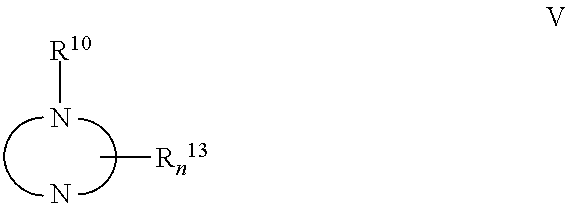Patents
Literature
76 results about "Poliovirus Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In immune individuals, antibodies against poliovirus are present in the tonsils and gastrointestinal tract (specifically IgA antibodies) and are able to block poliovirus replication; IgG and IgM antibodies against poliovirus can prevent the spread of the virus to motor neurons of the central nervous system.
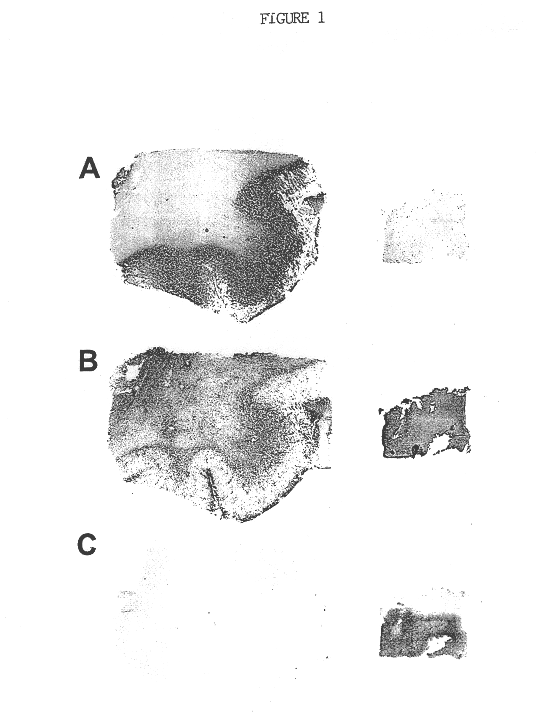
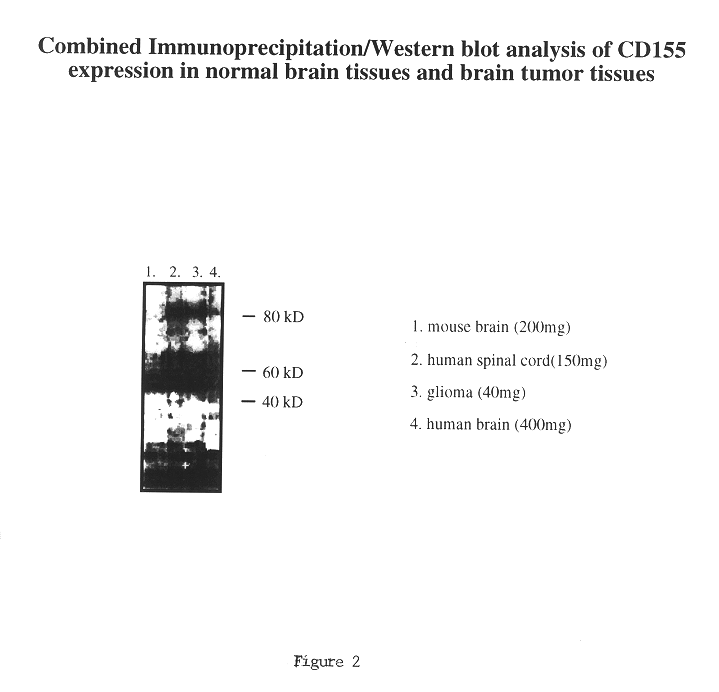
Method of detecting the presence of CD155 for diagnosis of cancer and to determine treatment
InactiveUS6518033B1SsRNA viruses positive-senseGenetic material ingredientsAbnormal tissue growthOncology
The present invention relates to a method of diagnosing, classifying and grading of tumor growths and to determine whether the use of chimeric polioviruses is a proper course for the treatment of the tumors. More particularly, the method is directed to the use of antibodies to a poliovirus receptor (PVR), CD155, to detect the presence of CD155 on tumor cells in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Carbon nanotube filter
Monolithic, macroscopic, nanoporous nanotube filters are fabricated having radially aligned carbon nanotube walls. The freestanding filters have diameters and lengths up to several centimeters. A single-step filtering process was demonstrated in two important settings: the elimination of multiple components of heavy hydrocarbons from petroleum, a crucial step in post-distillation of crude oil, and the elimination of bacterial contaminants such as Escherichia coli or the nanometer-sized poliovirus from drinking water. All the filtration processes were repeated several times with completely reproducible results. These nanotube filters can be cleaned repeatedly after each filtration process to regain their full filtering efficiency.
Owner:BANARAS HINDU UNIVERSITY +1
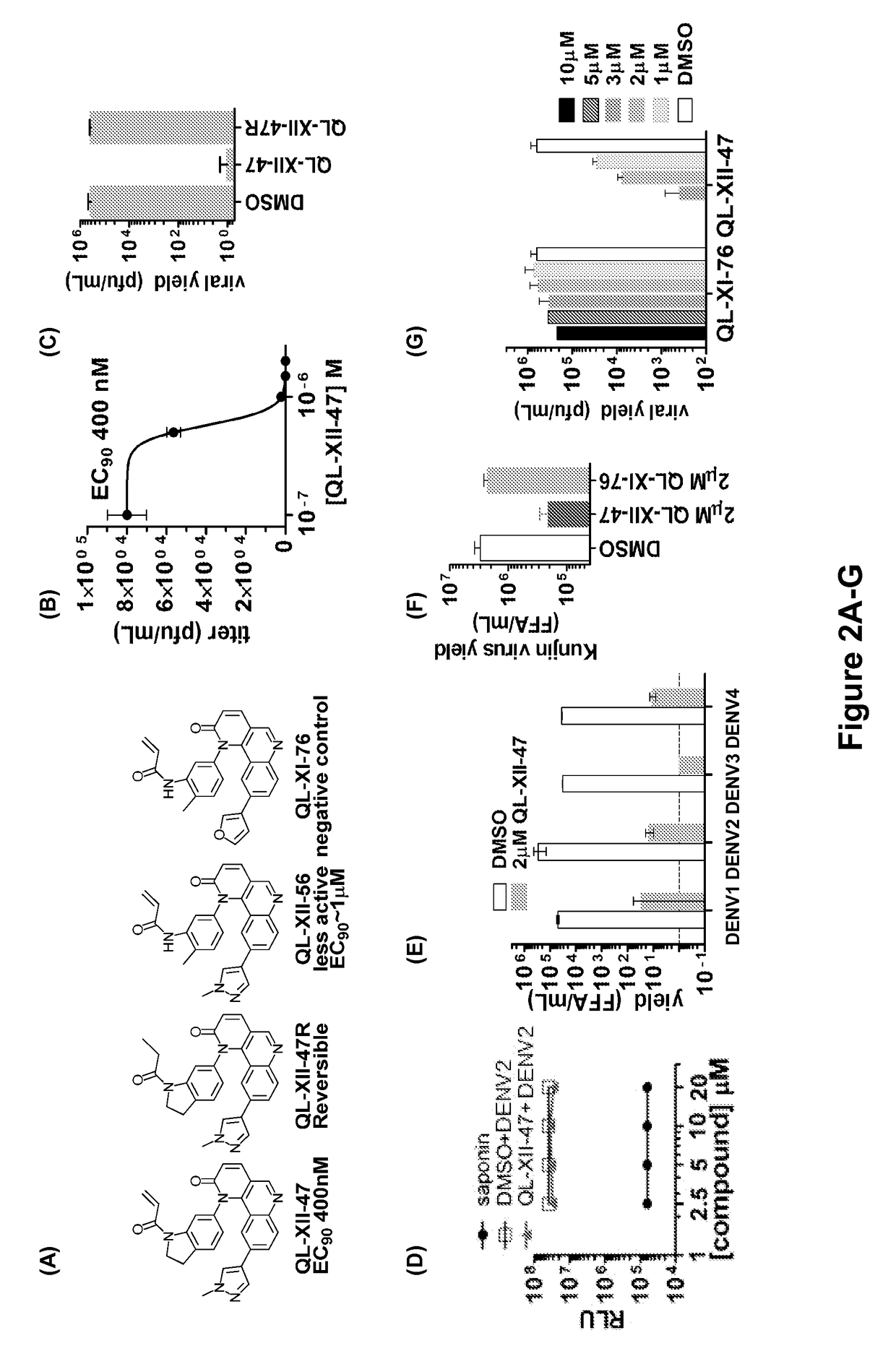
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
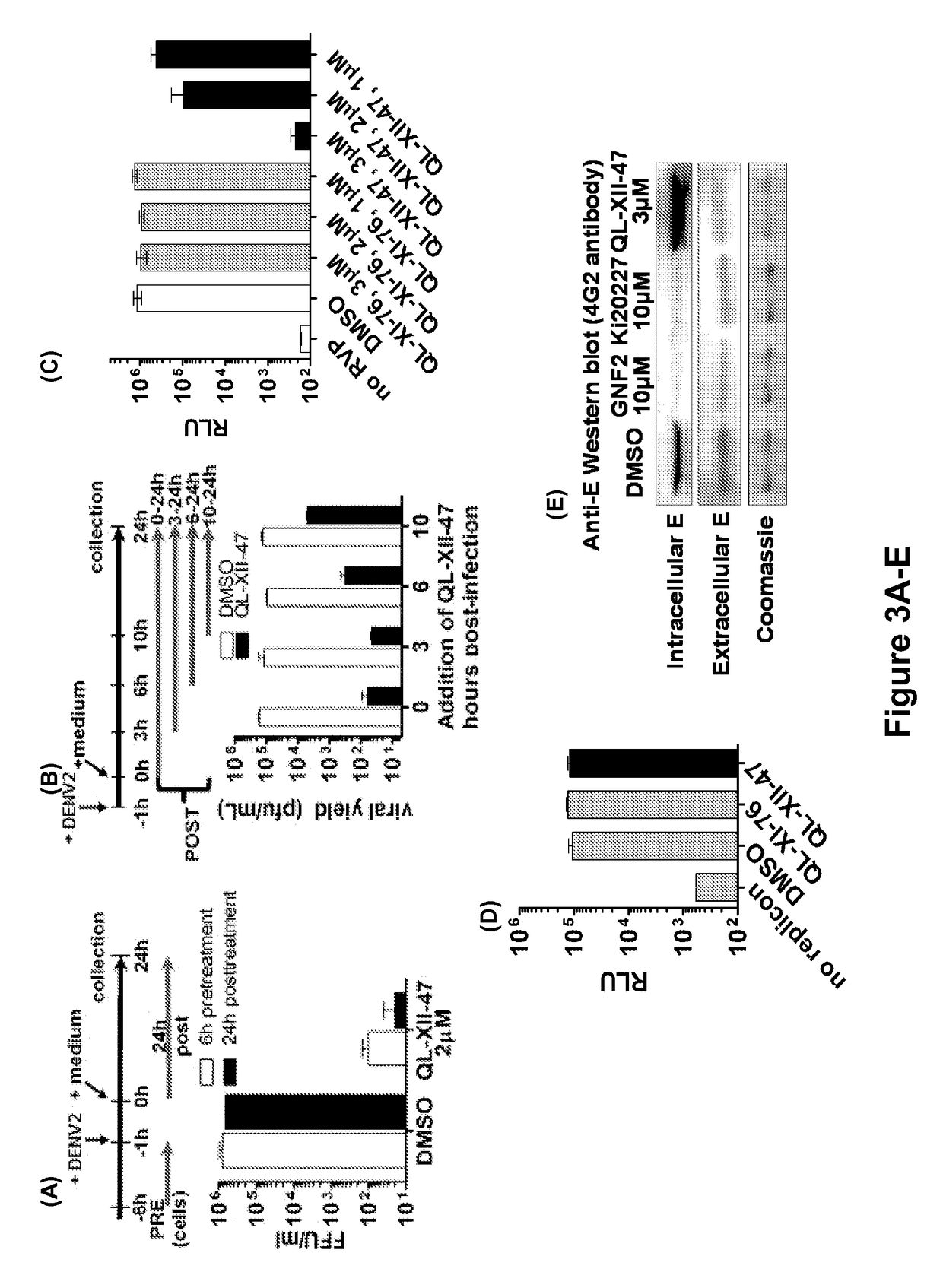
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
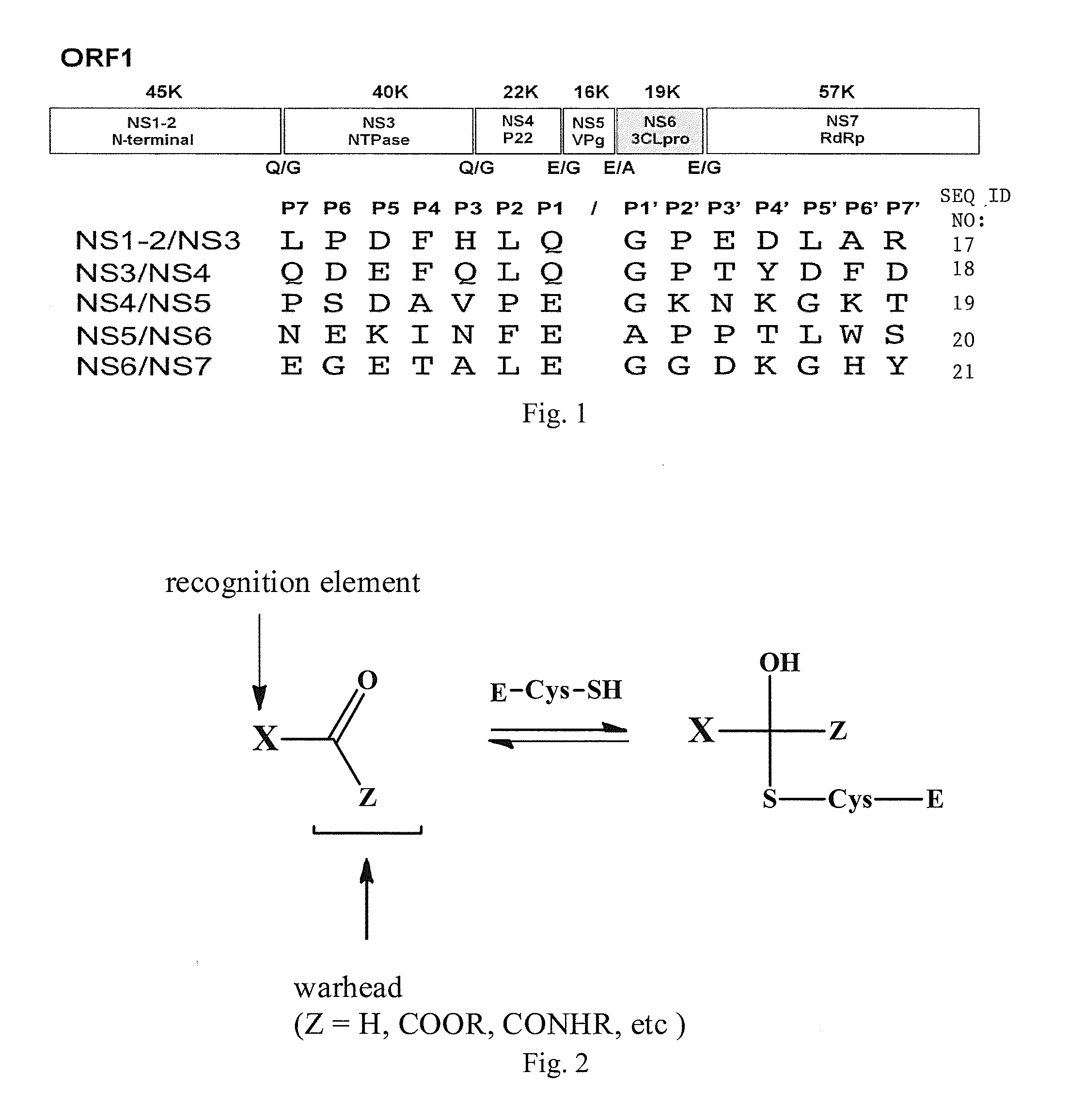
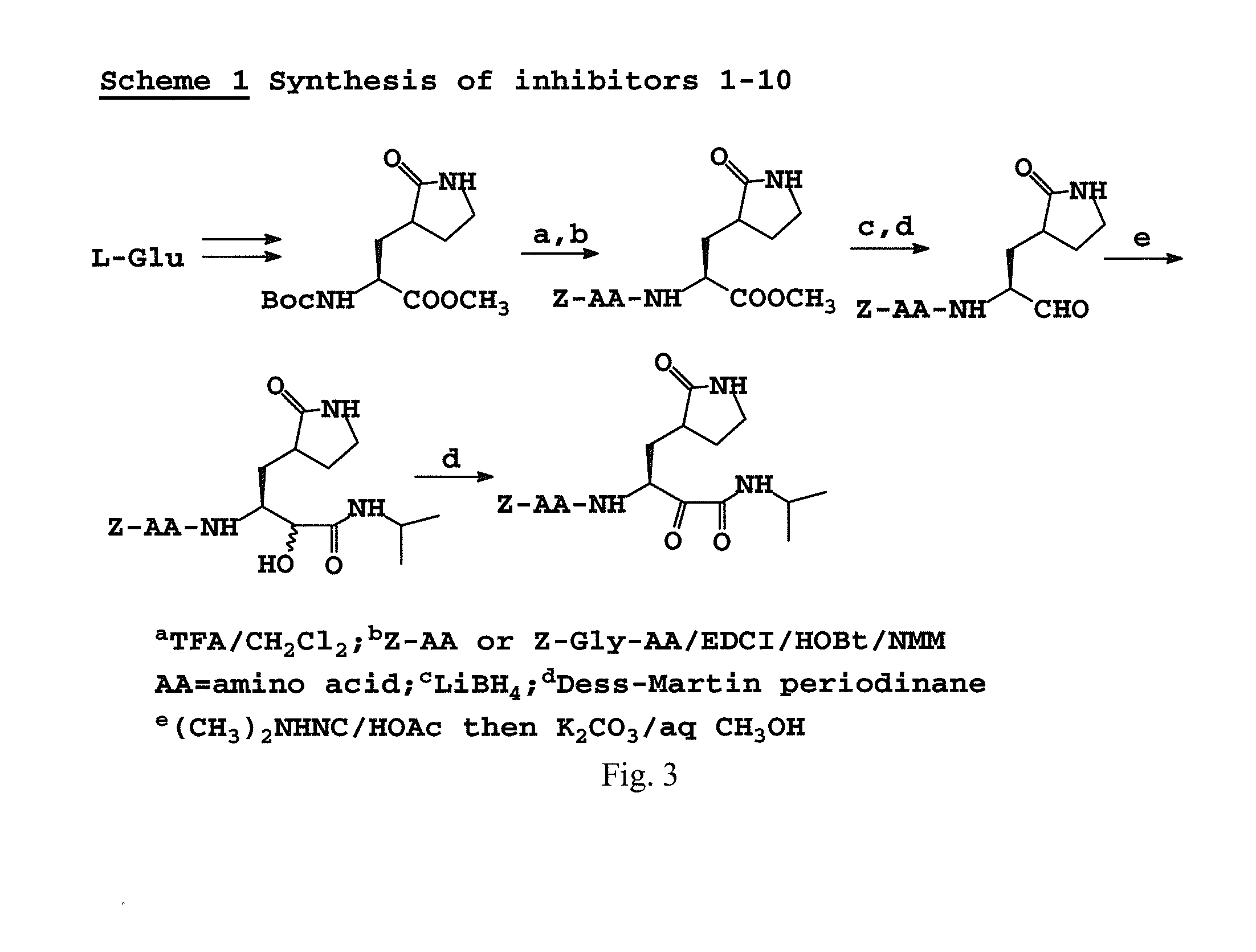
Broad-spectrum antivirals against 3c or 3c-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
ActiveUS20140243341A1Preventing and inhibiting replicationBiocideSsRNA viruses positive-senseEnterovirusDisease
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfite salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Vaccine
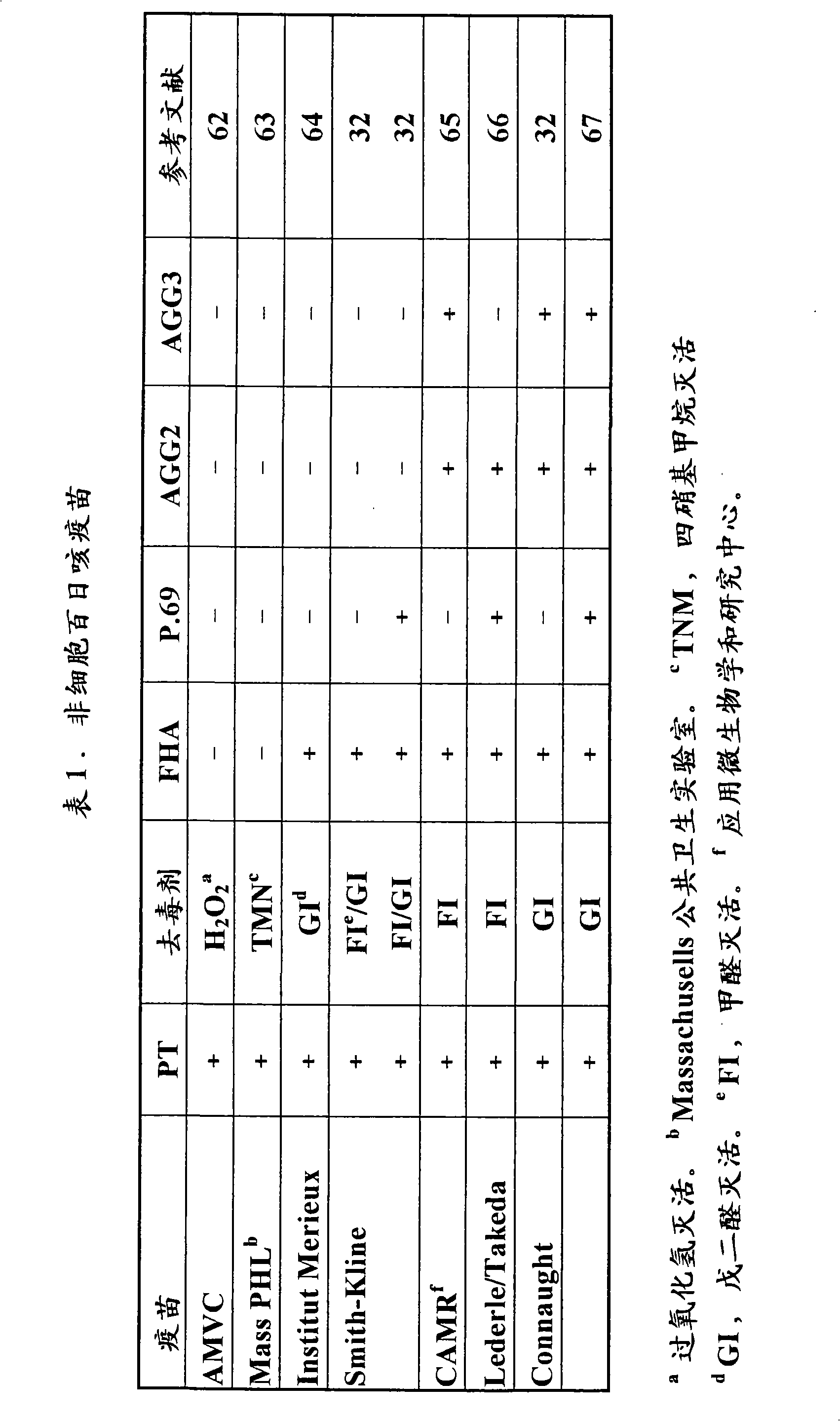
ActiveUS20100034850A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseReduced doseAntigen Unit
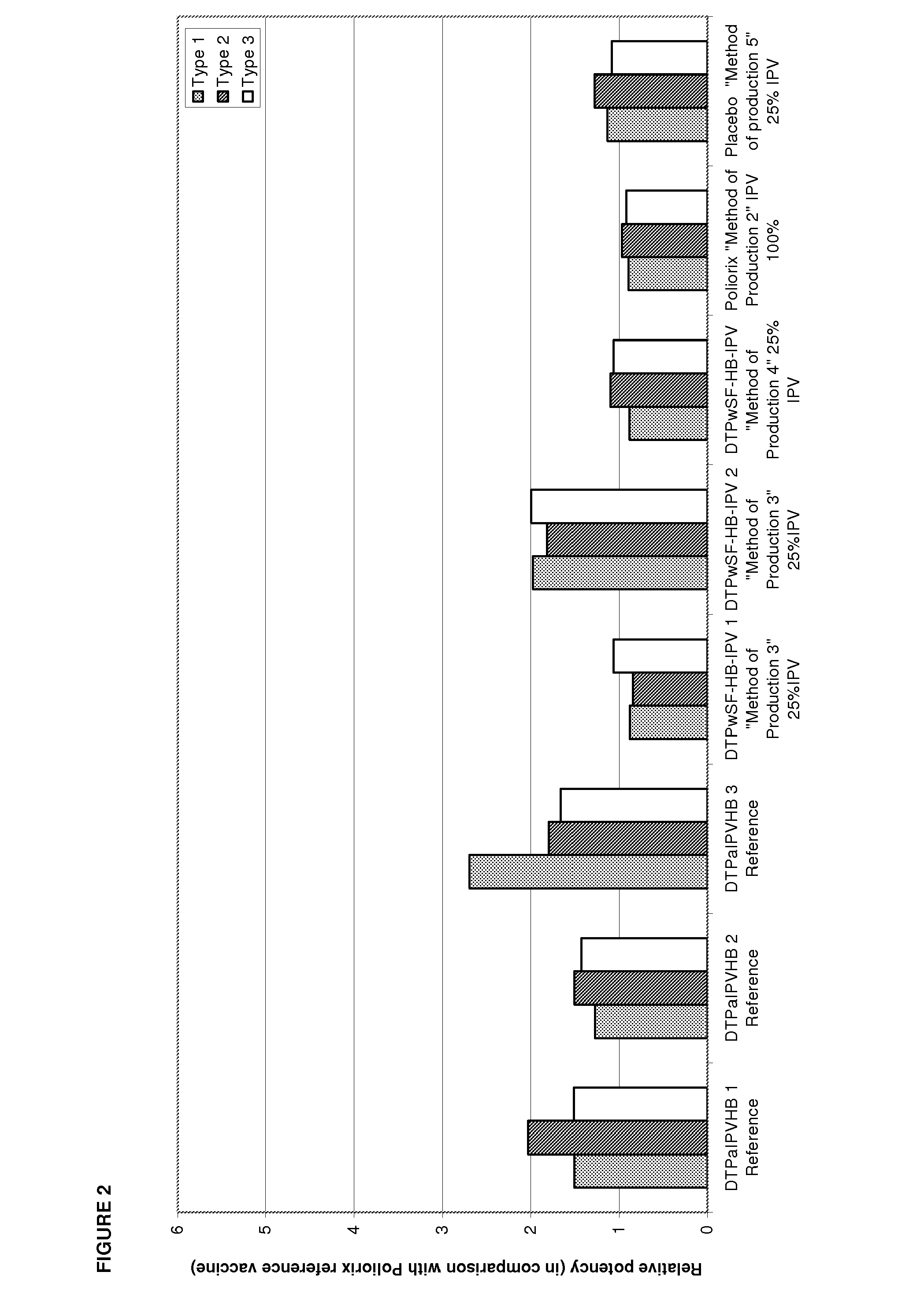
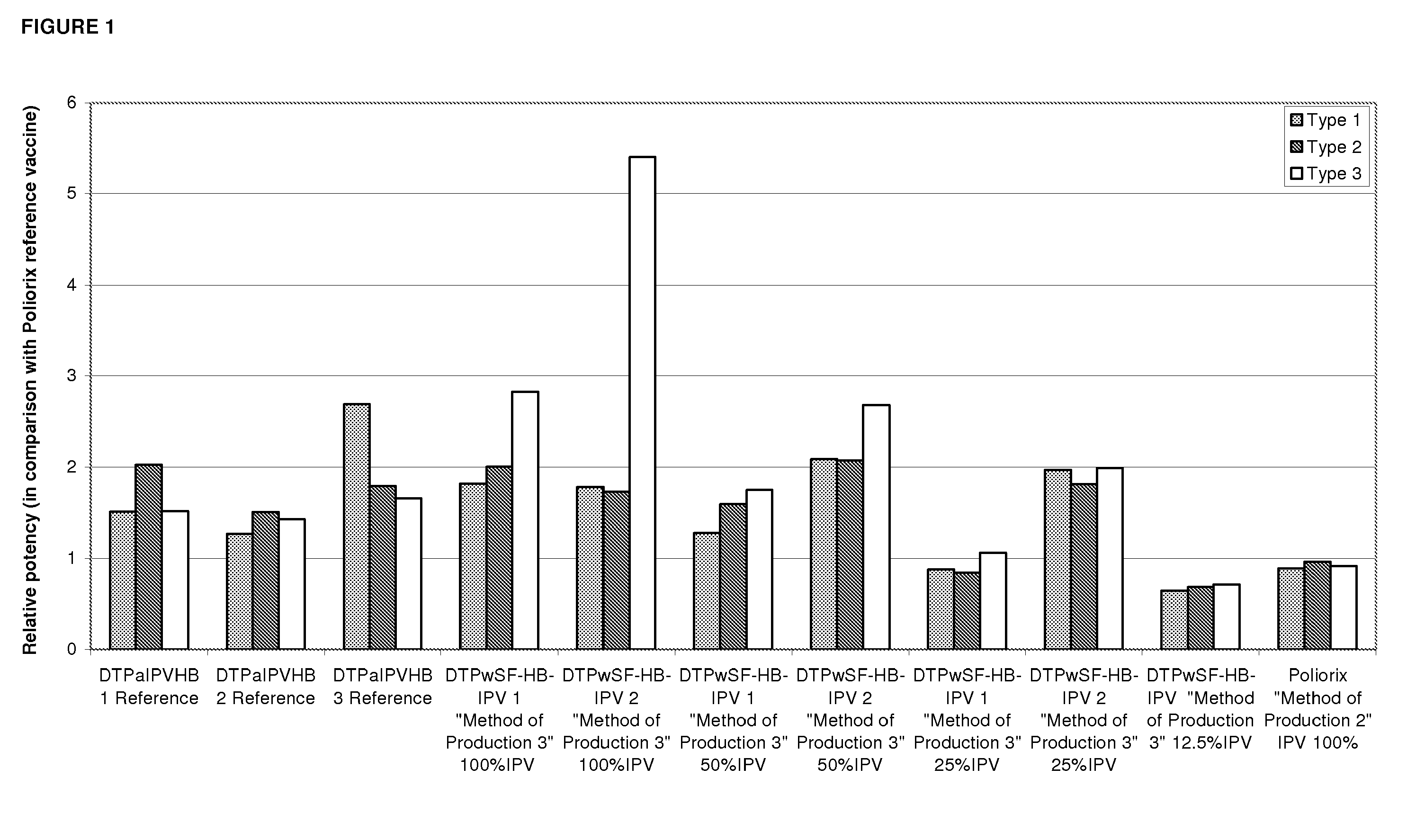
The standard dose of polio vaccines contains 40 D-antigen units of inactivated poliovirus type 1 (Mahoney), 8 D-antigen units of inactivated poliovirus type 2 (MEF-1), and 32 D-antigens units of inactivated poliovirus type 3 (Saukett). The present invention teaches that reduced doses of inactivated poliovirus can maintain adequate or improved level of protection against polio.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
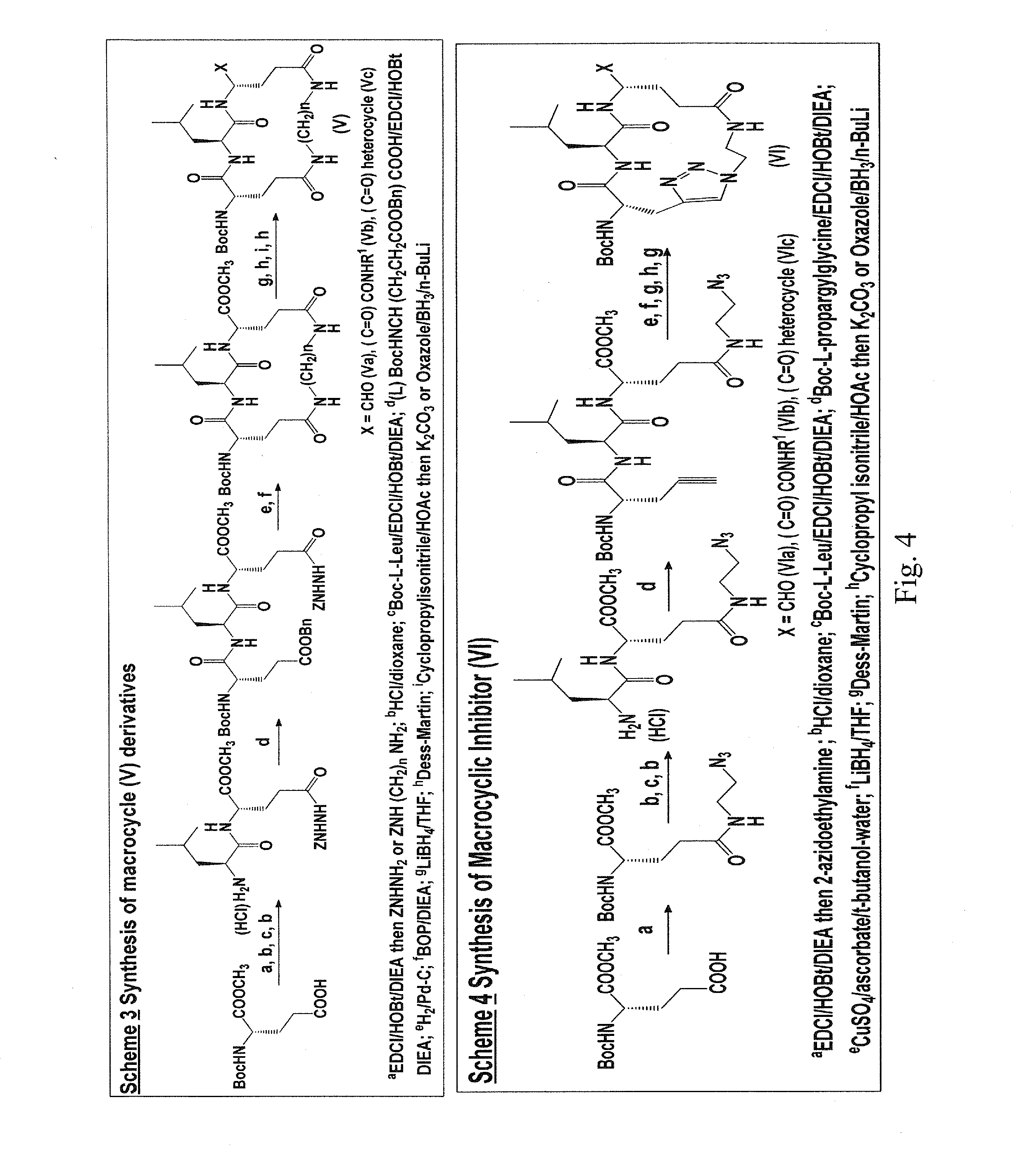
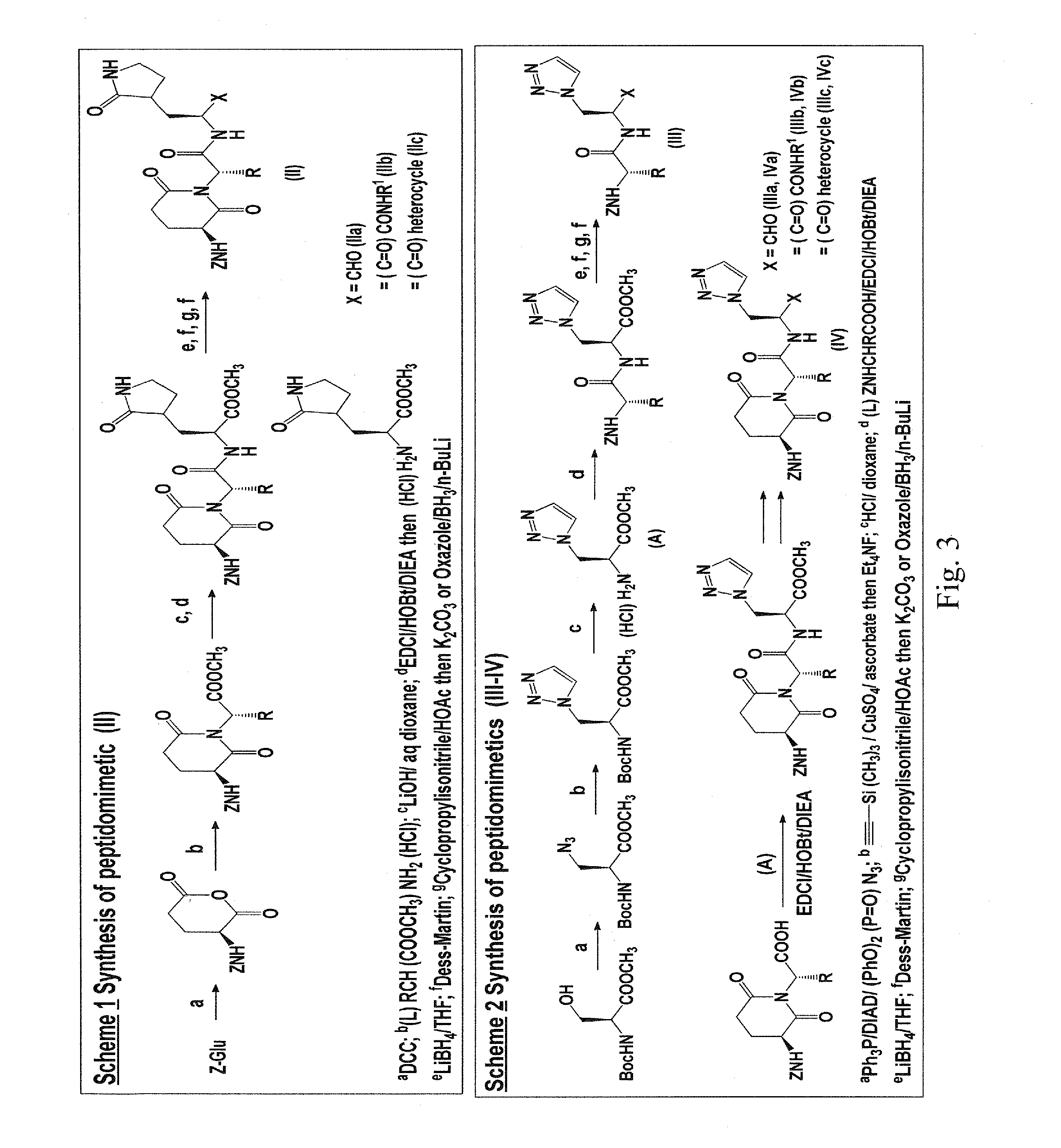
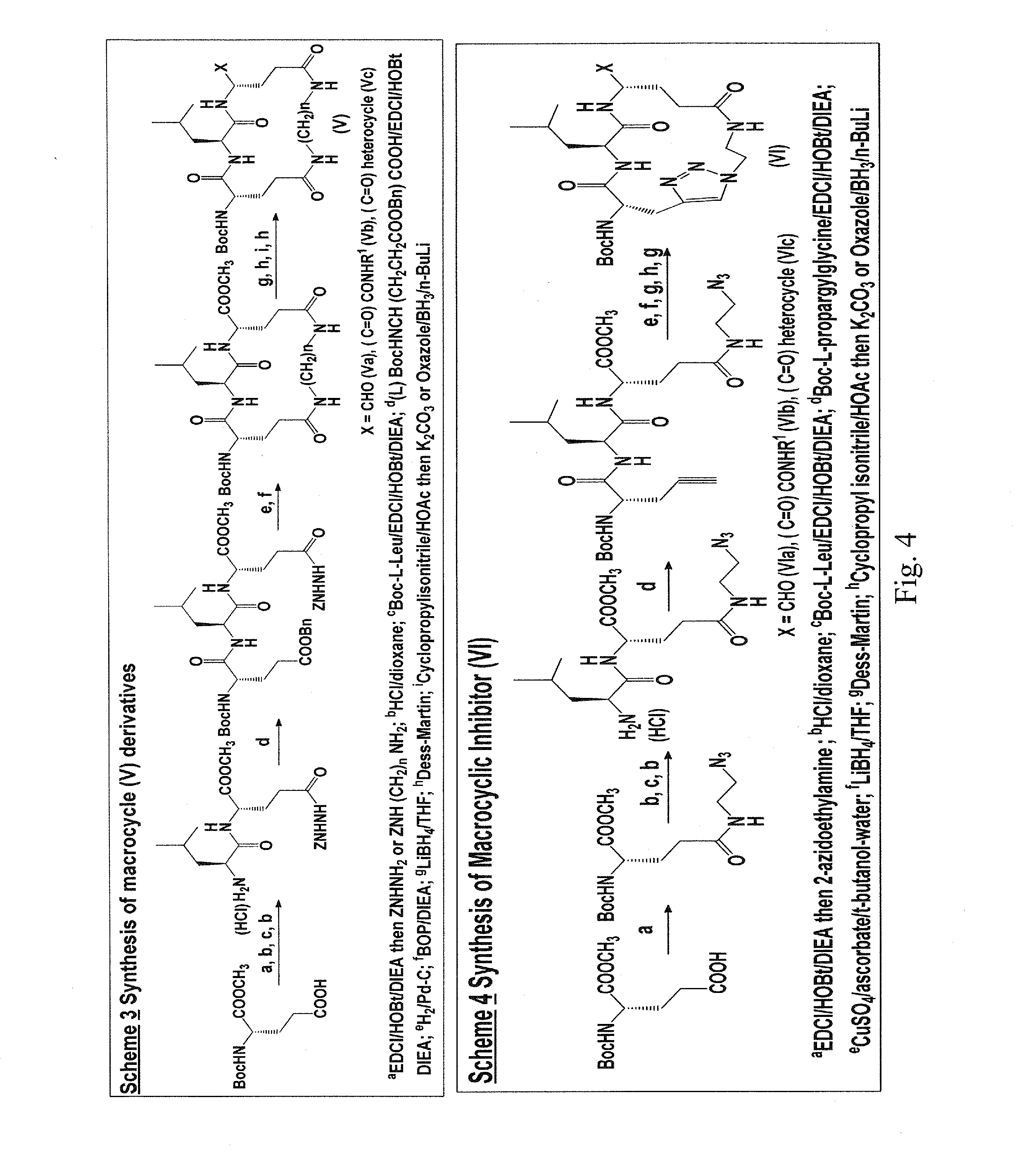
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3c or 3c-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +1
Broad-spectrum antivirals against 3C or 3C-like proteases of picornavirus-like supercluster: picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including peptidyl aldehydes, peptidyl α-ketoamides, peptidyl bisulfate salts, and peptidyl heterocycles, are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:WICHITA STATE UNIVERSITY +2
Recombinant poliovirus for the treatment of cancer
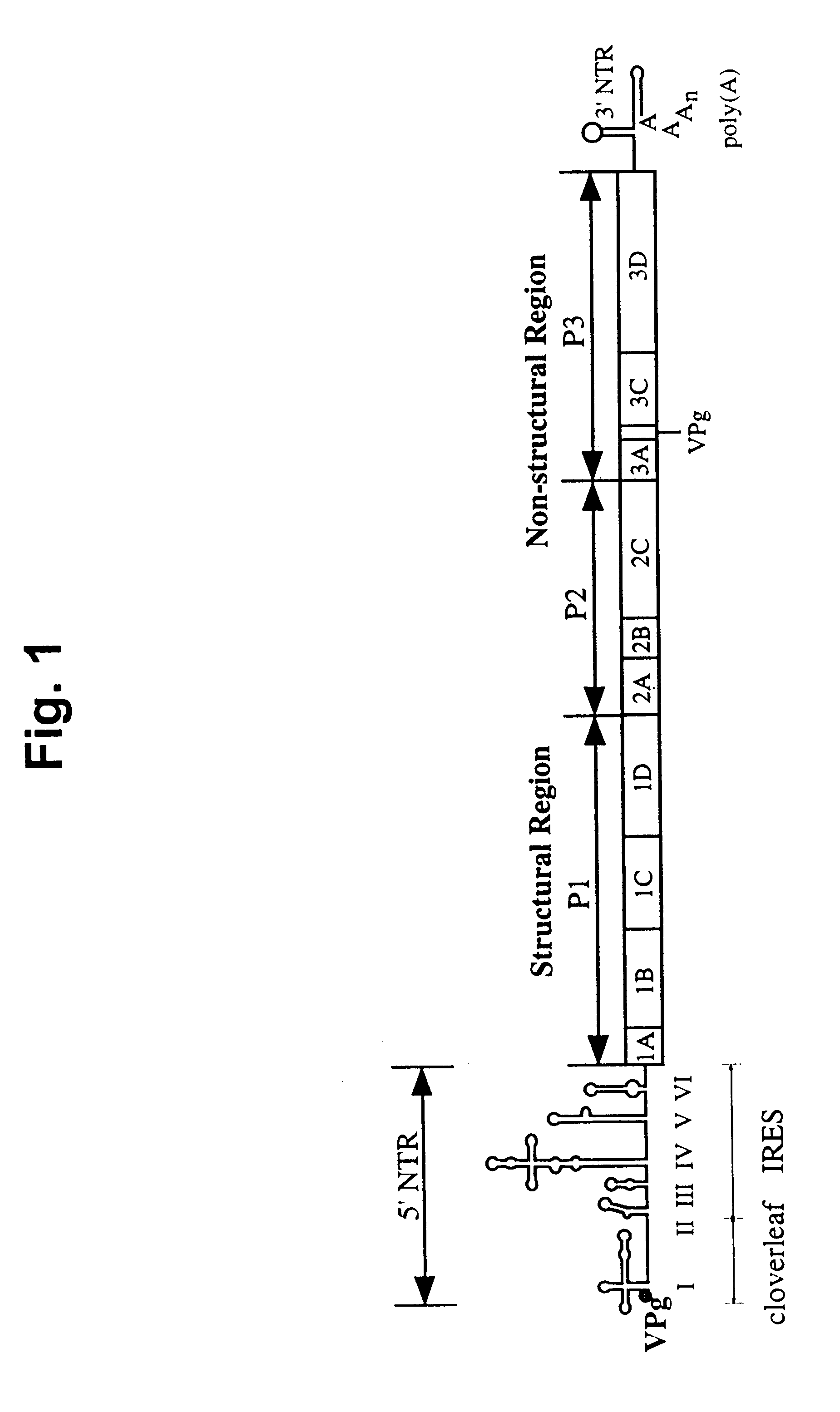
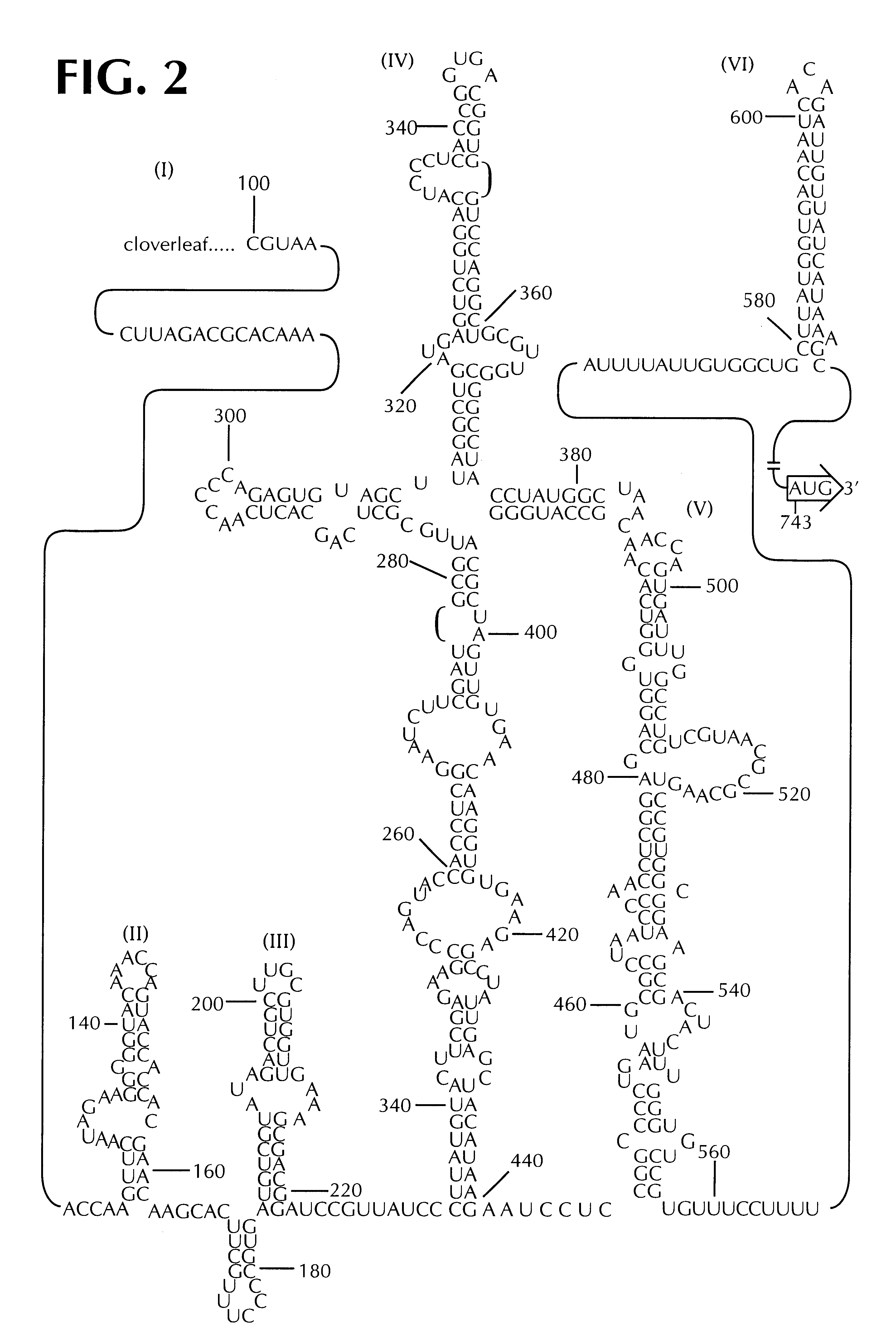
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:NEW YORK UNIV OF RES FOUND OF THE
Macrocyclic and peptidomimetic compounds as broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, caliciviruses and coronaviruses
Antiviral protease inhibitors, including macrocylic transition state inhibitors and peptidomimetics are disclosed, along with related antiviral compounds, and methods of using the same to treat or prevent viral infection and disease. The compounds possess broad-spectrum activity against viruses that belong to the picornavirus-like supercluster, which include important human and animal pathogens including noroviruses, sapoviruses, enteroviruses, poliovirus, foot-and-mouth disease virus, hepatitis A virus, human rhinovirus (cause of common cold), human coronavirus (another cause of common cold), transmissible gastroenteritis virus, murine hepatitis virus, feline infectious peritonitis virus, and severe acute respiratory syndrome coronavirus.
Owner:THE WICHITA STATE UNIV +1
Host targeted inhibitors of dengue virus and other viruses
ActiveUS9879003B2Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeOrganic chemistryHerpes simplex diseaseJunin virus
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Anti-viral compound
The present invention relates to compounds of Formula (I), which inhibit the growth of picornaviruses, Hepatitus viruses, enteroviruses, cardioviruses, polioviruses, coxsackieviruses of the A and B groups, echo virus and Mengo virus. In said Formula, A is phenyl, pyridyl, substituted phenyl, substituted pyridyl, or benzyl; R is hydrogen, COR4 or COCF; X is N-OH, O or CHR1 R1 is hydrogen, halo, CN, C -C alkyl -C≡ CH, CO(C -C alkyl), CO (C -C alkyl), or CONR2R3 R2 and R3 are independently hydrogen or C -C alkyl; A' is hydrogen, halo, C -C alkyl, benzyl, naphthyl, thienyl, furyl, pyridyl, pyrollyl, COR4 S(O)nR4 or a group of formula (II); R4 is C -C alkyl, phenyl, or substituted phenyl; n is 0,1, or 2; R5 is independently at each occurance hydrogen or halo; m is 1,2,3, or 4; and R6 is hydrogen, halo, CF, OH, CO H, NH, NO, CONHOCH, C -C alkyl, or CO (C -C alkyl), C -C alkoxy; or pharmaceutically acceptable salts thereof.
Owner:ELI LILLY & CO
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Antiviral Activity of Novel Bicyclic Heterocycles
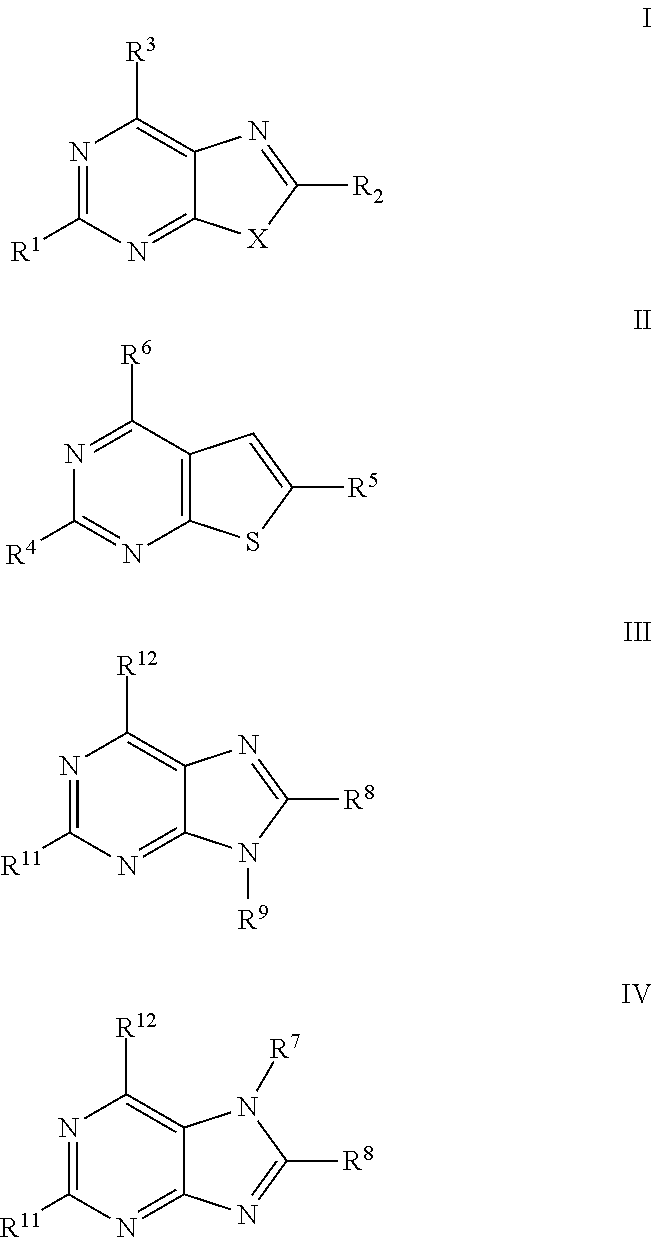
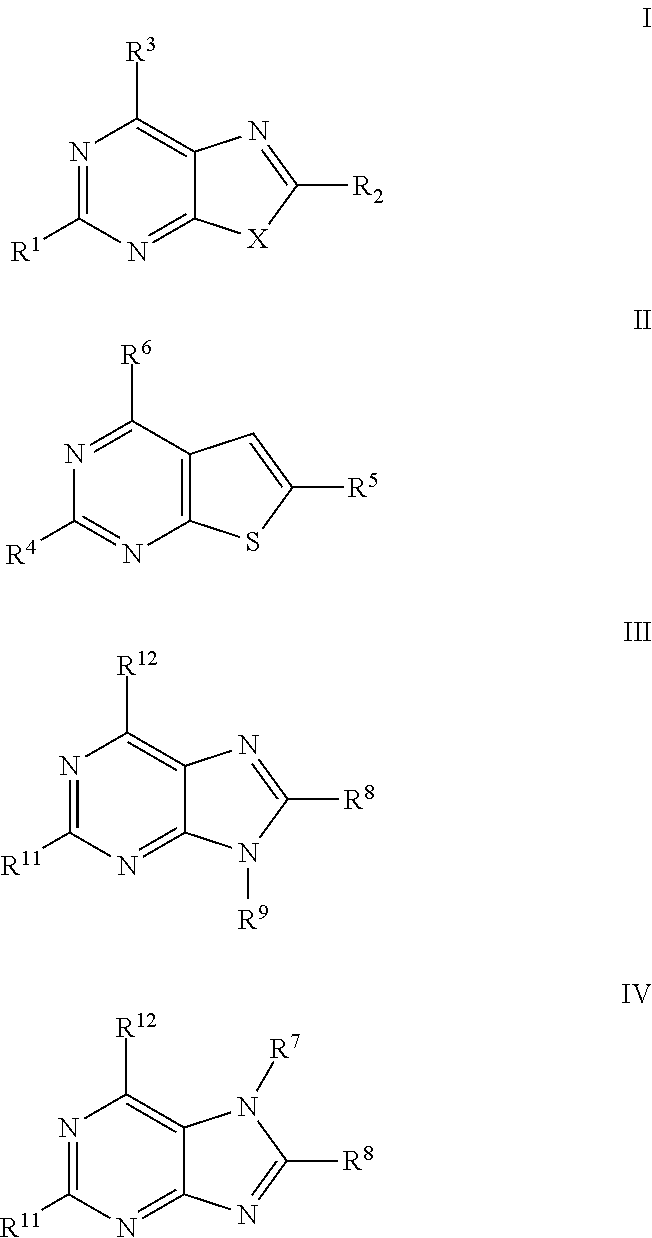
The present invention relates to compound of Formula I, II, III, or IV, and / or a pharmaceutical acceptable addition salt thereof and / or a stereoisomer thereof and / or a solvate thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R11, and R12 are as defined in the claim 1 or as described in detail in the description of the invention, and to the use of said compounds to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, particularly infections with RNA-viruses belonging to the family of the Retroviridae, the family of the Flaviviridae and the family of the Picornaviridae and more preferably infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus. The present invention also relates to pharmaceutical compositions of said compounds and the use of said pharmaceutical compositions to treat or prevent viral infections. The present invention further relates to the use of said compounds as biologically active ingredients, more specifically as medicaments for the treatment of viral disorders and pathologic conditions such as, but not limited to, viral infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus.
Owner:KATHOLIEKE UNIV LEUVEN
Attenuated poliovirus
ActiveUS8066983B2Enhanced replication propertyStably attenuatedBiocideSsRNA viruses positive-sensePoliomyelitisPoliovirus Receptor
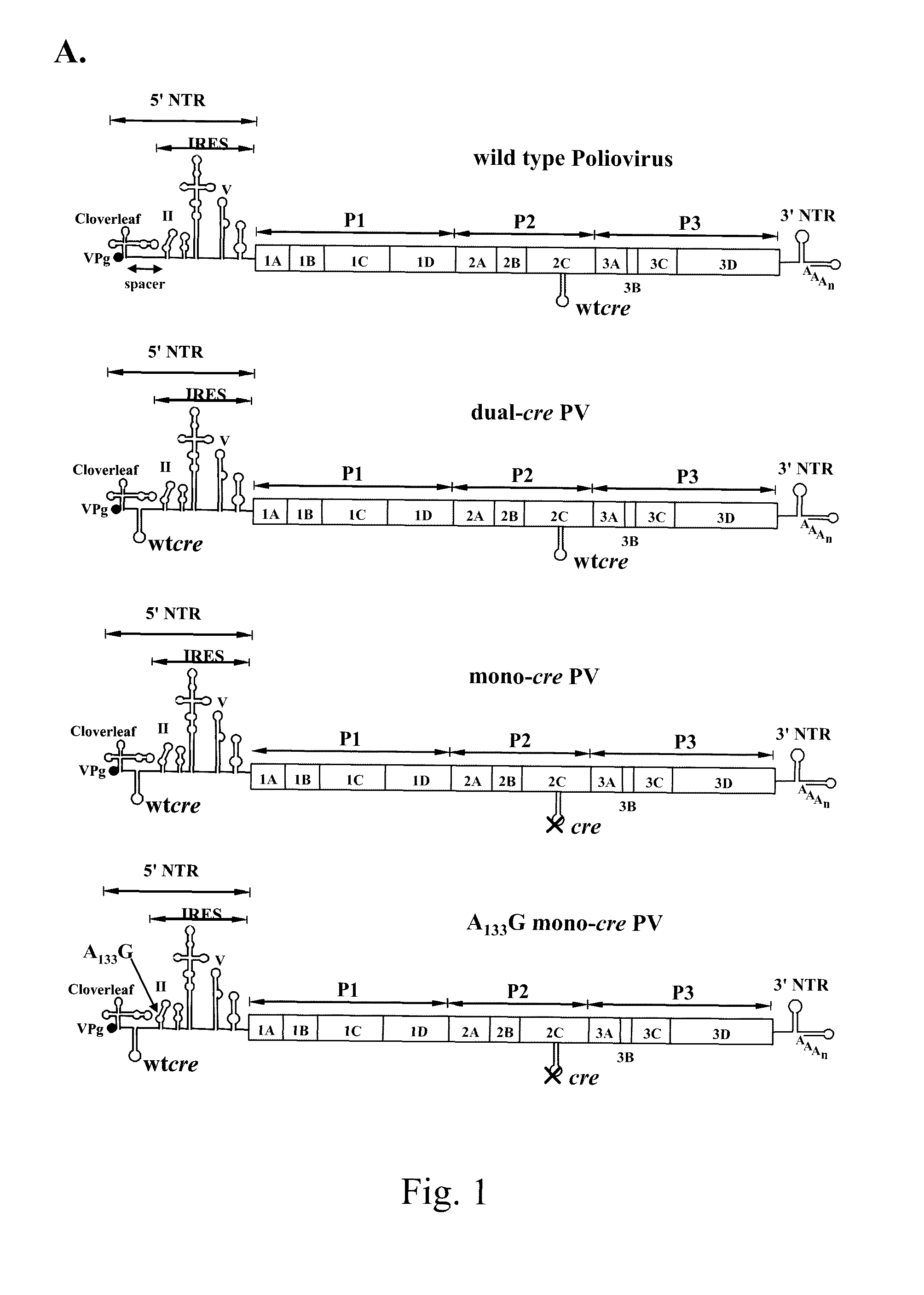
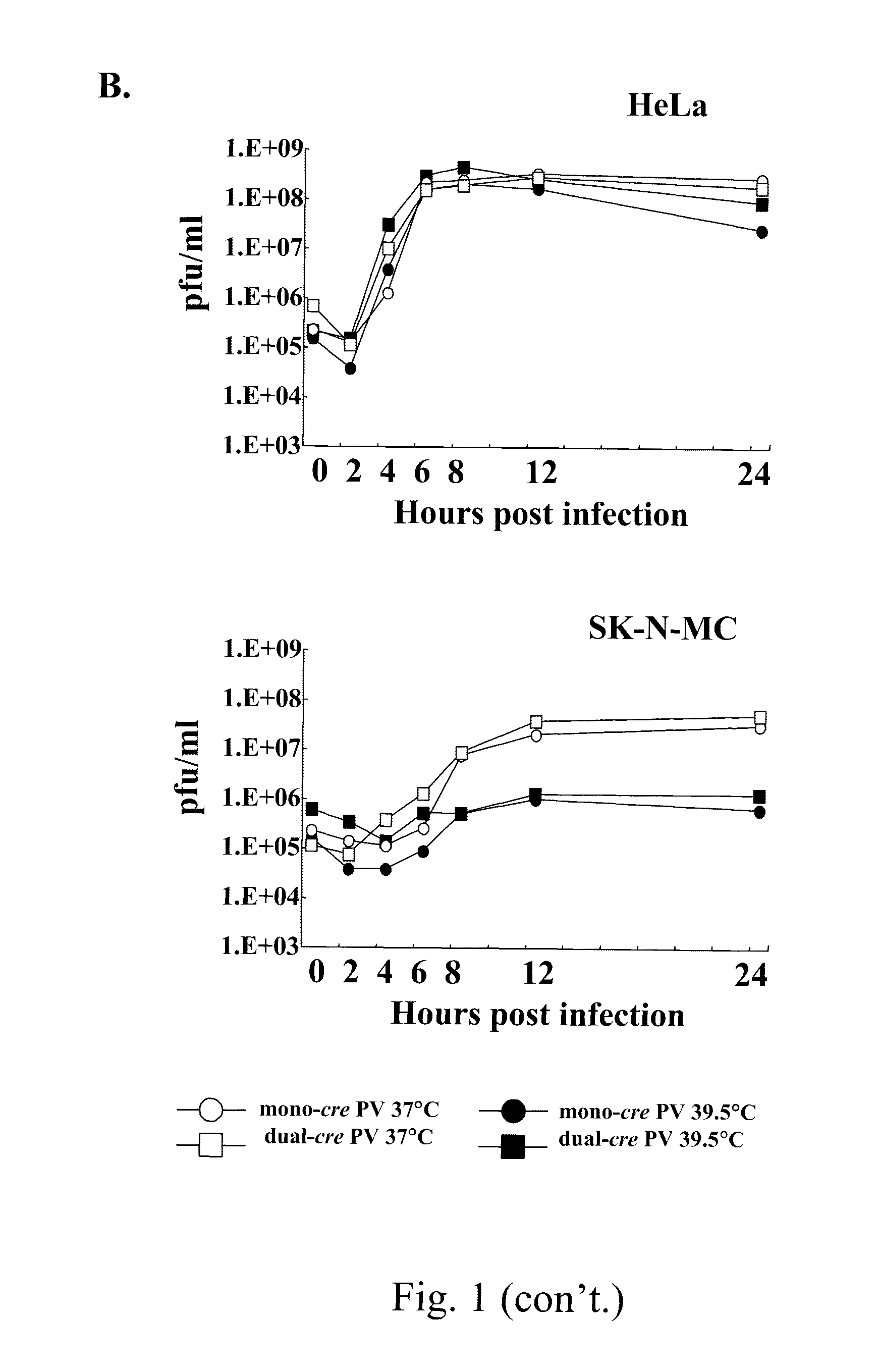
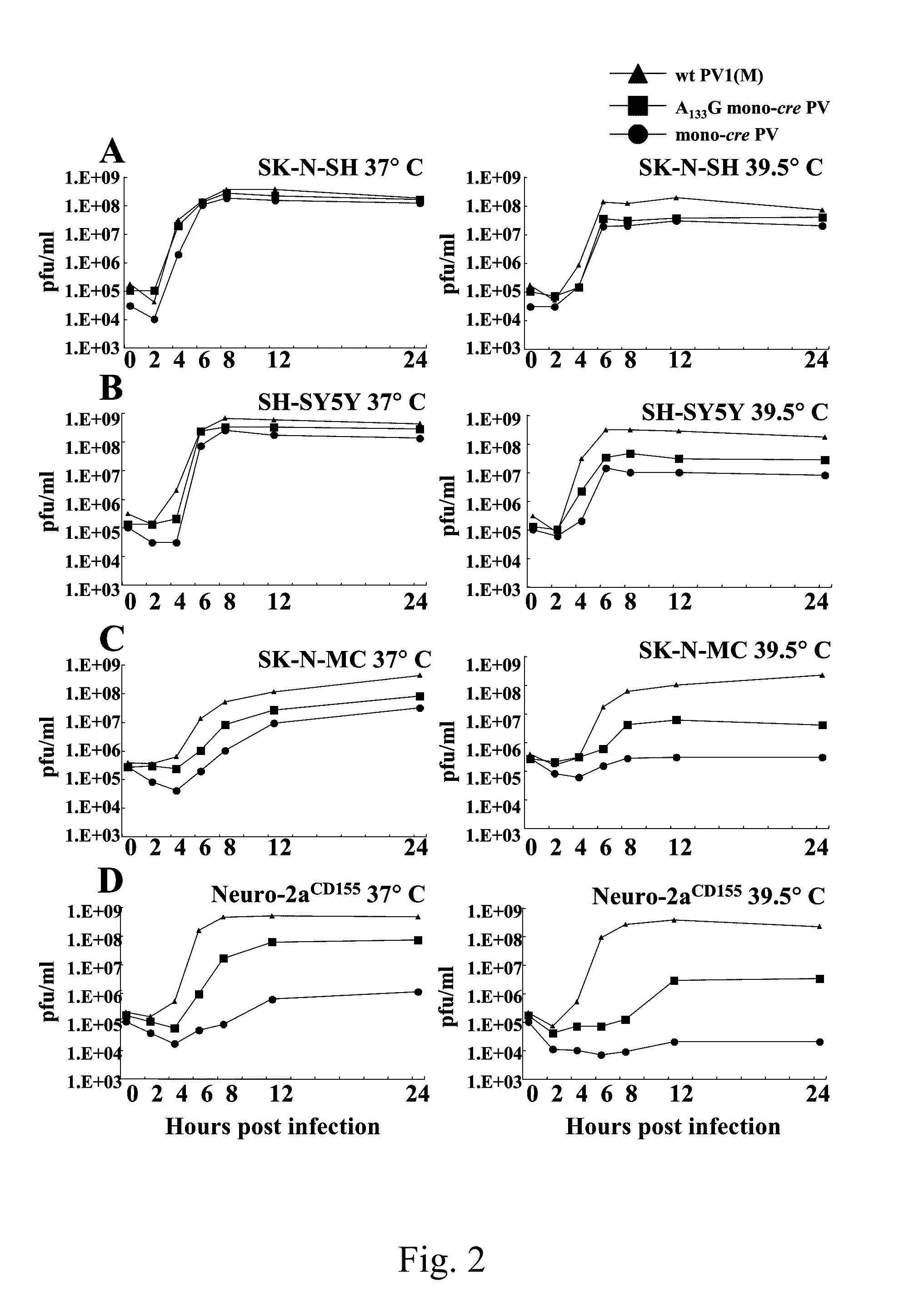
A novel and stable attenuated poliovirus, which replicates in neuroblastoma cells, is produced by engineering an indigenous replication element (cre), into the 5′ non-translated genomic region and inactivating the native cre element located in the coding region of 2C (mono-crePV). The stably attenuated poliovirus replicates in a neuroblastoma model (Neuro-2aCD155 tumors) expressing CD155, the poliovirus receptor, and is effective for oncolytic treatment and cure of solid tumors, such as neuroblastoma.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Clothing antibacterial softener preparation method
The invention relates to a clothing antibacterial softener preparation method, which comprises: weighing 5-10% by mass of dioctadecyl dimethyl ammonium chloride, 0.3-0.5% by mass of polydimethylsiloxane diquaternary ammonium salt, 7-15% by mass of luteolin, 2-10% by mass of ethylene glycol, and a proper amount of water, mixing, heating to a temperature of 55-60 DEG C, dissolving, heating to a temperature of 75 DEG C, adding 5-15% by mass of a thickening agent polyethylene glycol 6000, adding 0.5-1.0% by mass of eugenol, and cooling to obtain the product. According to the present invention, the luteolin is adopted as the main component so as to inhibit a variety of bacteria and viruses, such as staphylococcus aureus, escherichia coli, herpes simplex virus and poliovirus; the antibacterial function is strengthened under the premise of no influence on softening property and the like; and the clothing antibacterial softener with characteristics of good compatibility with the softener, stable performance, good antibacterial effect, capability of inhibition of odor due to bacterial breeding during a wearing process of clothing, no odor, no irritating, low toxicity, safety and no harm on environment is provided.
Owner:WUJIANG CITY LI DA LUSTRE FINISHED PROD
Multivalent DTP-POLIO vaccines
InactiveCN101310769AEffective preventionSsRNA viruses negative-senseAntibacterial agentsTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
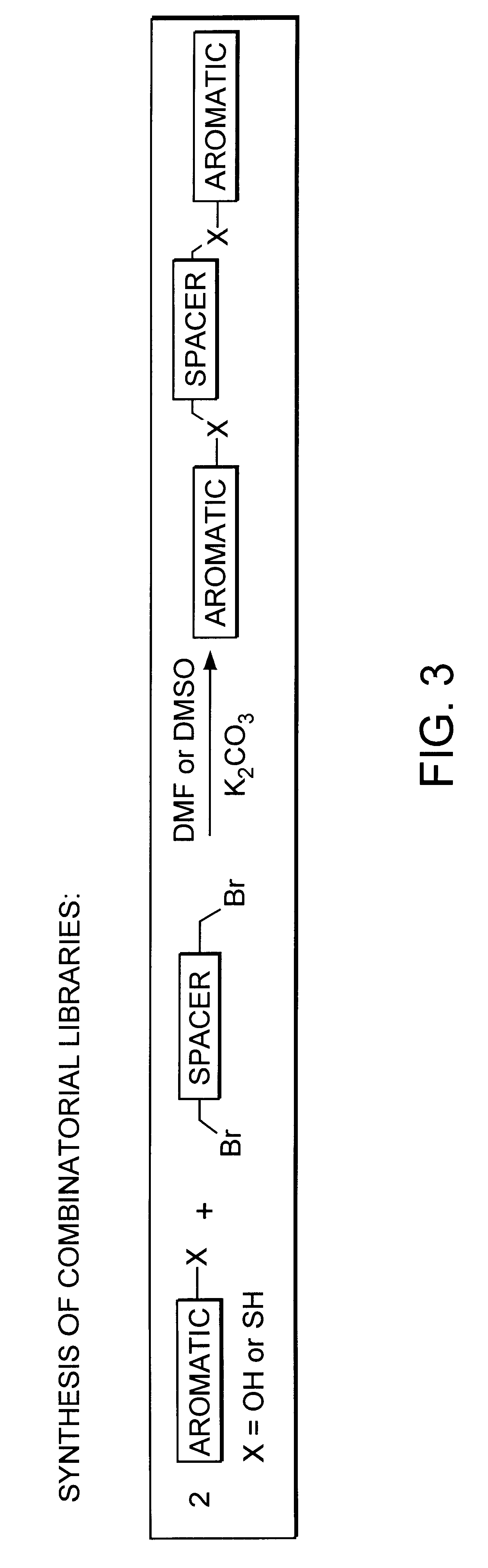
Anti-picornaviral ligands via a combinatorial computational and synthetic approach
The present invention provides structure-based combinatorial libraries of compounds containing the functional group minima of picornaviruses including poliovirus and rhinovirus. The libraries can be used to screen for therapeutical antiviral compounds, e.g., anti-picornaviral capsid-binding compounds.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Methods and compositions for stabilizing dried biological materials
ActiveUS20150030629A1Minimizing loss of activityImprove stabilityPowder deliveryBiocideVaccinationMagnesium salt
The present invention relates to methods for producing dried formulations of biopharmaceutical agents that aim to minimize the loss of activity of the agents upon drying and to provide dried formulations with an extended shelf life. The method comprises the step of drying an aqueous solution comprising, in addition to the biopharmaceutical agent, at least an amino acid, a polyol and a metal salt. Preferably the amino acid is glutamate, the polyol is sorbitol and optionally also mannitol and the metal salt is a magnesium salt. The solution is dried by vacuum drying or by lyophilization. The methods are particularly useful for preparing dried formulations of viruses such as poliovirus or respiratory syncytial virus to be used for vaccination. The invention also relates to dried formulations prepared in accordance with the methods of the invention and to their use as medicaments, e.g. as vaccines.
Owner:INTRAVACC BV
Vaccine
InactiveUS20100040647A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseDiseaseTetanus
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus are provided
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
IPV-DPT vaccine
InactiveUS8753646B2Efficient productionAntibacterial agentsBacterial antigen ingredientsProtective antigenTetanus toxoids
The invention provides a process for producing a combined vaccine containing an inactivated Sabin strain of poliovirus, a Bordetella pertussis protective antigen, a diphtheria toxoid and a tetanus toxoid, the process including a step of producing a high-titer Sabin strain poliovirus. The inventive process for producing a combined vaccine, including a step of culturing, in the presence of from about 4 g / L to about 6 g / L of a microcarrier, Vero cells to be inoculated with a Sabin strain of poliovirus, is useful as a process for efficiently producing a combined vaccine containing an inactivated Sabin strain of poliovirus.
Owner:TAKEDA PHARMA CO LTD +1
Multi-component vaccine to protect against disease caused by Haemophilus influenzae and Moraxella catarrhalis
InactiveUS6391313B1Enhanced primary anti-H91A Hin4 responseLower immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
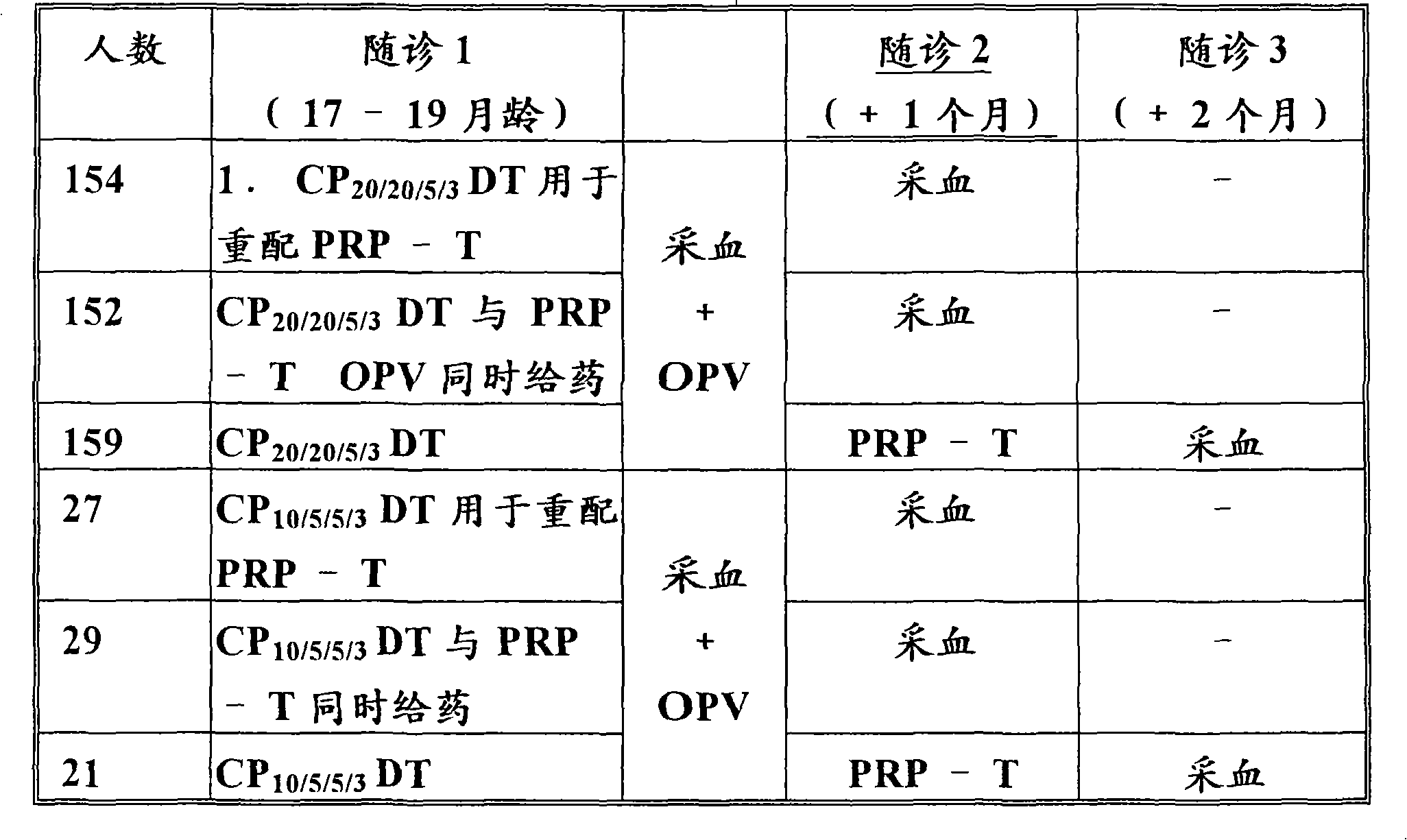
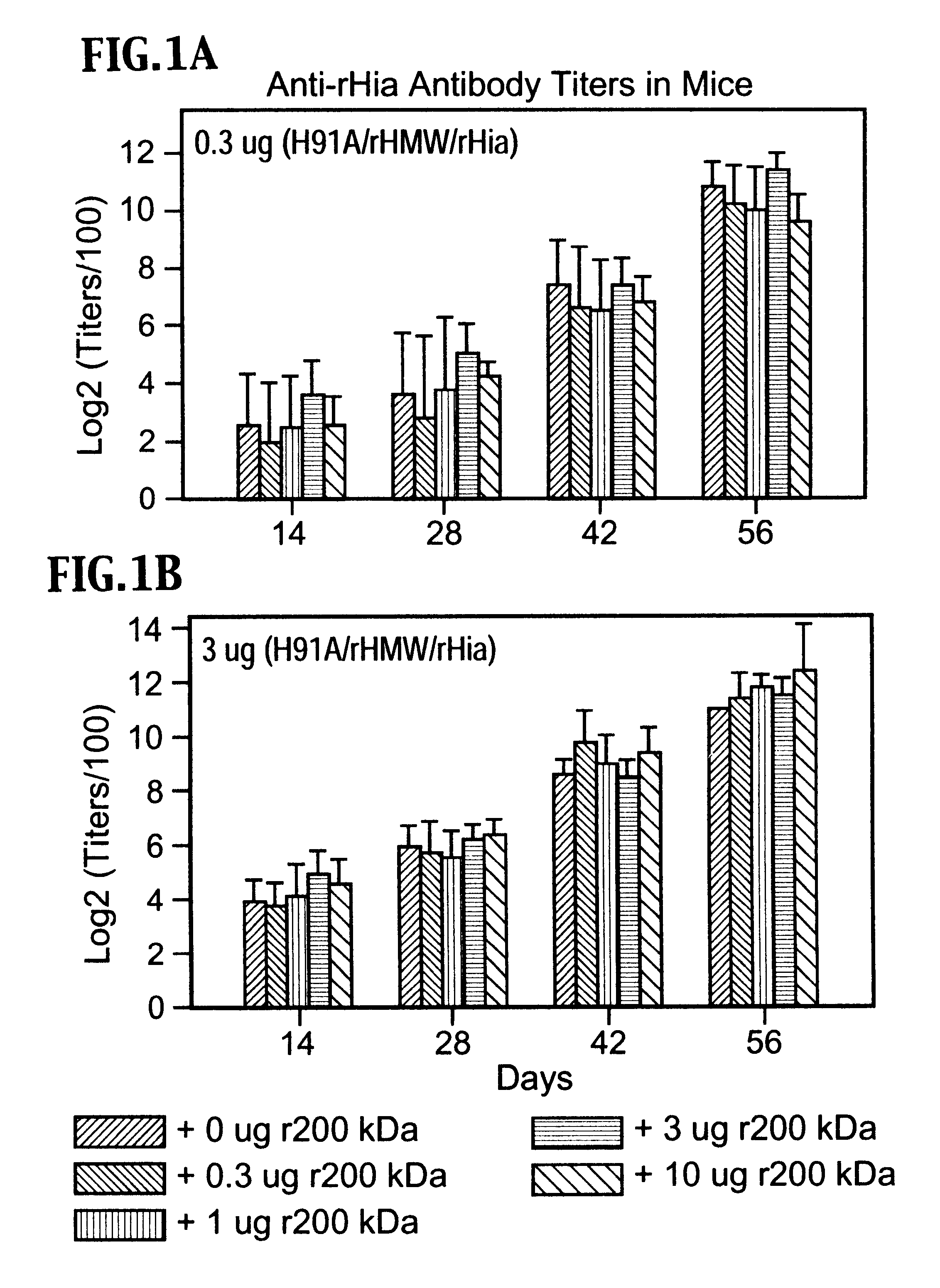
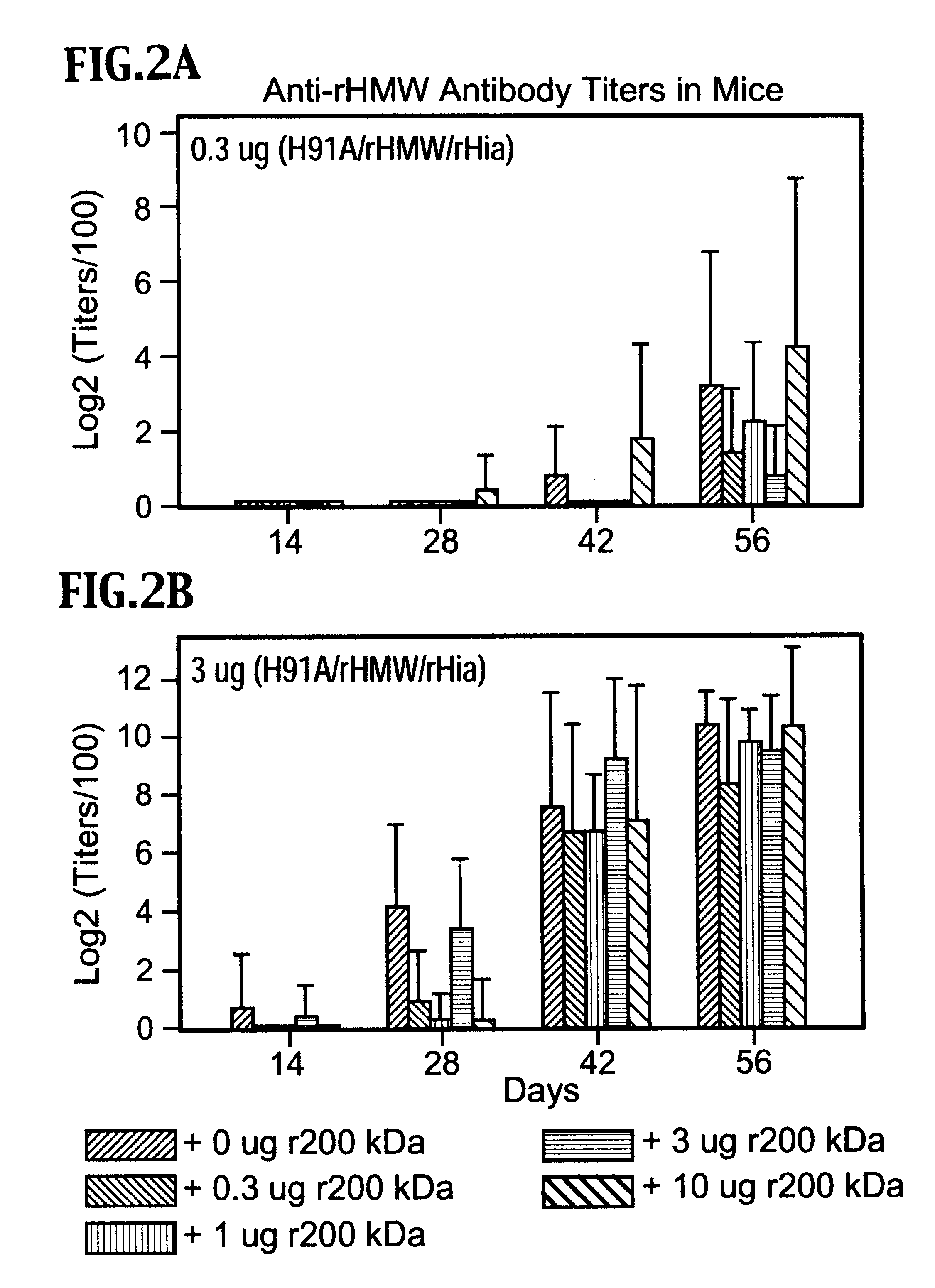
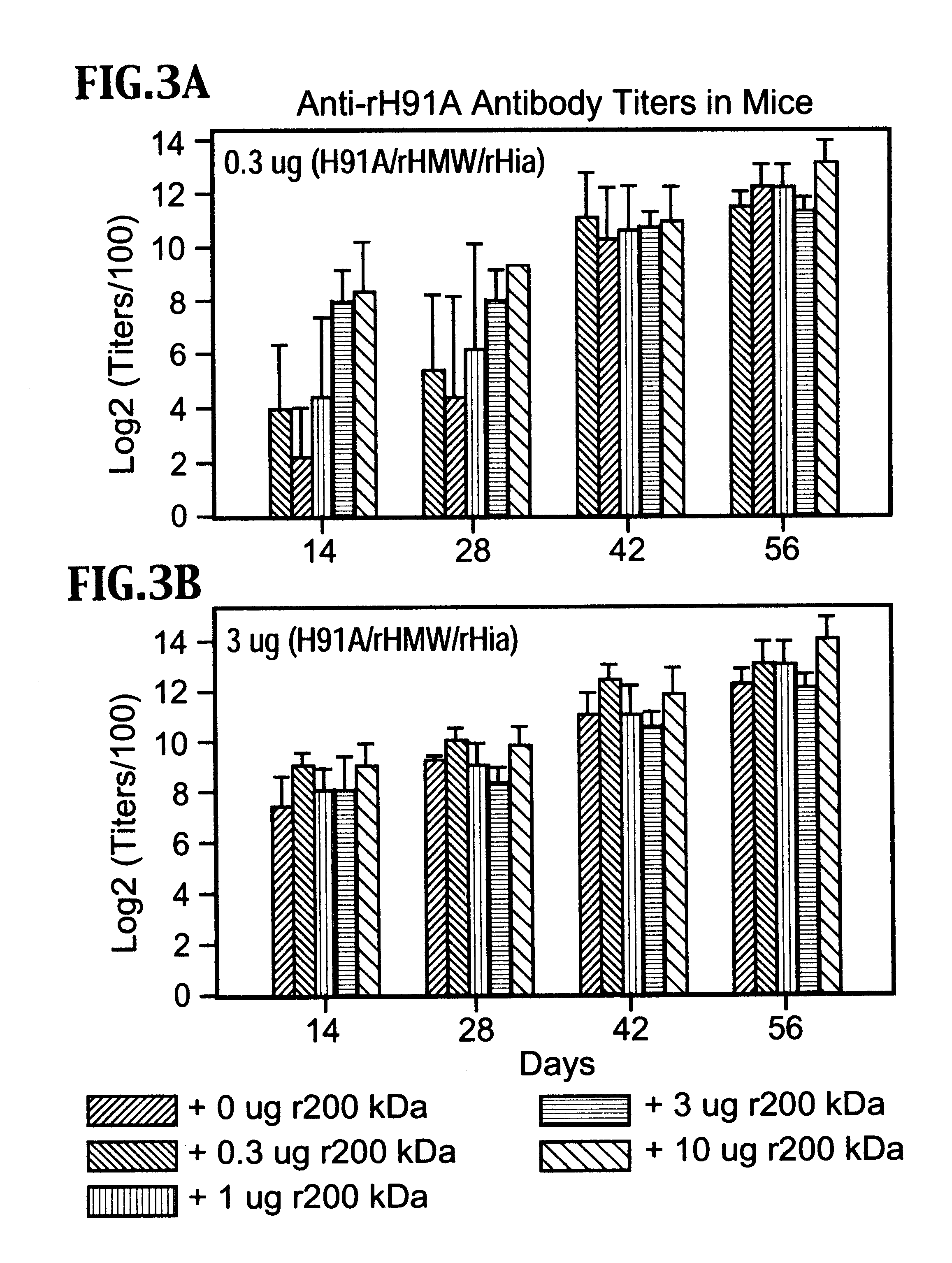
A multi-valent immunogenic composition confers protection on an immunized host against infection caused by both Haemophilus influenzae and Moraxella catarrhalis. Such composition comprises at least four antigens comprising at least one antigen from Haemophilus influenzae, and at least one antigen from Moraxella catarrhalis. Three of the antigens are adhesins. High molecular weight (HMW) proteins and Haemophilus influenzae adhesin (Hia) proteins of non-typeable Haemophilus and a 200 kDa outer membrane protein of Moraxella catarrhalis comprise the adhesin components while the other antigen is a non-proteolytic analog of Hin47 protein. Each component does not impair the immunogenicity of the others. The multi-valent immunogenic composition may be combined with DTP component vaccines, which may also include non-virulent poliovirus and PRP-T, to provide a component vaccine without impairment of the immunogenic properties of the other antigens.
Owner:AVENTIS PASTUER LTD
Fatty acid complex
ActiveCN108605933ABroad-spectrum bactericidal activityBroad-spectrum antiviral activityBiocideFruit and vegetables preservationEscherichia coliOctanoic Acids
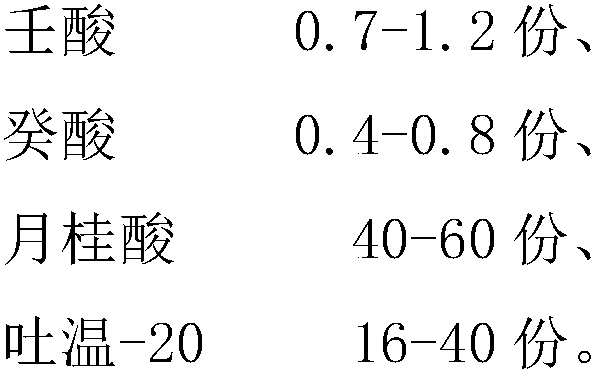
The invention discloses a fatty acid complex. The fatty acid complex comprises the following components in parts by weight: 2.2-3 parts of mixed fatty acid, 16-40 parts of a surfactant and 40-60 partsof auxiliary acid, wherein the mixed fatty acid is a mixture of octanoic acid, nonoic acid and capric acid; the auxiliary acid is lauric acid or a mixture of the lauric acid and citric acid. The fatty acid complex has broad-spectrum bactericidal and antiviral activity, can kill polioviruses, foot-and-mouth disease viruses, porcine reproductive and respiratory syndrome viruses and avian influenzaviruses, has the staphylococcus aureus and escherichia coli killing rate as high as 99.99999%; the fatty acid complex has excellent storage stability under a diluted condition, is applied to a food disinfectant, has an excellent food preserving function, is free of cleaning during disinfection, free of metal corrosion, practically non-toxic and non-irritant to skin, and is unlikely to volatilize;the fatty acid complex has acidic pH and a light coconut scent, disinfecting cleaning sewage does not pollute the environment, and the fatty acid complex can be reused in some disinfecting scenes.
Owner:上海康归生物科技有限公司
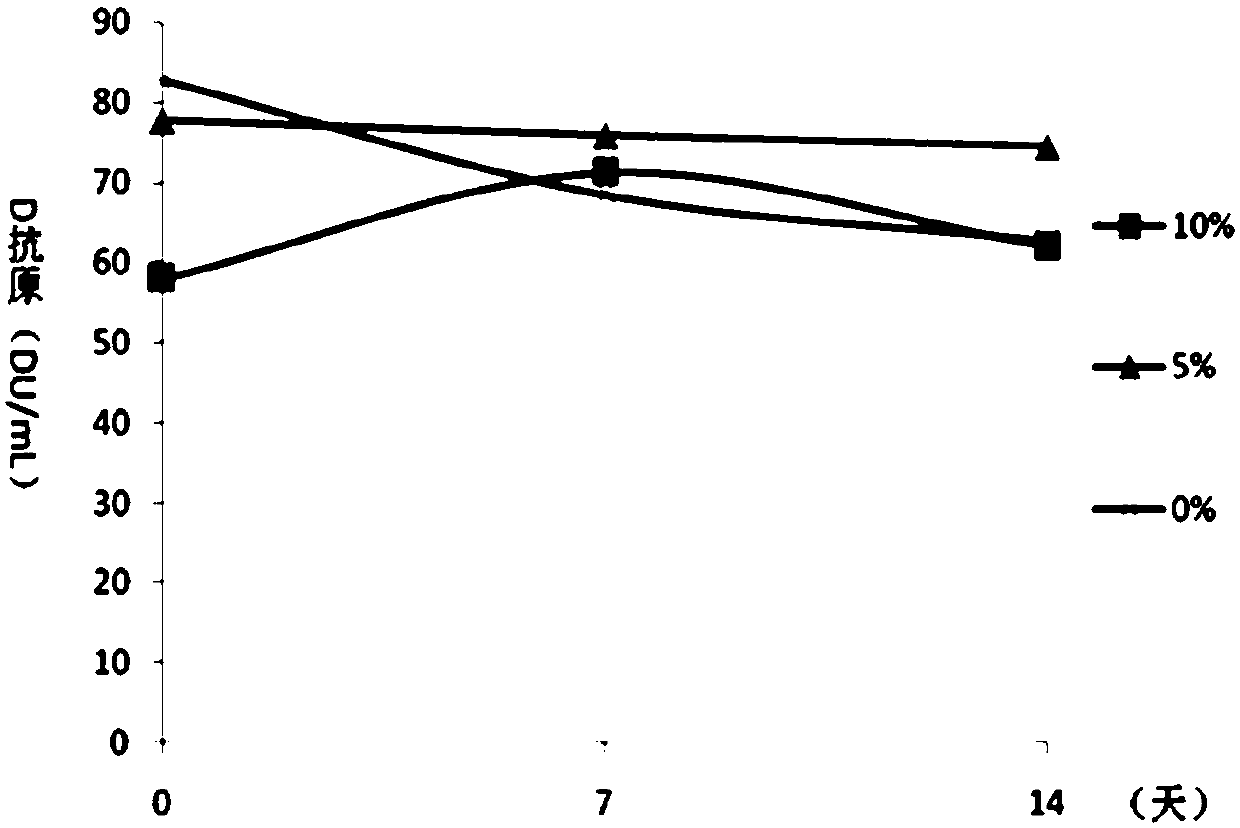
Method for detecting content of D antigen in poliovirus type III
InactiveCN106290886AQuantitatively accurateEasy to operateBiological material analysisMonoclonal antibodyPolyclonal antibodies
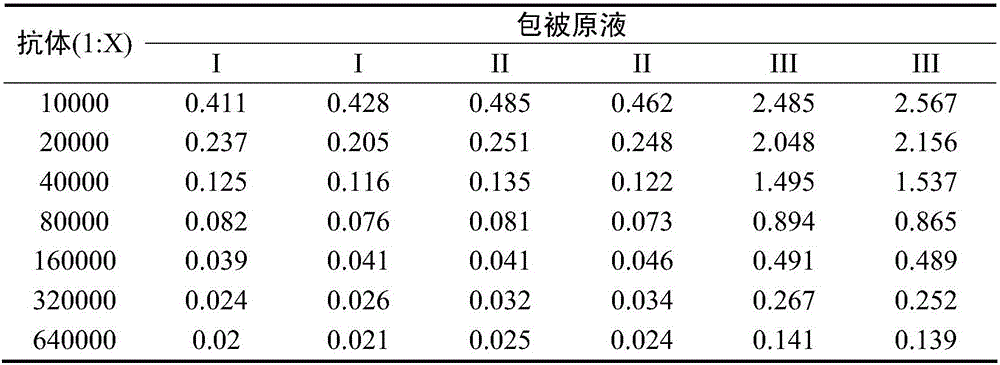
The invention provides a method for detecting the content of D antigen in poliovirus type III and belongs to the technical field of antigen detection. Through matching of a poliovirus type III specific polyclonal antibody and a monoclonal antibody, the content of D antigen in poliovirus type III can be detected in a highly targeted mode without detecting C antigen. The method is easy to operate, the detection result can truly reflect the content of effective antigen in poliovirus, a basis can be well provided for follow-up poliomyelitis vaccine immunogenicity testing usage and the final concentration of a vaccine product, and the quality of poliomyelitis vaccine can be improved finally. The method has a high economic value and a high market application value.
Owner:SINOVAC BIOTECH
Preparation method of dressing skin
InactiveCN104940982AImprove securityImprove inactivation efficiencyAbsorbent padsBandagesNeutral proteaseCuticle
The invention discloses a preparation method of a dressing skin. The preparation method comprises the following steps of: removing hairs of xenoskin raw materials, and scraping to remove fat and a cuticle so as to obtain a xenoskin material with the thickness of 0.3-0.5 mm; sequentially immersing by using NaCl solution, cleaning by using sterile water, immersing by using neutral protease with the mass fraction of 0.2-0.5% at 37 DEG C, and cleaning by using sterile water so as to obtain a xenoskin; and inactivating the xenoskin so as to obtain the dressing skin, wherein the inactivating step comprises the steps of heating the xenoskin at 50-60 DEG C for 60-120 MIN and irradiating the xenoskin by using gamma rays. The preparation method of the dressing skin is simple to operate and low in cost; four kinds of viruses including PRV (Pseudorabies Virus), VSV (Vesicular Stomatitis Virus), PV1 (Poliovirus 1) and PPV (Porcine Parvovirus) can be completely inactivated; the virus inactivating efficiency is obviously increased; the problem that PV1 cannot be completely inactivated only by irradiating gamma rays can be solved; and therefore, the safety of the dressing skin is increased.
Owner:CHANGSHA DARUIQI IND
Production of poliovirus at high titers for vaccine production
ActiveUS20110027317A1High potencyShort processNervous disorderSsRNA viruses positive-senseSerum freeVaccine Production
Provided is a process for the production of poliovirus, comprising the steps of: a) providing a serum-free suspension culture of cells, which are primary human retina (HER) cells that have been immortalized by expression of adenovirus E1 sequences, b) infecting the cells with poliovirus, at a cell density of between 2×106 cells / ml and 150×106 cells / ml, and c) harvesting poliovirus at a time of between 12 and 48 hours after infection.
Owner:JANSSEN VACCINES & PREVENTION BV
Adsorbed DTaP-IPV/Hib combined vaccine and preparation method thereof
ActiveCN109550046AImprove securityHigh biosecurityAntibacterial agentsBacterial antigen ingredientsMedicineHaemophilus influenzae type B antigen
The invention discloses an adsorbed DTaP-IPV / Hib combined vaccine and a preparation method thereof. The combination vaccine provided by the present invention includes an acellear pertussis antigen, adiphtheria antigen, a tetanus antigen, inactivated poliovirus and a haemophilus influenzae type b antigen, wherein the poliovirus is a Sabin strain. The method adopts the attenuated strain of the poliovirus Sabin strain to prepare the combined vaccine, and the combined vaccine has higher biosafety, less adverse reaction, and low production cost. By optimizing the antigen composition, adjuvants andstabilizers of the combined vaccine, the immunogenicity of various antigens in the combined vaccine provided by the invention are fully exerted, and the combined vaccine has high stability, and is suitable for popularization and application.
Owner:BEIJING MINHAI BIOTECH
Combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin inactivated poliovirus and preparation thereof
InactiveCN102178949ASimplified and expanded immunization programsReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseTetanus toxoids
The invention provides combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin (DTaP-sIPV) inactivated poliovirus and preparation thereof. The DTaP-sIPV is characterized in that each 100ml of the combined vaccine comprises the following components: 400-1800ug (PN) of acellular pertussis (AP) stock solution, 300-700Lf of tetanus toxoid (TT), 1000-2500Lf of diphtheria toxoid (DT), 126-154mg of Al(OH)<3>, 3000-6000DU of sIPV I, 5700-7100DU of sIPV II, 4500-9000DU of sIPV III, 765-935mg of NaCl, 0-600mg of 2-phenoxyethanol and the balance of H<2>O. Compared with the existing products, the DTaP-sIPV has the advantages of higher biological safety, better side reaction and the like; and the DTaP-sIPV has the beneficial effects of preventing a plurality of target diseases, reducing inoculating needles, simplifying immunization programs, improving inoculation rate, reducing opportunity of cross infection, being popular with a majority of parents and children, saving various expenses and facilitating smooth promotion of immunization plan.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Combination heptavalent vaccine
ActiveUS20130280293A1Reduce in quantityImprove satisfactionBacterial antigen ingredientsSsRNA viruses positive-senseClostridium tetaniImmunogenicity
The invention provides a stable immunogenic composition for prevention and prophylaxis of infections caused by rota virus, poliomyelitis virus, Haemophilius influenza, Hepatitis B, Corynebacterium diphtheriae, Clostridium tetani, Bordatella pertusis (acellular) in a single combined vaccine. The invention also provides for a bivalent immunogenic composition against rota virus and polio virus. The process of making such compositions of the multivalent antigens are also disclosed. The present invention also relates to the production and use of such vaccines for prophylaxis against the infections mentioned above.
Owner:BHARAT BIOTECH INTERNATIONAL
Oncolytic poliovirus for human tumors
Human clinical use of a chimeric poliovirus construct has demonstrated excellent anti-tumor effect. The mechanism of action is believed to involve both viral oncolysis as well as immune recruitment, both of which lead to necrosis in the area of the tumor. No adverse effects have been observed.
Owner:DUKE UNIV
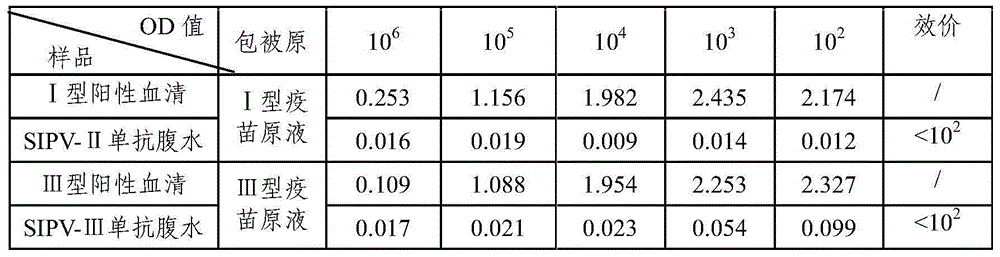
Sabin strain poliovirus type II monoclonal antibody and application thereof
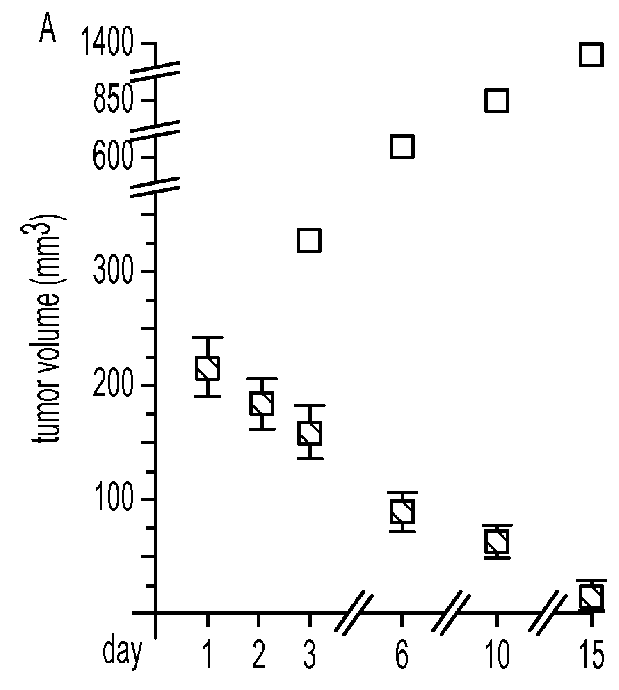
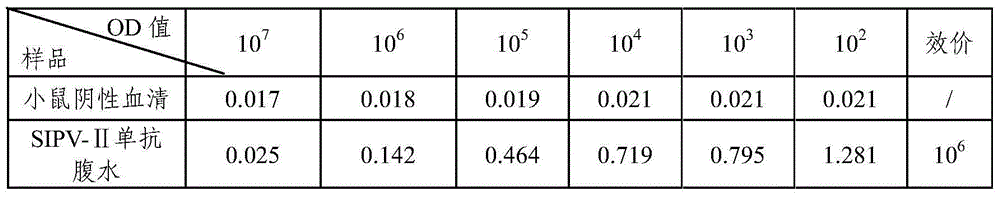
ActiveCN104371979AHigh titer reactivityHigh School and ValenceMicroorganism based processesImmunoglobulins against virusesEnterovirusMicrobiology
The invention provides a Sabin strain poliovirus type II monoclonal antibody and an application thereof, and belongs to the immunology field. After a mouse is immunized and inoculated with Sabin strain poliovirus type II, mouse spleen cells are fused with mouse myeloma cells, a hybridoma cell strain producing the anti-Sabin strain poliovirus type II specific monoclonal antibody is screened and has the preservation number of CGMCC No.9232, and the secreted monoclonal antibody has high titer, has strong neutralizing activity, and can effectively block infection of the poliovirus type II; at the same time, the antibody can specifically distinguish the poliovirus type II from poliovirus type I, poliovirus type III and other various enteroviruses, and can be used for preparing type II antigen content detection kits and antibody detection kits of the poliovirus type II and a poliomyelitis inactivated vaccine, also can be used for identification detection of the poliovirus type II, and has the broad application prospect.
Owner:SINOVAC BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com