Oncolytic poliovirus for human tumors
a technology of human tumors and oncolytic poliovirus, applied in the field of antitumor therapy of oncolytic virus, can solve problems such as unpredictable efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
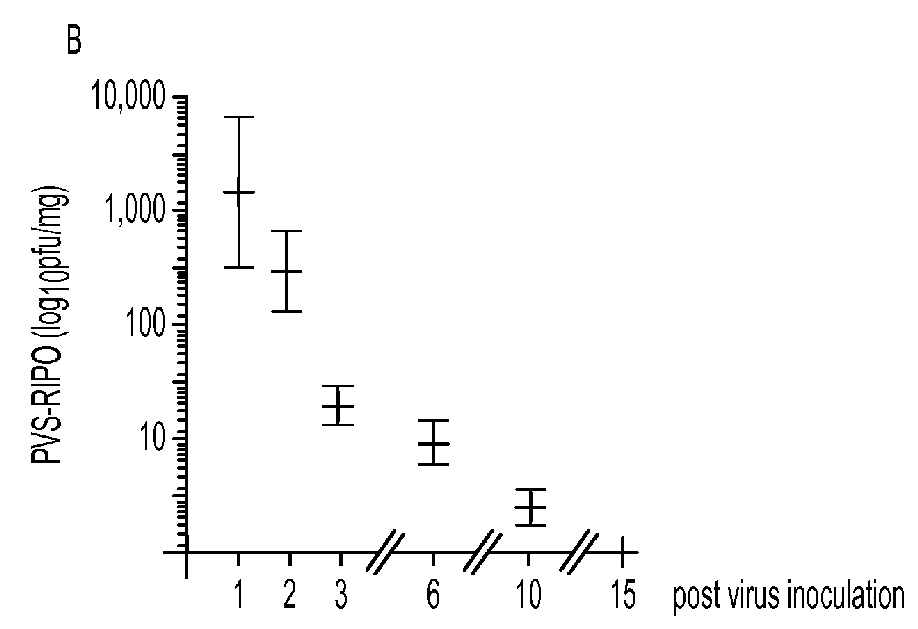
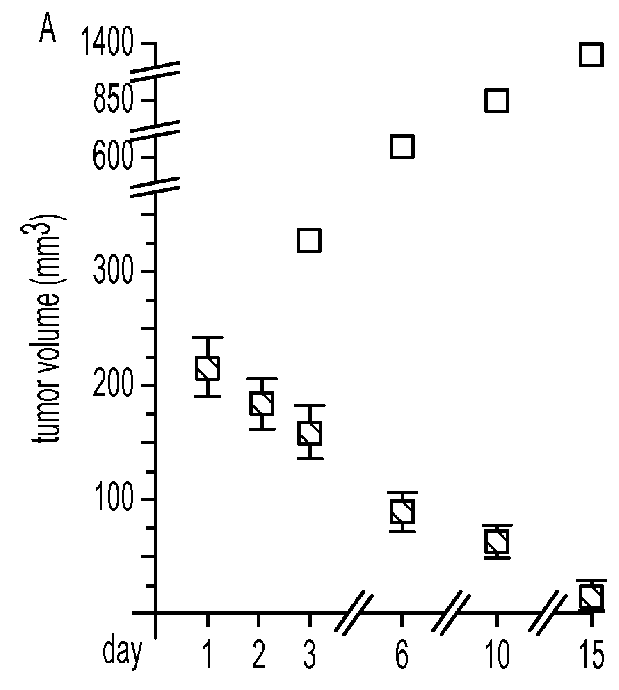
[0025]Animal tumor models. An IND-directed efficacy trial of PVS-RIPO was conducted in the HTB-15 GBM xenograft model in athymic mice. PVS-RIPO (from the clinical lot) was administered at the ‘mouse-adjusted’, FDA-approved max. starting dose [the FDA-approved max. starting dose (10e8 TCID) was adjusted for the reduced tumor size in mice (to 6.7×10e6 TCID)]. Delivery mimicked the intended clinical route, i.e., slow intratumoral infusion. Under these conditions, PVS-RIPO induced complete tumor regress in all animals after 15 days (FIG. 8A). While virus was recovered from treated tumors until day 10, the levels were modest at best, indicating that direct viral tumor cell killing alone cannot account for the treatment effect (FIG. 8B)
[0026]Evidence from animal tumor models suggests that intratumoral inoculation of PVS-RIPO causes direct virus-induced tumor cell killing and elicits a powerful host immunologic response against the infected / killed tumor (3, 7, 10). The response to virus in...
example 2
[0027]Clinical trials. IND no. 14,735 ‘Dose-finding and Safety Study of PVSRIPO Against Recurrent Glioblastoma’ was FDA-approved on Jun. 19, 2011 and IRB-approved on Oct. 27, 2011. A phase I / II clinical trial in patients with recurrent glioblastoma (GBM) (NCT01491893) is currently enrolling patients.
[0028]Two human subjects have so far been treated with PVS-RIPO per IRB-approved protocol. Preliminary findings from the first subject are described in Example 3.
example 3
[0029]Preliminary findings with first human subject. The patient is a 21-year-old female nursing student diagnosed with a right frontal GBM (WHO grade IV). She was first diagnosed in June 2011, at the age of 20 years, following a history of severe headaches and unsuccessful treatment for a suspected sinus infection. Brain imaging was obtained on Jun. 17, 2011 and showed a large right frontal mass, measuring ˜5×6 cm. She underwent a subtotal resection of the right frontal mass on Jun. 22, 2011, with pathology confirming GBM (WHO grade IV). Given the young age of the patient, her excellent performance status and the subtotal tumor resection, it was decided to treat her aggressively with a combination of six weeks of radiation therapy with concurrent Temodar chemotherapy at 75 mg / m2 by mouth daily and bevacizumab (antiangiogenic agent) administered every 2 weeks. The patient completed six weeks of treatment on Sep. 18, 2011. On Oct. 3, 2011, the patient initiated adjuvant therapy with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com