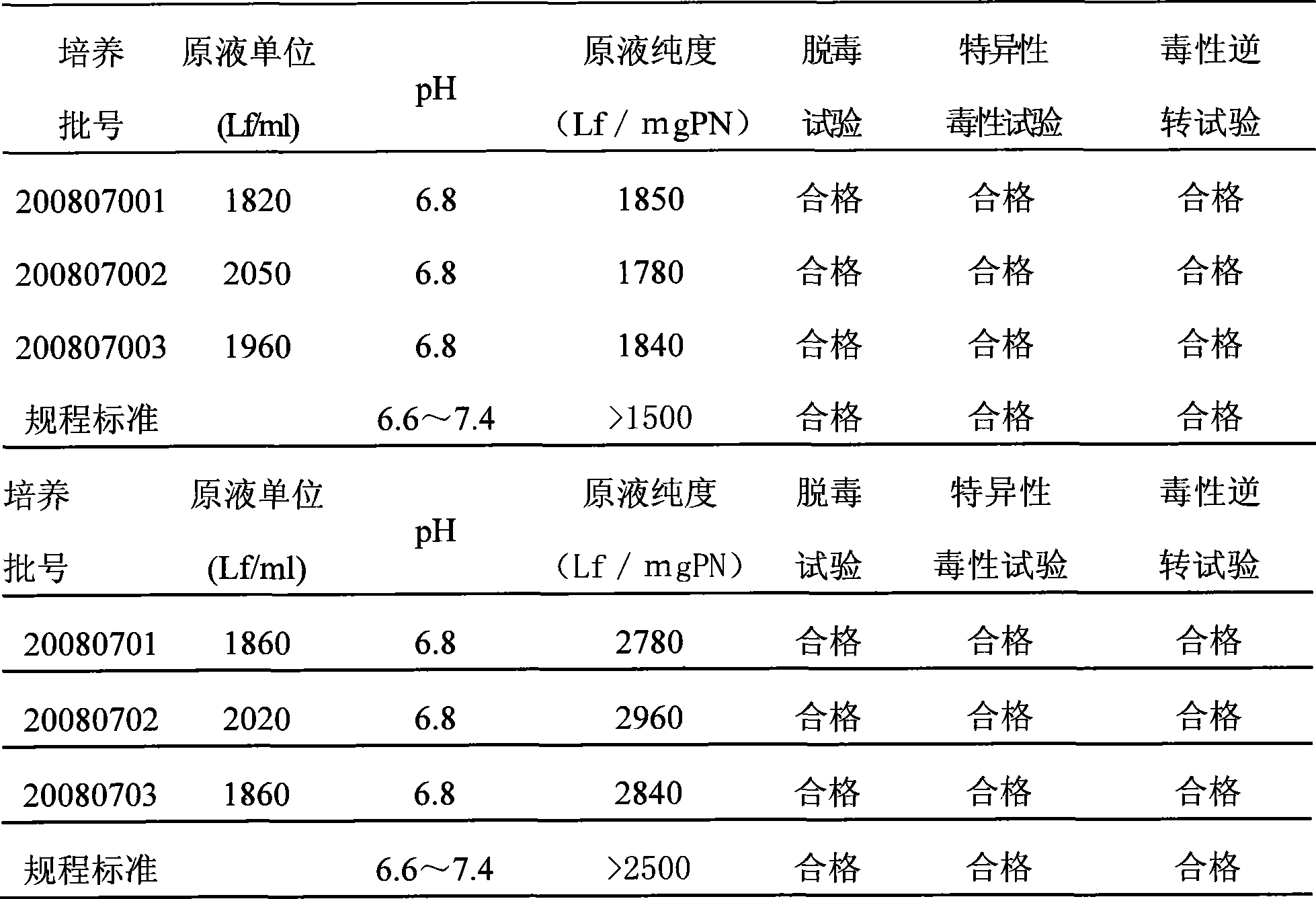
ActiveCN101503725AHigh purityReasonable process routeAntibacterial agentsDepsipeptidesLiquid productUltrafiltration
The invention relates to a process for improving the purity of diphtheria toxin. The manufacture process comprises the following steps of solid culture medium transfer, culture in a liquid culture medium fermentor, removal of thalli by centrifugation, primary salting-out, secondary salting-out, ultrafiltration desalting, gel filtration, aseptic filtration, removal of toxin, detection and storage stock solution, preparation of semi-finished products, finished products, wherein the liquid culture medium in the liquid culture medium fermentor is treated to remove proteins with the molecular weight of more than 50,000, and the culture products are almost diphtheria toxin with the molecular weight of more than 50,000; and by salting out and gel filtration, the proteins and other impurity proteins of which the molecular weight is less than 50,000 in the liquid product are further removed, so that the diphtheria toxin with high purity is obtained.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
Combination vaccines with low dose of hib conjugate
InactiveUS20090208526A1Facilitates correct measurementImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsTetanusDiphtheria vaccination
Provided herein are combination vaccines comprising antigens for protecting a subject against at least diphtheria, tetanus, pertussis and Hib, wherein: (a) the antigen for protecting against Hib is a conjugate of a Hib capsular saccharide; (b) the concentration of the Hib conjugate in the vaccine is <15 μg / ml; and (c) the Hib conjugate has never been lyophilised.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multi-component vaccine comprising at least three antigens to protect against disease cased by Haemophilus influenzae
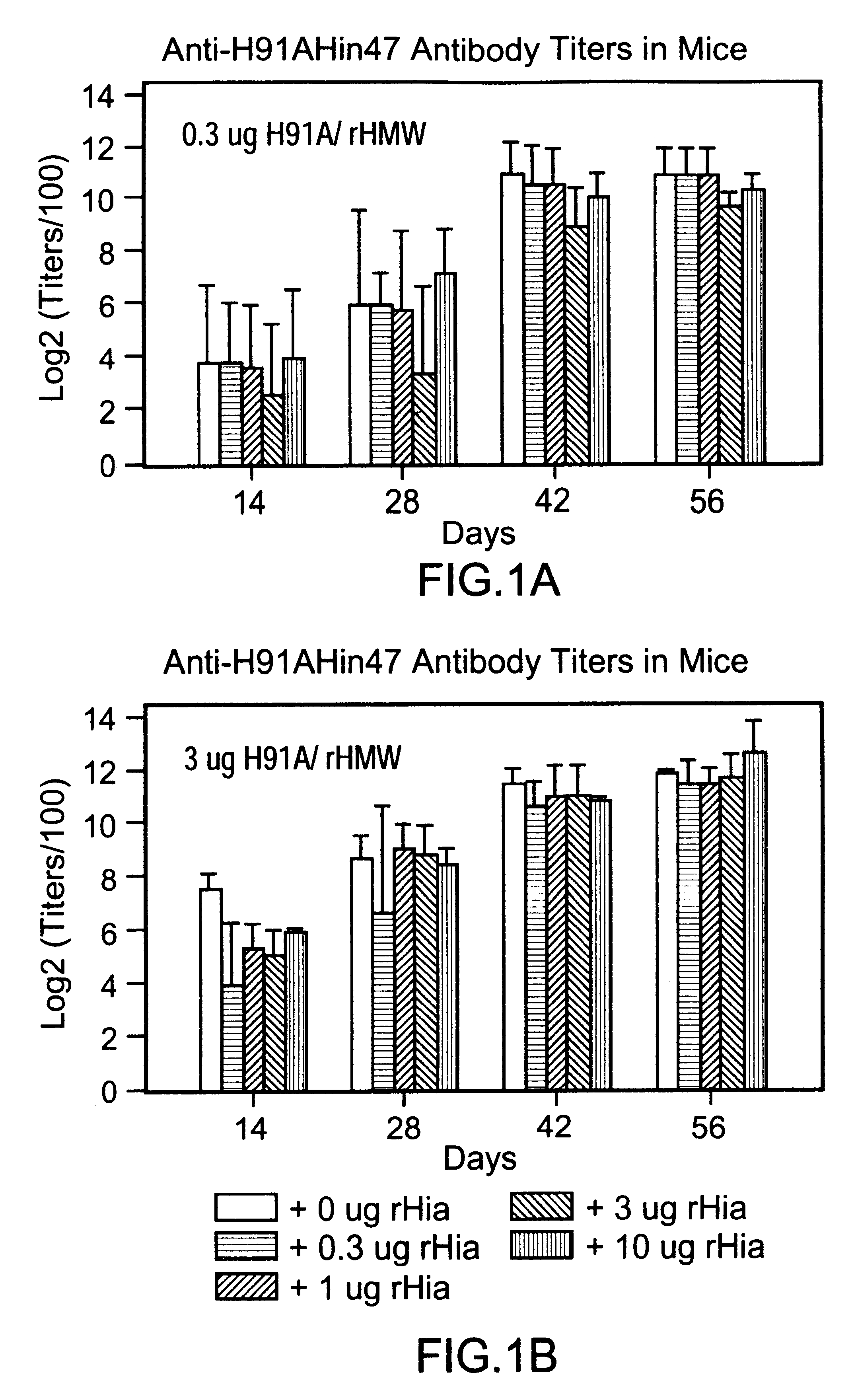
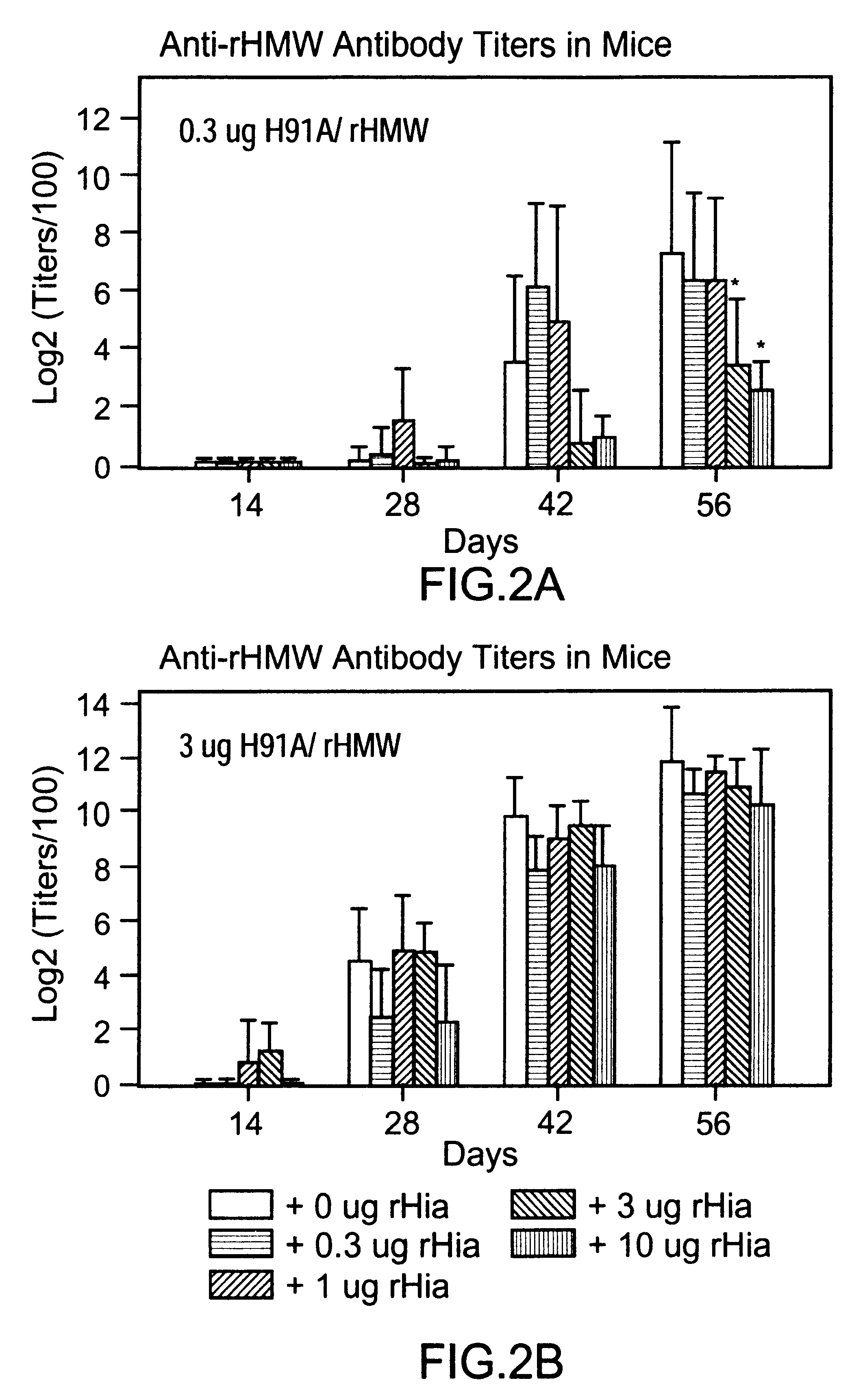
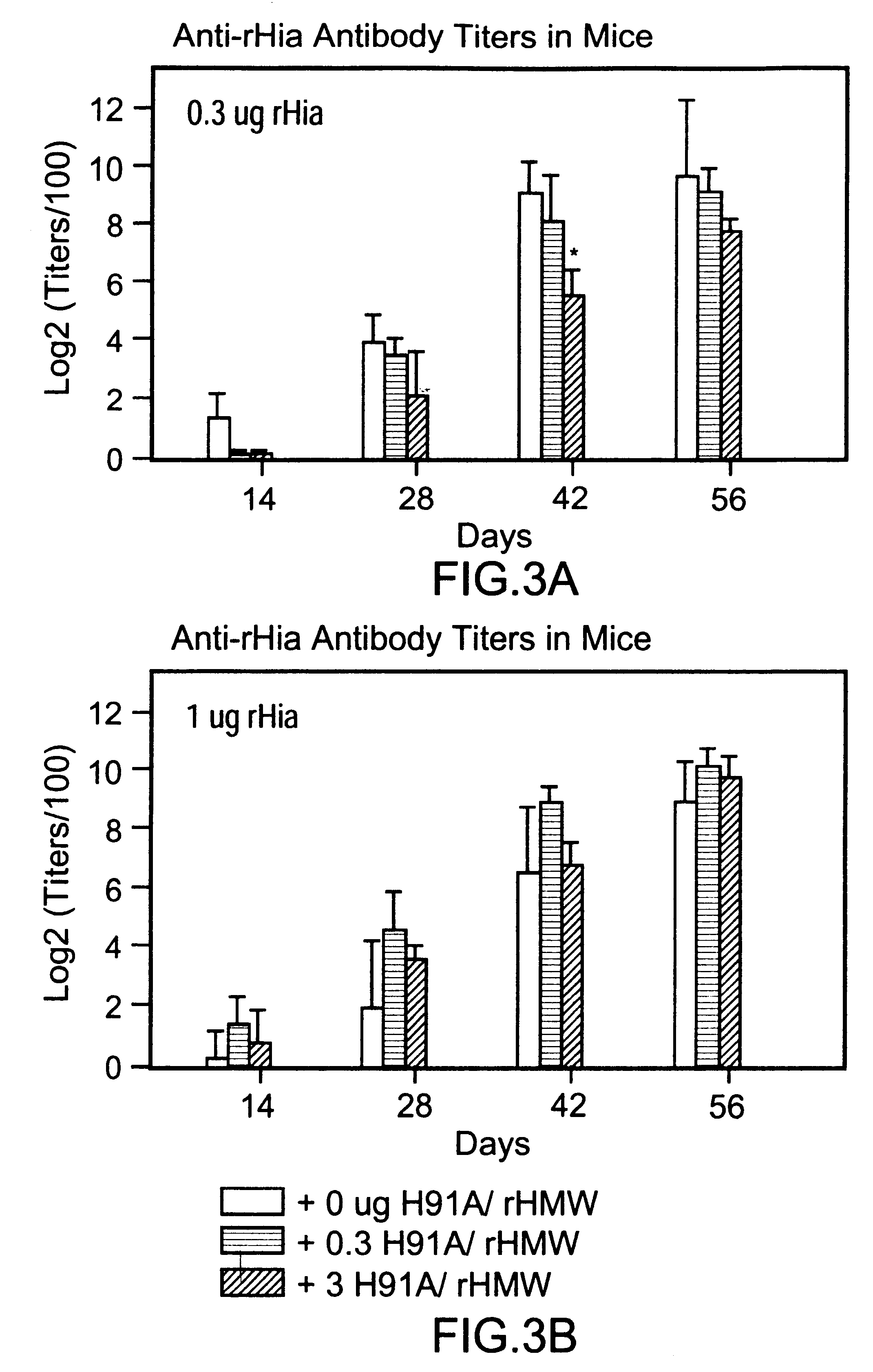
InactiveUS6342232B1No suppression of anti-rHMW responseStimulate immune responseAntibacterial agentsSenses disorderProteolysisDiphtheria vaccination
A multi-component immunogenic composition confers protection on an immunized host against infection caused by Haemophilus influenzae. Such composition comprises at least three different antigens of Haemophilus influenzae, two of which are adhesins. High molecular weight (HMW) proteins and Haemophilus influenzae adhesin (Hia) proteins of non-typeable Haemophilus influenzae comprise the adhesin components while the other antigen is a non-proteolytic analog of Hin47 protein. Each component does not impair the immunogenicity of the others. The Haemophilus vaccine may be combined with DTP component vaccines, which may contain inactivated poliovirus, including type 1, type 2 and / or type 3, and / or a conjugate of a capsular polysaccharide of Haemophilus influenzae and tetanus or diphtheria toxoid, including PRP-T, to provide a multi-valent component vaccine without impairment of the immunogenic properties of the other antigens.
Owner:AVENTIS PASTUER LTD
Throat powder formula for treating throat and oral diseases
InactiveCN1748733AEasy to prepareGood treatment effectInorganic boron active ingredientsHydroxy compound active ingredientsDiseaseOral disease
The medicine for treating throat and oral cavity diseases has the recipe comprising eight kinds of Chinese medicinal materials, including natural indigo, pearl, musk, borneol, potassium nitrate, catechu, etc. in certain weight proportion. The medicine has the functions of clearing away heat and toxic material, removing blood stasis, dissipating mass, eliminating swelling, relieving pain and astringing. It is used in treating swollen and rigid tongue, double tongue, tonsillitis, diphtheria, swelling and sore throat and other throat and oral cavity diseases, and has simple preparation process, high treating effect, fast acting and no toxic side effect.
Owner:谢焕青
Production of diphtheria toxin
InactiveCN1894399AAntibacterial agentsBacterial antigen ingredientsNitrogen sourceDiphtheria vaccination
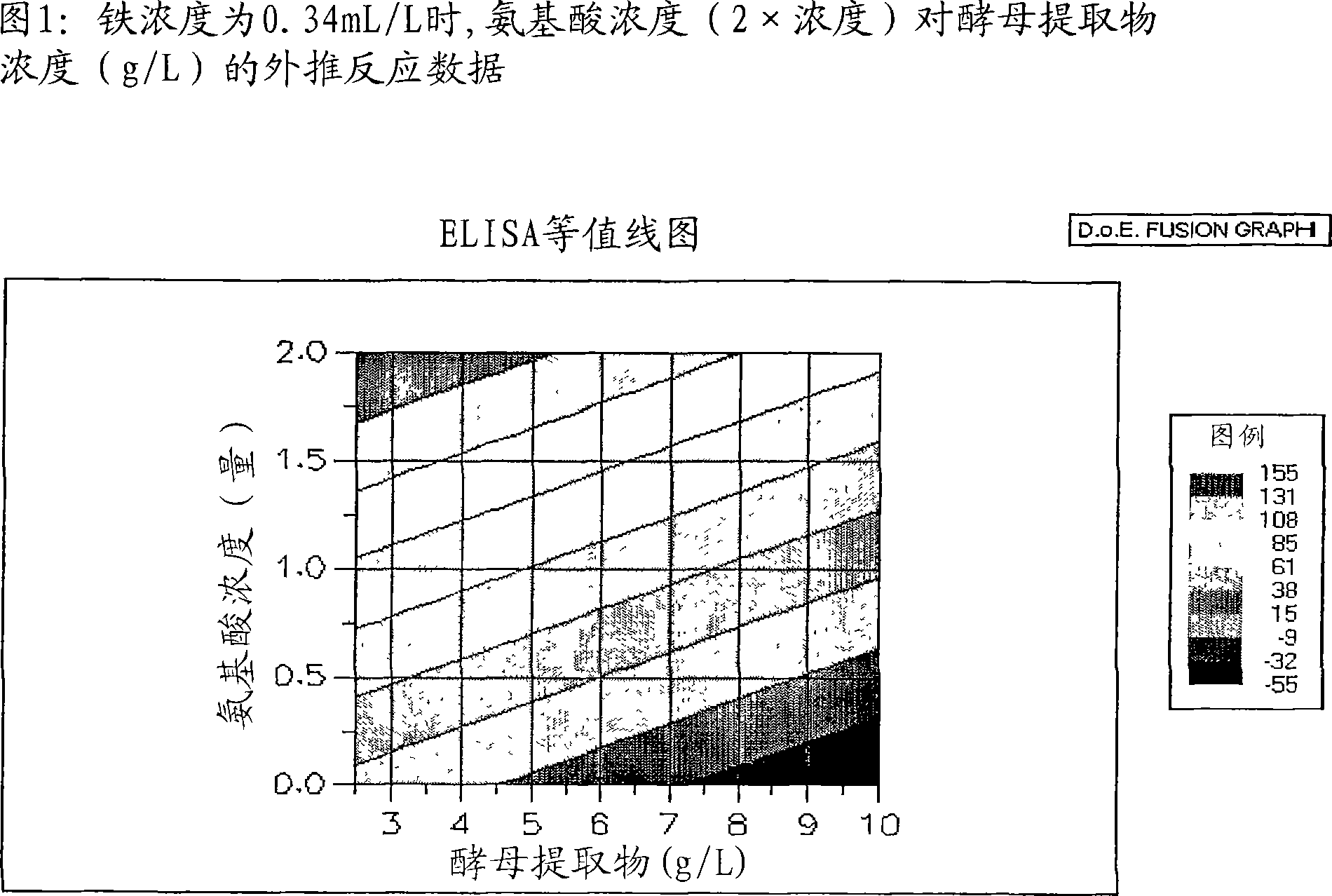
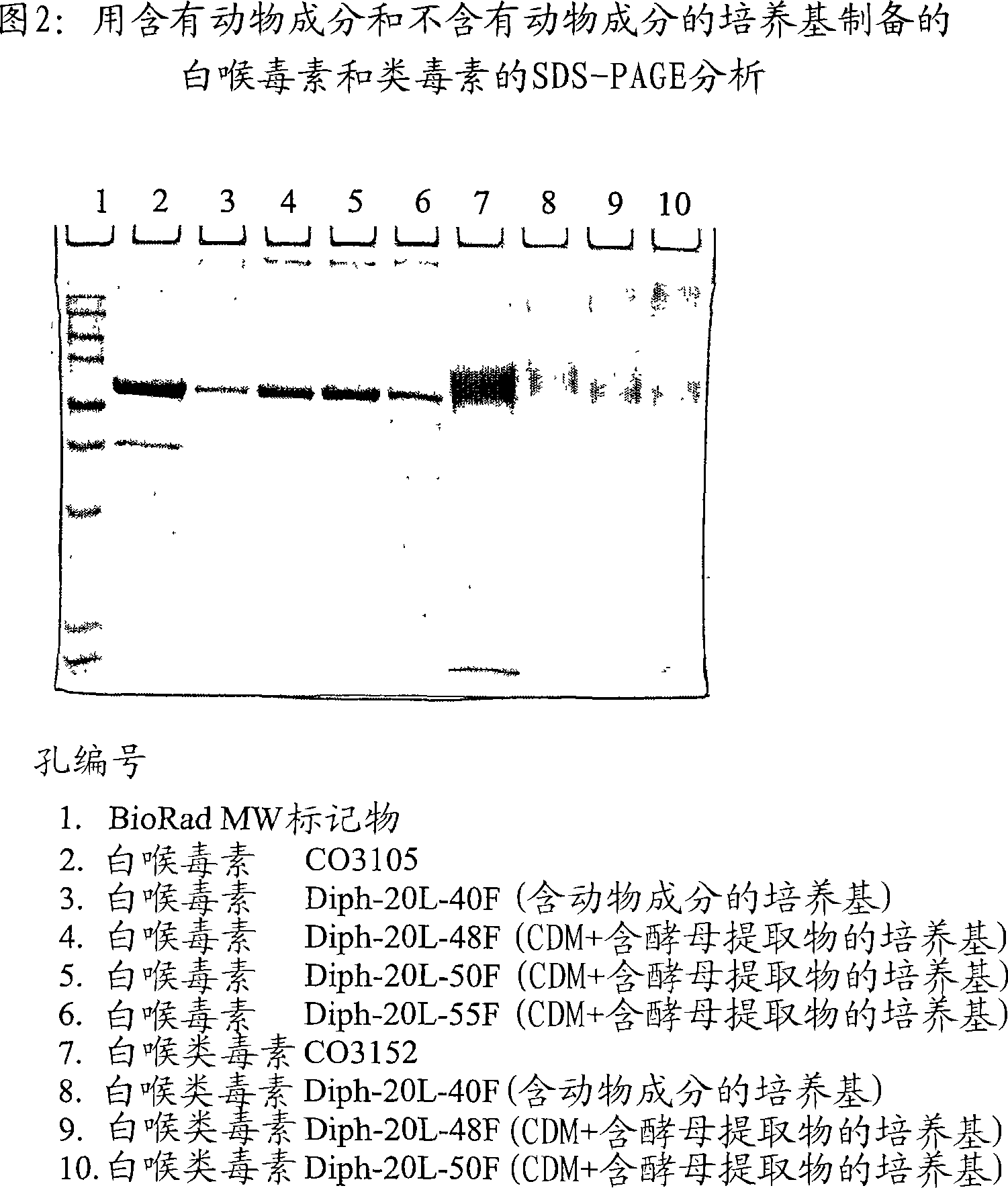
A Corynebacterium diphtheriae culture medium for the production of diphtheria toxin and methods for producing the toxin are provided. The medium is substantiallty free of animal-derived products and comprises water, a carbohydrate source, a nitrogen source and a number of free amino acids in an initial concentration wherein the initial concentration of each free amino acid is not limiting for the production of the toxin.
Owner:圣诺菲·帕斯图尔公司
Pastevula mulfocida capsular polysaccharide-protein conjugate vaccine and preparation method thereof
ActiveCN108721616AIncrease virulencePlay the role of cross protectionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineHeterologous
Belonging to the technical field of biology, the invention in particular relates to a pastevula mulfocida capsular polysaccharide-protein conjugate vaccine and a preparation method thereof. The pastevula mulfocida capsular polysaccharide-protein conjugate vaccine provided by the invention is formed by connection of pastevula mulfocida capsular polysaccharide and diphtheria toxoid carrier protein through nano-microspheres. The combination of pastevula mulfocida capsular polysaccharide with the carrier protein diphtheria toxoid enhances the immune efficacy of the vaccine, and also reaches a cross protection effect on the infection of homologous strains and heterologous strains. The preparation method of the pastevula mulfocida capsular polysaccharide-protein conjugate vaccine provided by theinvention has the characteristics of simple operation and cost saving, and has good application prospect.
Owner:广州渔跃生物技术有限公司
Acellular pertussis combined vaccine and preparation method thereof
InactiveCN104707134AClear ingredientsLittle side effectsAntibacterial agentsBacterial antigen ingredientsHemagglutininSide effect
The invention discloses an acellular pertussis combined vaccine and a preparation method thereof and belongs to the technical field of production and preparation of vaccines. The acellular pertussis combined vaccine is prepared from the following raw material components: 5-40Mu g of pertussis toxin, 5-40Mu g of filamentous hemagglutinin, 2-10Mu g of pertussis adhesin, 10-25lf of diphtheria toxoid, 4-10lf of tetanus toxoid, 1.0-2.0mg / ml of aluminium hydroxide and 7.5-9.5g / L of sodium chloride. The preparation method of the acellular pertussis combined vaccine comprises the following steps: preparing monovalent vaccine original fluids, mixing and diluting. The acellular pertussis combined vaccine is clear and definite in ingredients, quality control can be easily realized, the side effect is small, and the safety is high; and the preparation method of the acellular pertussis combined vaccine is simple to operate, convenient in preparation and low in cost, so that the acellular pertussis combined vaccine is applicable to industrial mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
METHOD OF PRODUCING MENINGOCOCCAL MENINGITIS VACCINE FOR NEISSERIA MENINGITIDIS SEROTYPES A, C, Y, and W-135
InactiveUS20080318285A1Yield maximizationYield minimizationAntibacterial agentsBacteriaDiseaseSynechococcus
Owner:REDDY JEERI R
Preparation method of diphtheria vaccine
ActiveCN104027797AHigh purityImprove controllabilityAntibacterial agentsBacterial antigen ingredientsFiltrationUltrafiltration
The invention provides a preparation method of a diphtheria vaccine and aims at solving the problems of long production period of the diphtheria vaccine, ammonium sulfate pollution generated in production process, excessive impurity protein in the final product and over-large side reaction of the product in the prior art. The preparation method of the diphtheria vaccine is characterized by comprising the following steps of 1) carrying out resuscitation and fermentation cultivation of a corynebacterium diphtheriae strain, thereby preparing a diphtheria bacterial solution; 2) centrifuging the diphtheria bacterial solution, carrying out triple filtration treatment, and then carrying out ultrafiltration concentration, and dialysis and elution, thereby obtaining a diphtheria toxin; 3) detoxifying the diphtheria toxin, and then carrying out ultrafiltration concentration, and dialysis and elution by use of a tangential flow ultrafiltration system; 4) purifying the detoxified diphtheria toxin. High-speed centrifuging is used for removing bacteria, and ultrafiltration concentration and elution are used instead of adsorption by use of active carbon and salting out by use of ammonium sulfate, and the whole experimental steps can be finished by only one day, and therefore, the process steps are simplified, the production period is shortened, the production efficiency is improved and the controllability of the operation steps is improved.
Owner:SHANDONG YIDU BIOTECH
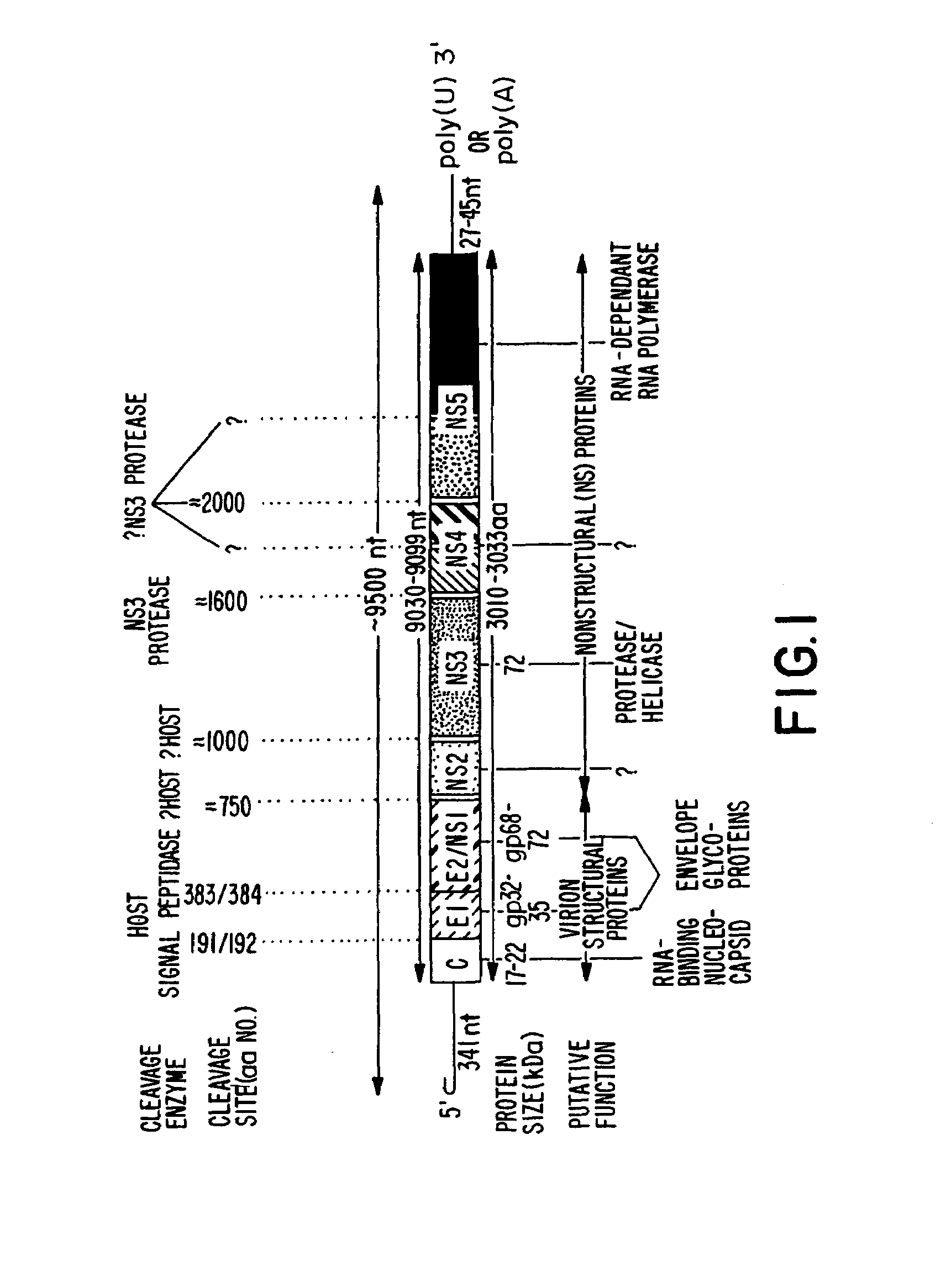
Conserved motif of hepatitis C virus E2/NS1 region
Immunogenic compositions comprising an immunogenic polypeptide and a pharmaceutically acceptable vehicle are described. The immunogenic polypeptide comprises the amino acid sequence Xaa-Thr-Xaa-Val-Thr-Gly-Gly-Xaa-Ala-Ala-Arg-Thr-Thr-Xaa-Gly-Xaa-Xaa-Ser-Leu-Phe-Xaa-Xaa-Gly-Xaa-Ser-Gln-Xaa-Ile-Gln-Leu-Ile (SEQ ID NO:8). The immunogenic polypeptide can be coupled to a pharmaceutically acceptable carrier, such as a diphtheria toxoid.
Owner:CHIRON CORP
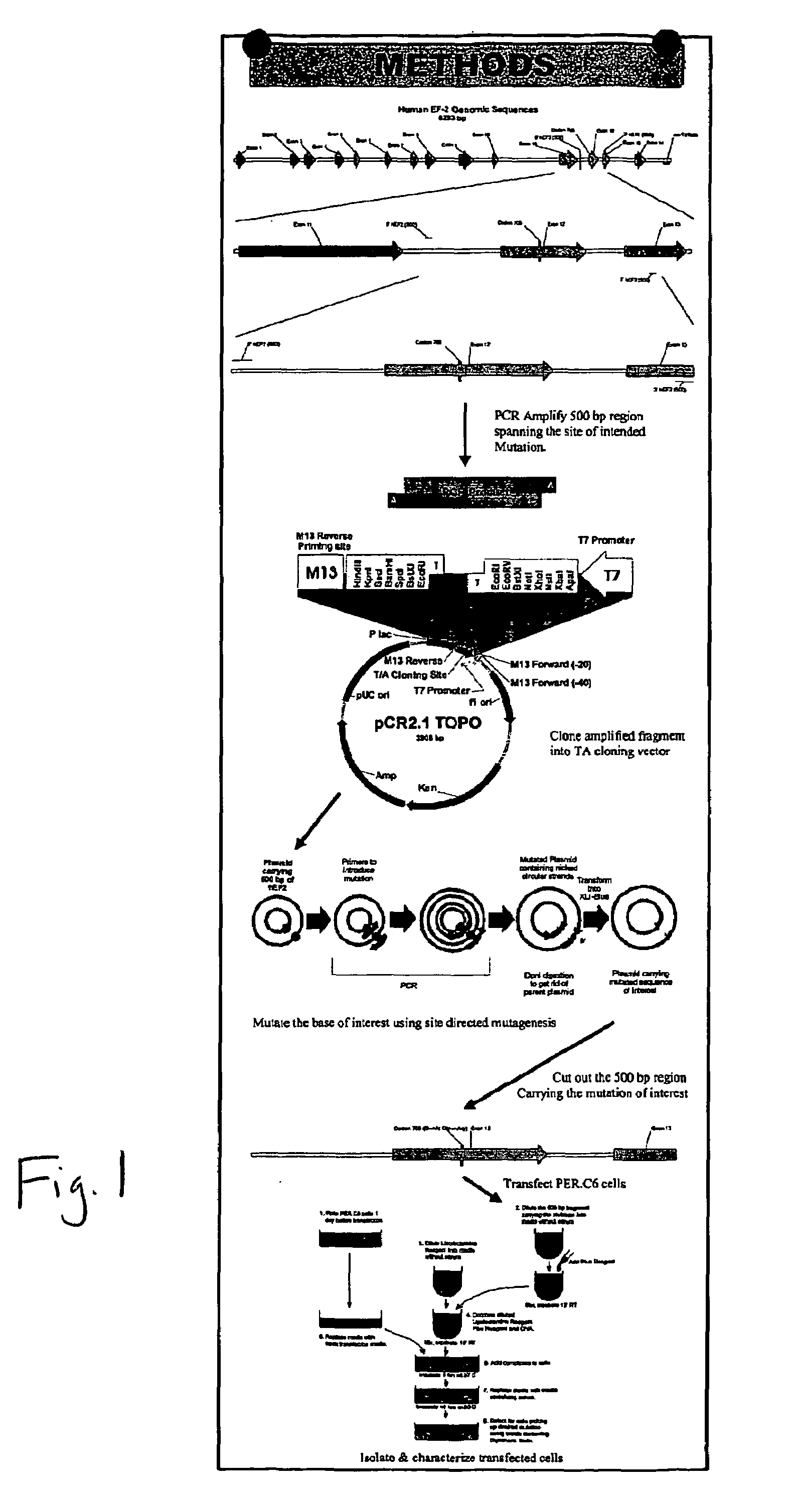
Packaging cell line for diphtheria toxin expressing non-replicating adenovirus
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Manufacturing Method of Combined Vaccine
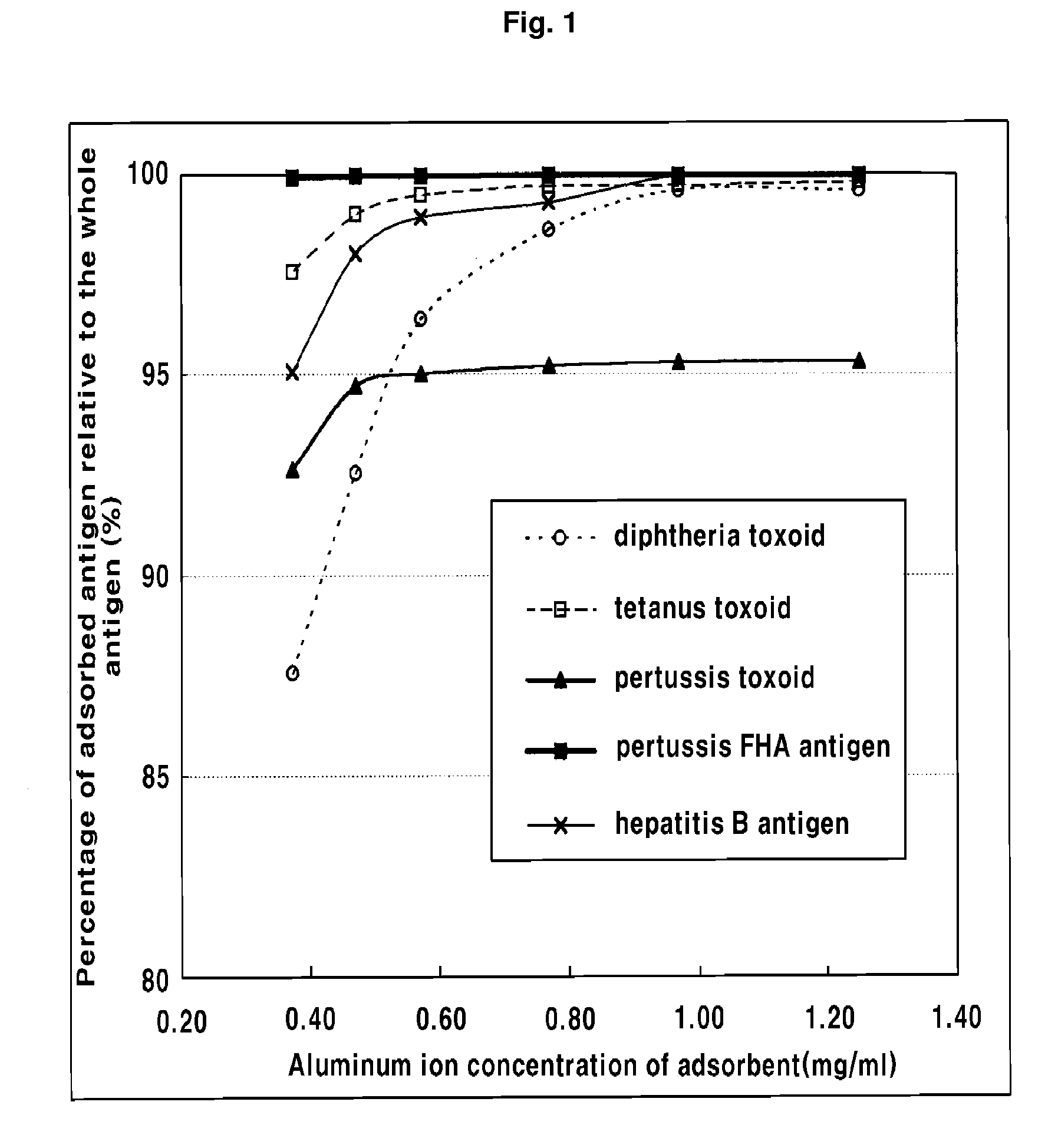
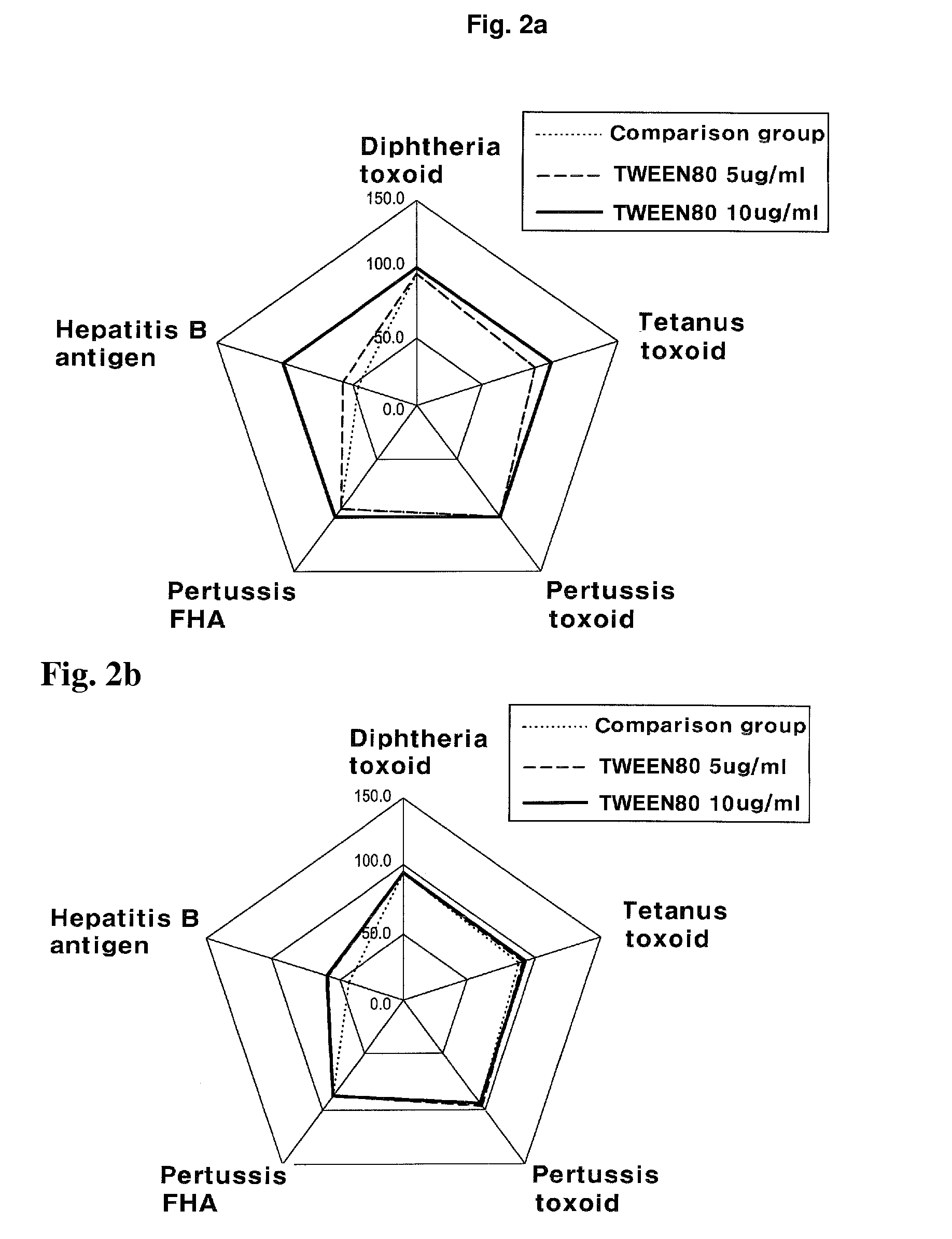
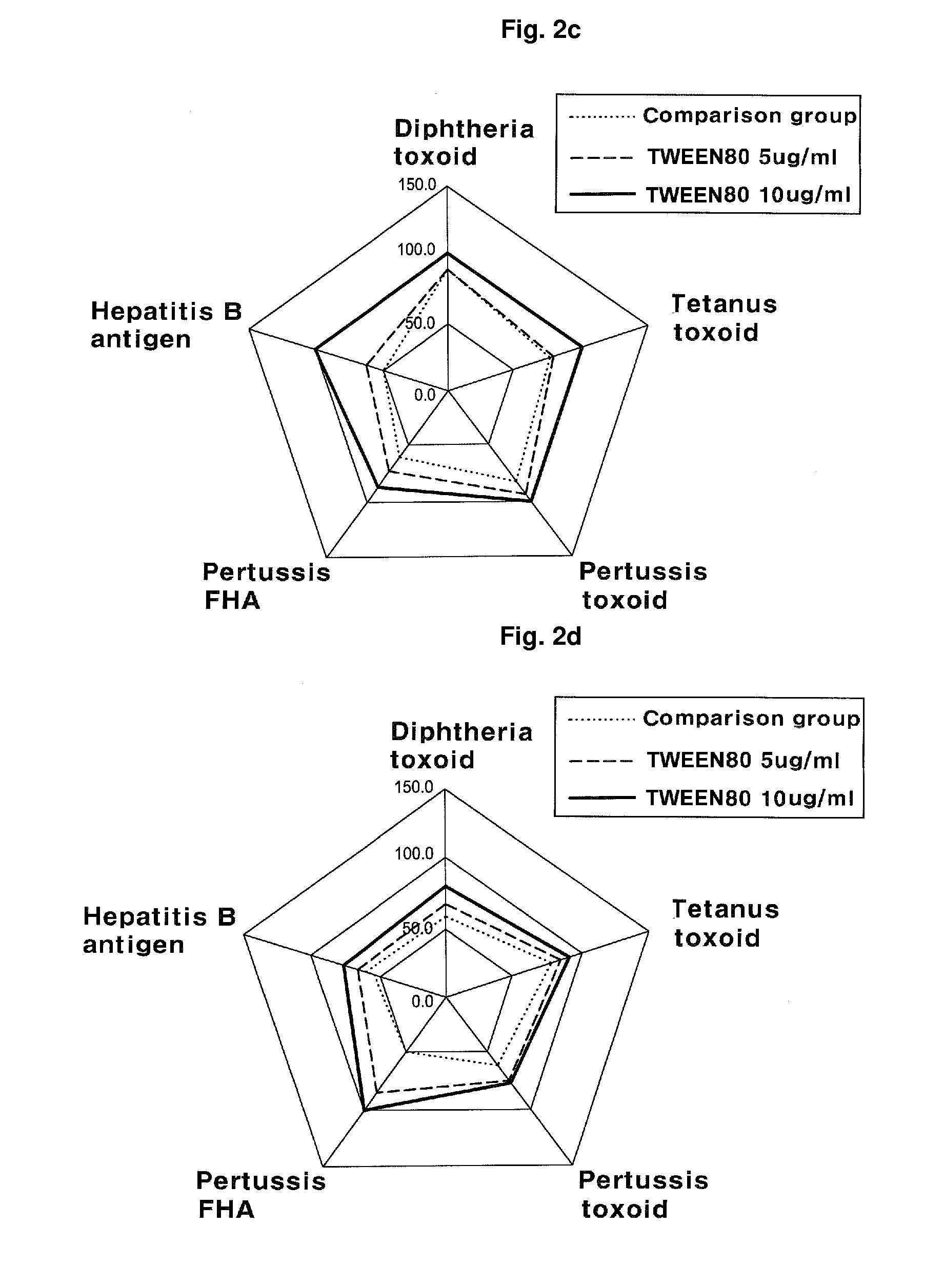
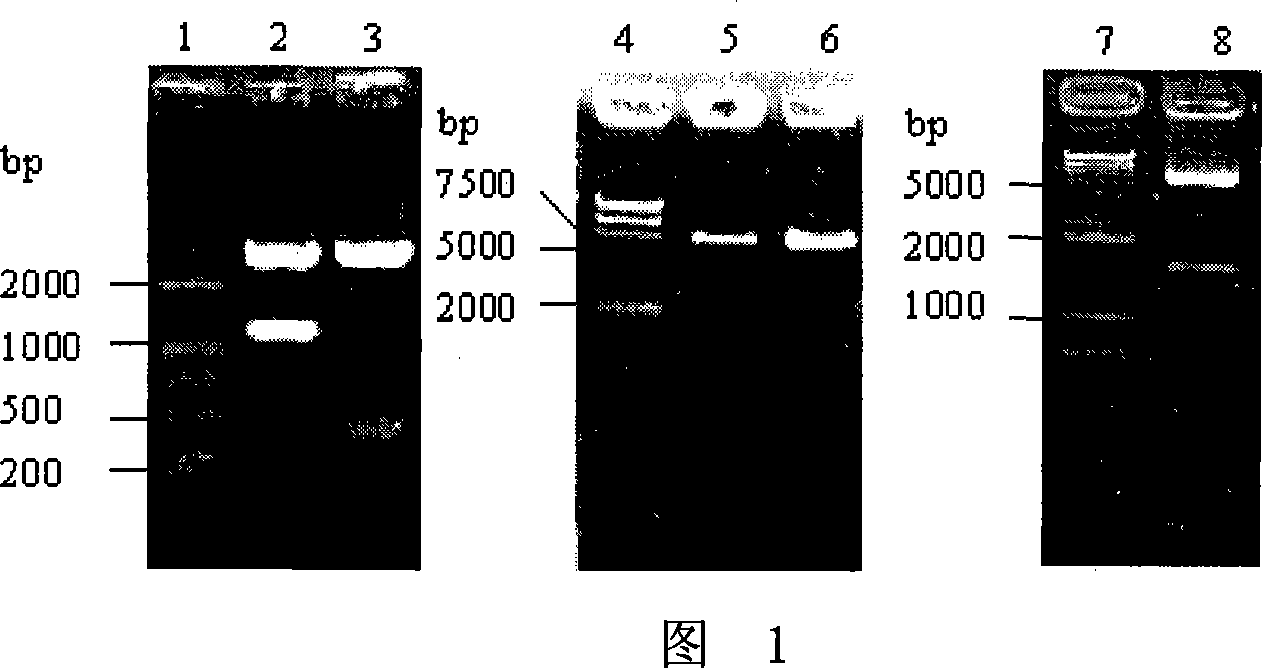
The present invention relates to a method for manufacturing a combined vaccine capable of concurrently preventing multiple diseases such as diphtheria, tetanus, pertussis, and hepatitis B which should be prevented in an infant. The method for manufacturing a combined vaccine according to the present invention includes the steps of independently adsorbing each protective antigen to an adsorbent of a aluminum hydroxide gel with respect to various diseases such as diphtheria, tetanus, pertussis, and hepatitis B which should be prevented in the infants, and combining each protective antigen adsorbed to the adsorbent after the adsorption. In the present invention, it is possible to concurrently prevent multiple diseases such as diphtheria, tetanus, pertussis, and hepatitis B which should be prevented in the infant using a combined vaccine manufactured according to the present invention.
Owner:LG LIFE SCI LTD
Interfusion protein between diphtheria toxin and GM CSF mutant, coded gene and application
InactiveCN101050239AKeep aliveHigh expressionBacteriaPeptide/protein ingredientsMyeloid leukemiaDiphtheria vaccination
This invention discloses fusion protein of diphtherin and GM-CSF, its coding gene, and its application. The fusion protein is selected from: (a) the protein shown in SEQ ID No.2; (b) the protein derived from SEQ ID No.2 by substituting, deleting and / or adding one or more amino acid residues, which can kill acute myeloid leukemia cells. The fusion protein can kill target cells, and has a high expression level.
Owner:中国疾病预防控制中心病毒病预防控制所
METHOD OF PRODUCING MENINGOCOCCAL MENINGITIS VACCINE FOR NEISSERIA MENINGITIDIS SERO TYPES A,C,Y, and W-135
InactiveUS20080020002A1Yield maximizationYield minimizationAntibacterial agentsBacteriaConjugate vaccineSynechococcus
Methods for producing quadrivalent meningococcal meningitis polysaccharide and conjugate vaccines for serotypes A, C, Y and W-135 disclosed. Neisseria meningitidis fastidious medium was designed to maximize the yield of capsular polysaccharides and generate minimal cellular biomass and endotoxin in a short duration of fermentation. The crude polysaccharides are isolated, purified, and mechanically depolymerized by sonication. These purified polysaccharides were found in human clinical trials to be safe and immunogenic against meningococcal disease caused by N. meningitidis A, C, Y and W-135 serogroups in sub-Saharan Africa. In the preferred embodiment, the polysaccharides are conjugated to carrier proteins of diphtheria or tetanus toxiod to an average molecular size of 5100 to 9900 Daltons and provide broad spectrum protection to humans of all ages. Accelerated polysaccharide production and the efficacy of the resulting vaccine are demonstrated.
Owner:REDDY JEERI R
Traditional Chinese medicine powder for treating diphtheria
InactiveCN101085067ASignificant effectAntibacterial agentsHydroxy compound active ingredientsCurative effectDiphtheria vaccination
Owner:牟培锐
Integration of meningococcal conjugate vaccination
ActiveUS9402915B2Antibacterial agentsCarrier-bound antigen/hapten ingredientsCarrier proteinDiphtheria vaccination
Conjugated meningococcal capsular saccharides will be introduced into immunization schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunizing a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunized with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
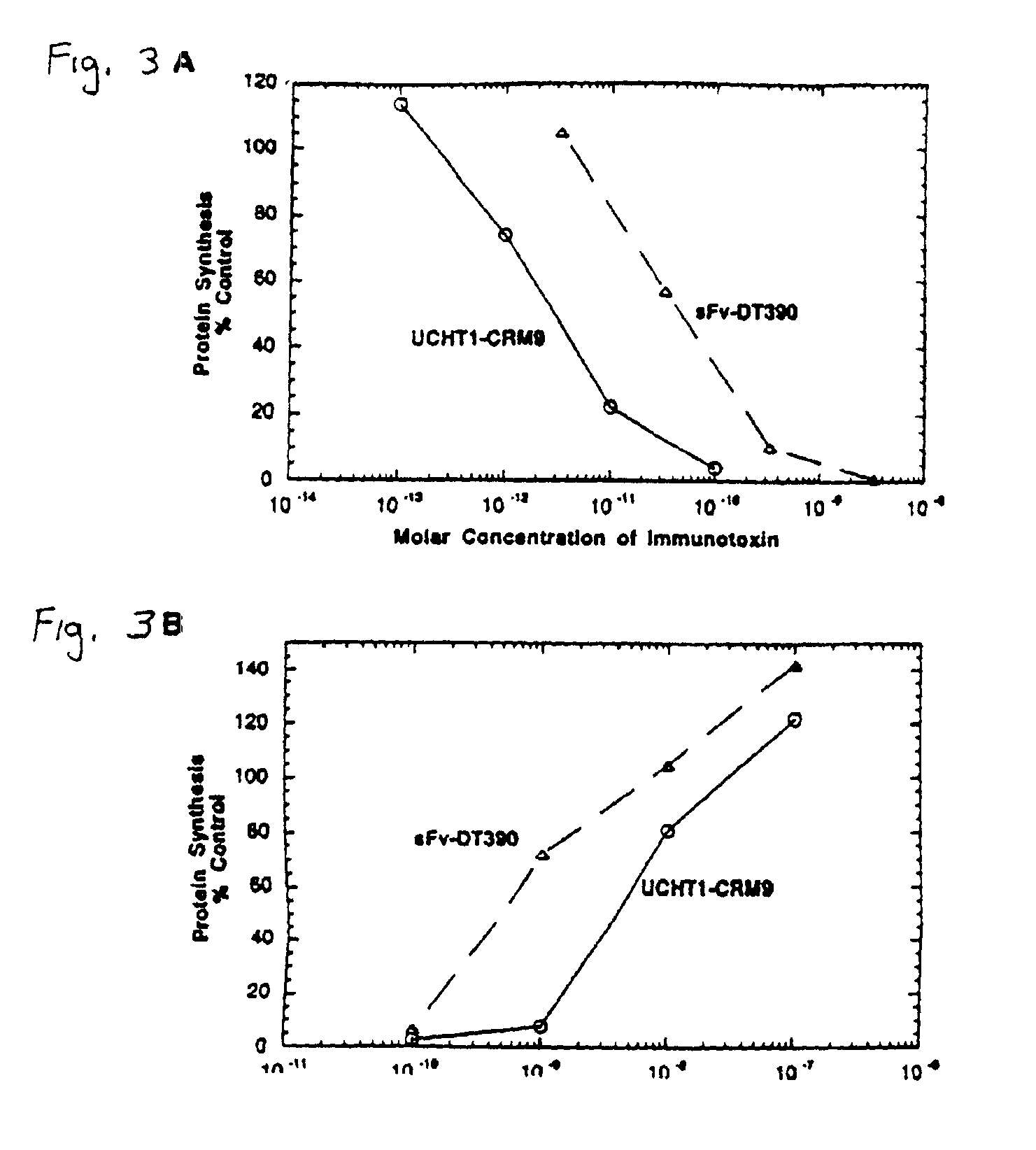
Methods of inducing immune tolerance using immunotoxins
InactiveUS7125553B1Induce immune toleranceSnake antigen ingredientsAntibody ingredientsPresent methodBinding site
Provided is a method of treating an immune system disorder not involving T cell proliferation, comprising administering to the animal an immunotoxin comprising a mutant diphtheria toxin moiety linked to an antibody moiety which routes by the anti-CD3 pathway, or derivatives thereof under conditions such that the disorder is treated. Thus, the present method can treat graft-versus-host disease. Also provided is a method of inhibiting a rejection response by inducing immune tolerance in a recipient to a foreign mammalian donor tissue or cells, comprising the steps of: a) exposing the recipient to an immunotoxin so as to reduce the recipients's peripheral blood T-cell lymphocyte population by at least 80%, wherein the immunotoxin is anti-CD3 antibody linked to a diphtheria protein toxin, wherein the protein has a binding site mutation; and b) transplanting the donor cells into the recipient, whereby a rejection response by the recipient to the donor organ cell is inhibited, and the host is tolerized to the donor cell.
Owner:UNITED STATES OF AMERICA +1
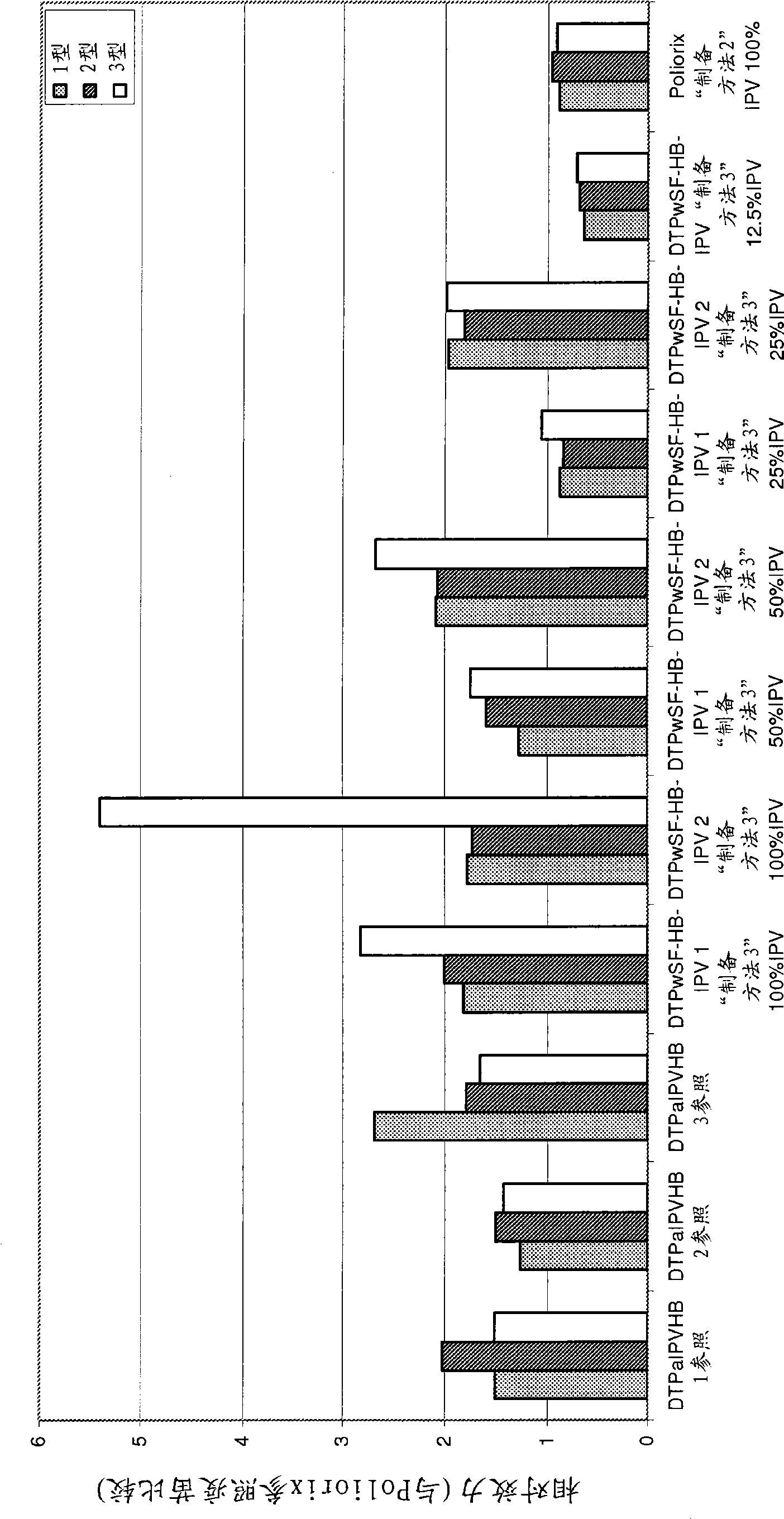
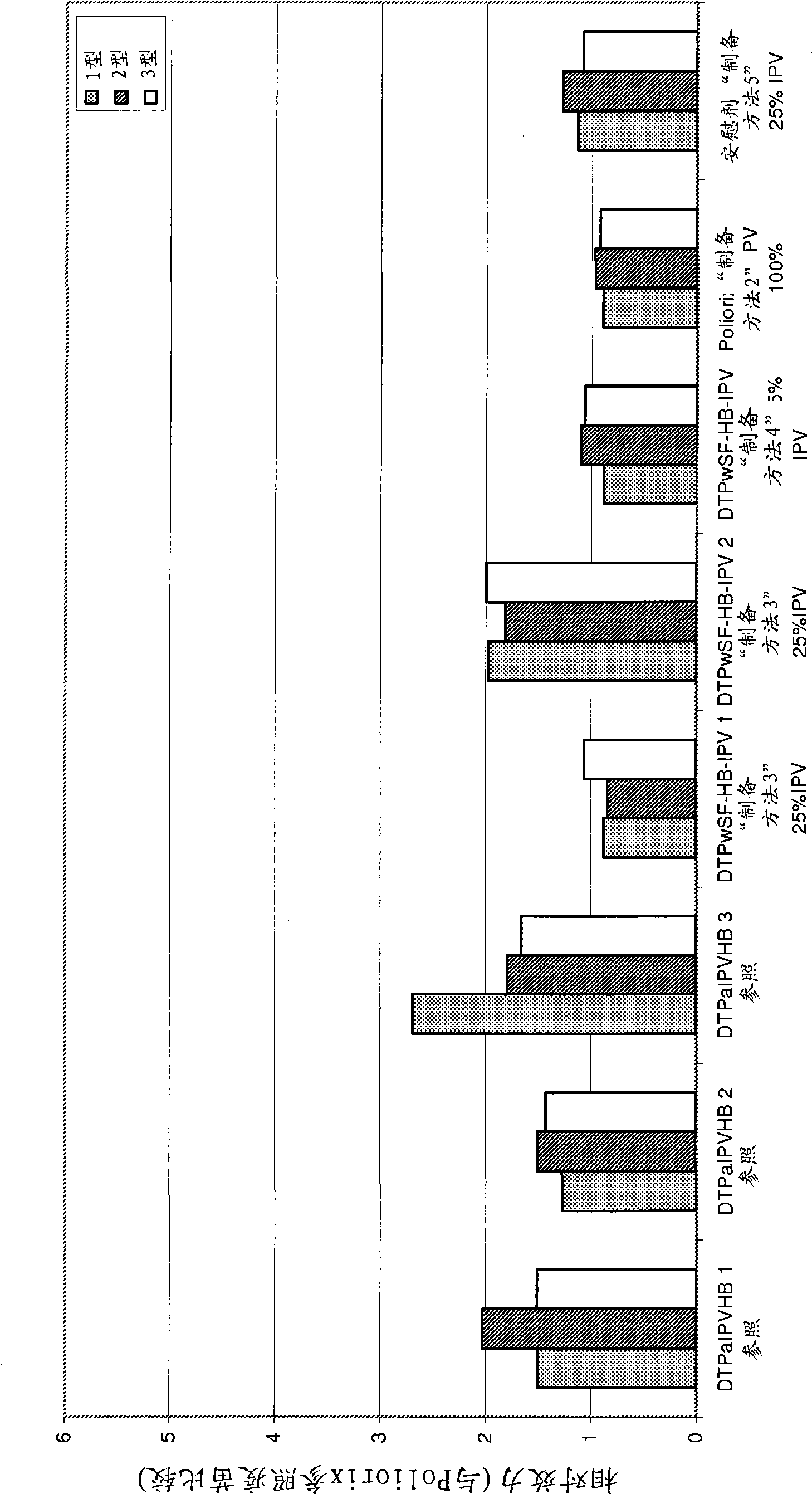
Vaccine
InactiveCN101534854AIncrease doseIncreased level of protectionViral antigen ingredientsAgainst vector-borne diseasesDiseaseTetanus
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus (IPV) is provided, which can maintain an adequate or improved level of protection against polio.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Traditional Chinese medicine air disinfectant for clinical laboratory and preparation method thereof
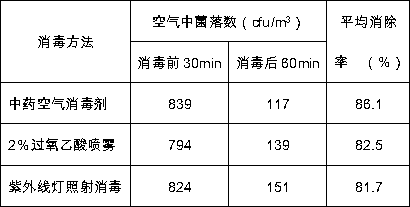
The invention discloses a traditional Chinese medicine air disinfectant for a clinical laboratory and a preparation method thereof, and belongs to the field of traditional Chinese medicine. The medicine effective components of the air disinfectant comprise the following raw materials: pyrola calliantha, folium mori, haliotidis concha, eupatorium fortune, pyrrosia lingua, dendranthema indicum, gentiana scabra, glycyrrhiza uralensis, achyranthes aspera, dark plum, plantago asiatica, fructus forsythia, adina pilulifera, agastache rugosa, rhus chinensis, atractylodes rhizome, folium artemisiae argyi, platycladus orientalis, coriandrum sativum, piper longum, cortex moutan radicis, rubia cordifolia, rosmarinus officinalis and aristolochia debilis. The air disinfectant belongs to a pure traditional Chinese medicine material preparation, is poisonless and harmless, has obvious bacteriostatic or bactericidal effect on staphylococcus aureus, pneumococcus, mycobacterium tuberculosis, typhoid bacillus, corynebacterium diphtheria, escherichia coli and pseudomonas aeruginosa, has a certain inhibiting effect on other pathogenic bacteria, influenza virus and fungi, is good in sterilization effect,does not have stimulation for human body, does not have extraneous odor and has vegetation fragrance, can perform refreshment and purify and refresh the air, and does not affect the normal work of inspection personnel after sterilization.
Owner:宋瑞红
Traditional Chinese medicine for treating diphtheria
InactiveCN101757426AEffective treatmentQuick effectAntibacterial agentsRespiratory disorderDiphtheria vaccinationSophora
The invention discloses a traditional Chinese medicine for treating diphtheria, belonging to the technical field of medicaments. Gypsum, rhizoma anemarrhenae, fritillaria thunbergii, radix isatidis, subprostrate sophora, viola yedoensis, honeysuckle, dried rhizome of rehmannia, radix scrophulariae, forsythia, ophiopogon japonicus, radix paeoniae alba, cortex moutan, mint, liquorice and fresh olive are used as raw materials; and the raw materials are prepared according to different characteristics and different proportioning of each traditional Chinese medicine. The traditional Chinese medicine has unique prescription, is used for treating the diphtheria with obvious effect, and has simple and convenient processing and preparation and convenient taking.
Owner:李琪
Preparation method of traditional Chinese medicine spray
The invention discloses a preparation method of a traditional Chinese medicine spray, and in particular relates to a preparation method of a traditional Chinese medicine spray related with traditional Chinese medicine. The traditional Chinese medicine spray provided by the invention comprises the following traditional Chinese medicine raw materials of eupatorium, Agastache rugosus, rhizoma atractylodis, Mangnolia officinalis, radix sophorae flavescentis and folium artemisiae argyi at the weight ratio of 10 to 10 to 20 to 10 to 20 to 7. The medicine liquid prepared by the invention is capable of effectively killing and restraining multiple viruses such as a common cold virus, a staphylococcus, a streptococcus, a dysentery bacillus, a Bacillus anthraci, alpha-streptococcus hemolyticus, a corynebacterium diphtheria, a lung class diplococcus, staphylococcus aureus; the use is safe, the smell is fragrance, the medicine liquid is harm for a human body, and does not corrode an article.
Owner:刘平
Biological immunopotentiator composition for treating cancers
InactiveCN101669973AImprove the quality of lifeGood value for tumor treatmentOrganic active ingredientsBacteria material medical ingredientsLymphatic SpreadTetanus toxoids
The invention relates to a biological immunopotentiator composition for treating cancers. The composition medicament for treating the cancers mainly comprises 0.5 to 15 billions of inactivated human salmonella typhi, 1 to 10 billions of inactivated bordetella pertussis, and diphtheria toxoid, tetanus toxoid or staphylococcus aureus enterotoxin C. The novel composition is helpful for activating the immune system of a tumor patient, improving the quality of life, and inhibiting tumor growth and metastasis.
Owner:马逸冰
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com