Combination vaccines with low dose of hib conjugate
a conjugate and low dose technology, applied in the field of conjugate vaccines, can solve the problems of inability to survive prolonged storage, unstable hib conjugate in aqueous media, and low production cost of hib conjugate antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
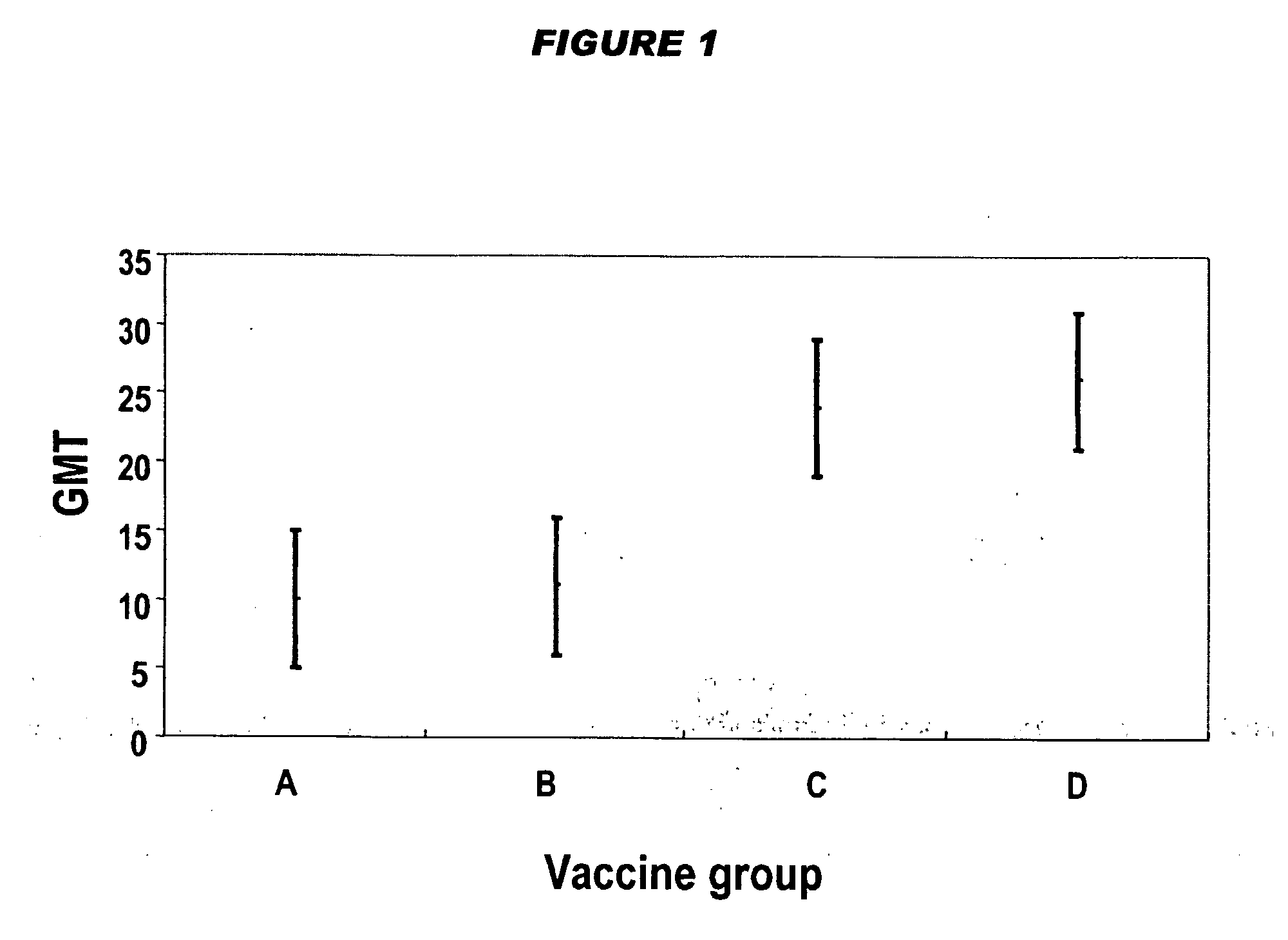
[0121]Four DTwP-Hib vaccine formulations were prepared, differing only in their dose of Hib-CRM97 conjugate. Vaccines were prepared as 0.5 ml doses with the following antigenic compositions:
ABCD [175]Hib-CRM197 conjugate (μg saccharide1.252.5510per dose)Diphtheria toxoid (Lf per dose)15Tetanus toxoid (Lf per dose)3.2Inactivated B. pertussis organisms (OU15per dose)Aluminium phosphate adjuvant (mg Al3+0.3per dose)NaCl (mg per dose)4.5MerthiolateTraceHib fraction relative to ref. 175⅛¼½1
[0122]The production process was essentially as follows: start with wfi; add the aluminium phosphate adjuvant; add the D component; add the T component; add the wP component; add NaCl; check and adjust pH; and add the Hib component. Contrary to the statement in reference 175, the Hib component does not adsorb to the adjuvant.
Stability
[0123]Two stability studies were performed: one under normal storage conditions at 2-8° C. for 2 years, and another under accelerated conditions at 37° C. for 14 days. Vac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com