Vaccine
A vaccine and combined vaccine technology, applied in the field of vaccines for poliomyelitis prevention, can solve the problems of complicating the development of multi-component vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1: Testing of Low Dose IPV Formulations
[0206] For all formulations of Example 1, except IPV (which was added but not adsorbed), the antigen was adsorbed by addition of aluminum salts prior to preparation.
[0207] The table below shows the adsorption methods for D, T, Pw and HBsAg.
[0208] AlPO 4
[0209] |
[0210] D (7.5Lf / 0.075mg Al 3+ )
[0211] |
[0212] Stir at room temperature for 15 to 20 minutes.
[0213] |
[0214] Adjust pH to pH5.1+ / -0.1
[0215] |
[0216] Stir at room temperature for 15 to 20 minutes.
[0217] |
[0218] Calibration pH5.1+ / -0.1
[0219] |
[0220] Stir at room temperature for 15 up to 45 minutes.
[0221] |
[0222] Mature at 37°C + / -1°C for 7 days + / - 8 hours without stirring (glass con...
Embodiment 2
[0374] Example 2: Feasibility of not using thimerosal in the vaccine of the present invention
[0375] The Preservative Efficiency Test (PET) enables the detection of antimicrobial activity of test vaccines. The test includes:
[0376] In the final container step of the vaccine formulation, the vaccine formulation is challenged with a designated inoculation of suitable microorganisms,
[0377] Store the inoculated formulation at the indicated temperature
[0378] Samples are withdrawn from the container at regular intervals and the organisms in the withdrawn samples are counted.
[0379] PET assay procedures are described in the European Pharmacopoeia (5.1.3) and USP (). According to these guidelines, antimicrobial activity was evaluated by comparing the reduction in the number of viable microorganisms with the criteria mentioned in the table below (Table 7)
[0380]
[0381] Table 7. EP and USP Standards
[0382] Nru * : not recycled
[0383] Ni * : not increased ...
Embodiment 3
[0384] Example 3: Effect of Hib components on IPV potency and IPV stability over time
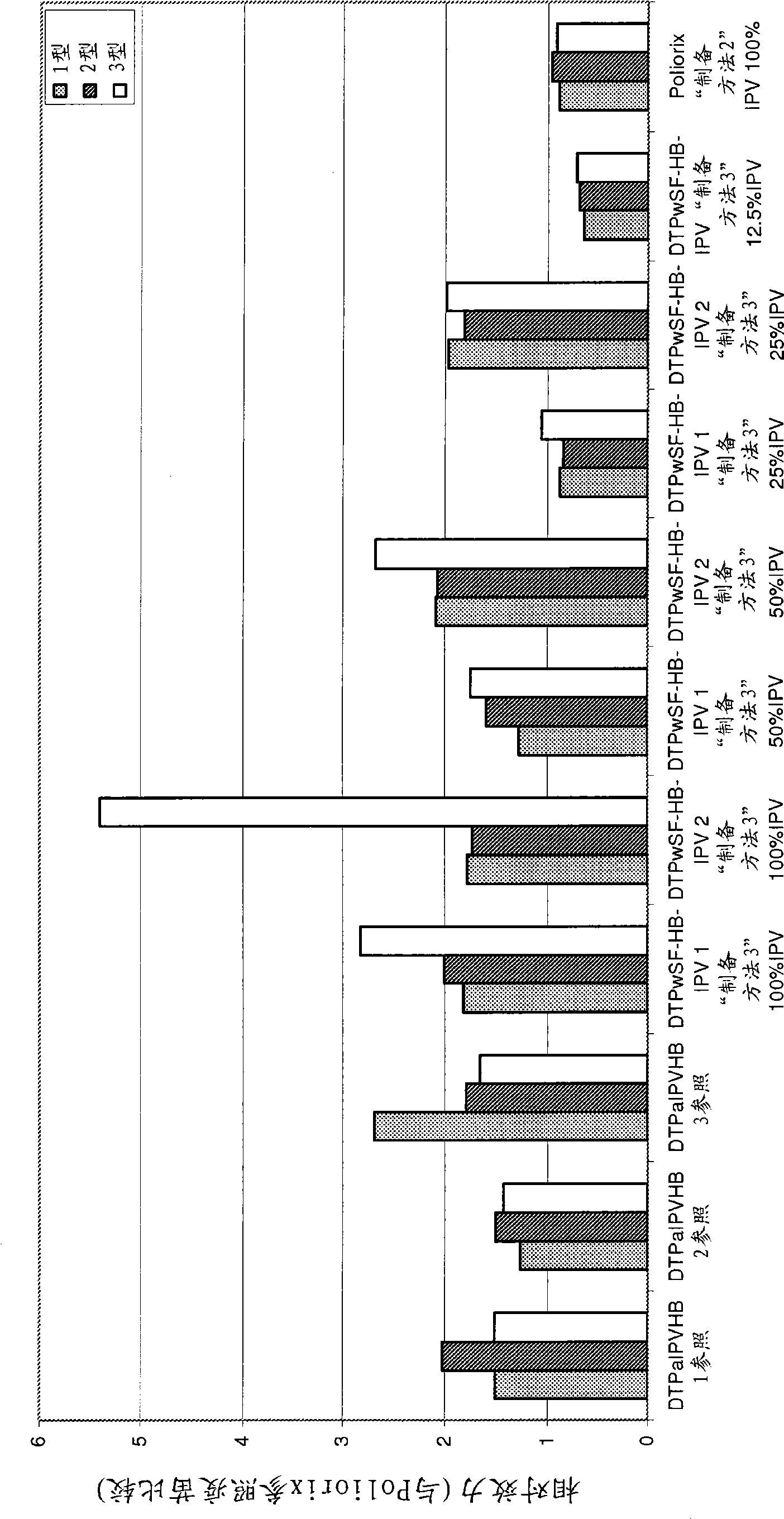
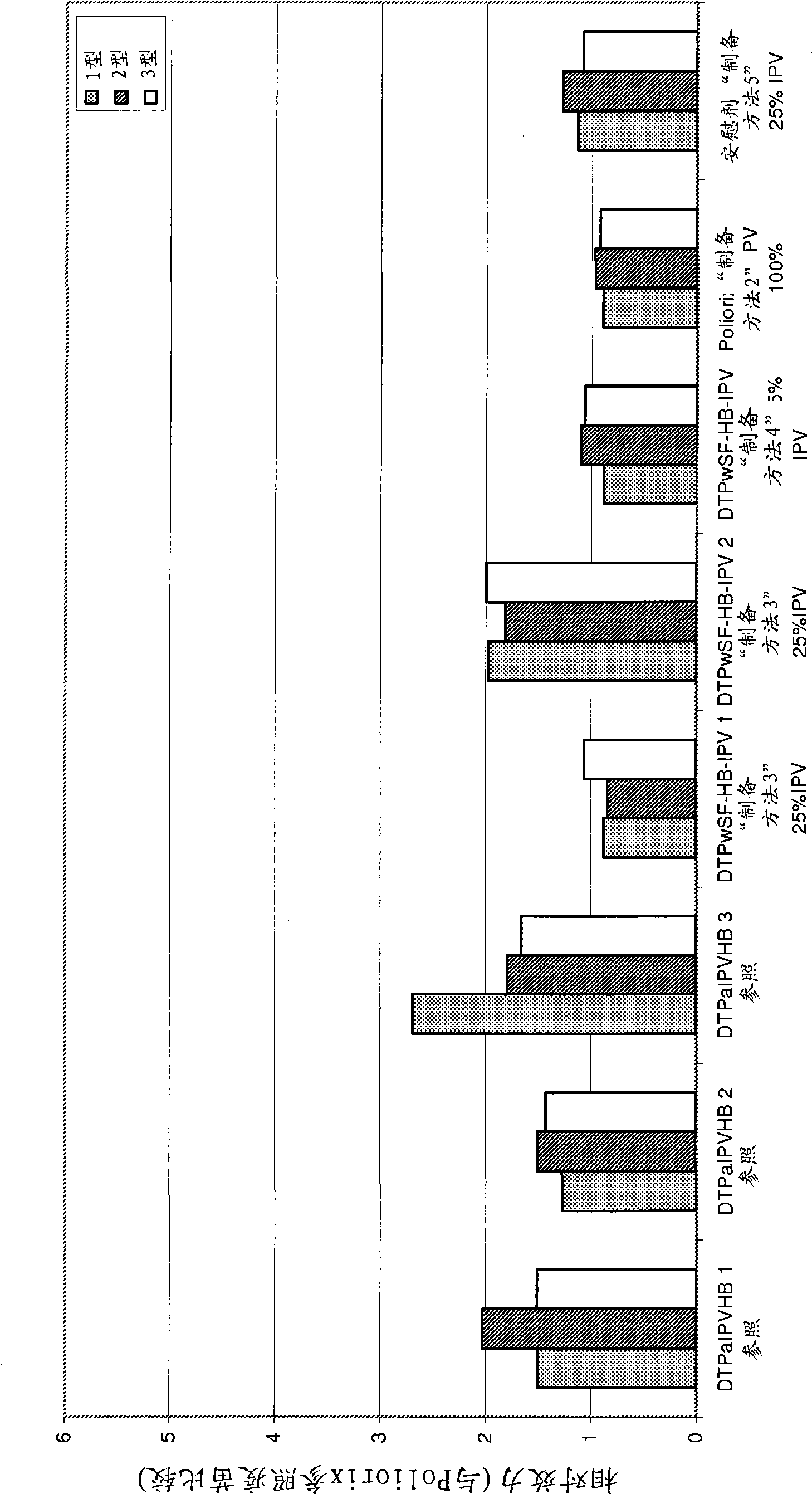
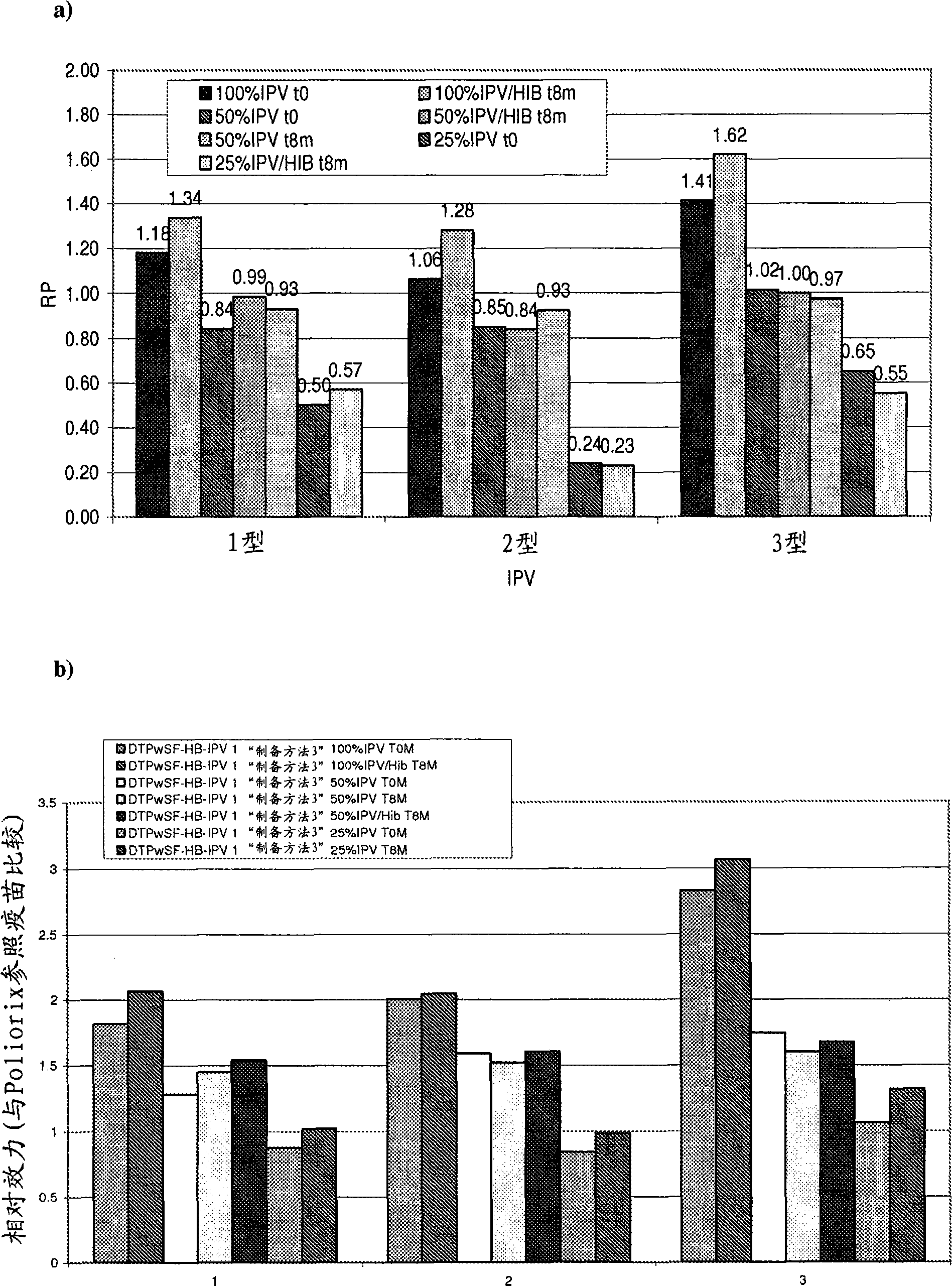
[0385] The relative potency of IPV was tested as described in Example 1 to determine the effect of the Hib component on IPV potency and to evaluate the stability of IPV over time under different IPV dose conditions. The vaccines studied were DTPwHBIPV(40-8-32) with reconstituted DTPwHBIPV and stored for 8 months, DTPwHBIPV(20-4-16), with reconstituted DTPwHBIPV (20-4-16) and stored for 8 months , DTPwHBIPV (20-4-16) and stored for 8 months, DTPwHBIPV (10-2-8) and DTPwHBIPV (10-2-8) containing reconstituted Hib and stored for 8 months. Measured relative to DTPaIPVHB (Pediarix) ( image 3 a) or Poliorix ( image 3 b) RP value. The Hib component was found to have no effect on IPV potency. The relative potency of IPV was found to be maintained at month 8 ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com