Patents
Literature
44 results about "Bacterial contaminants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bacterial contamination is a situation which occurs when bacteria end up in a location where they are not supposed to be. It is often used to refer to contamination of food by bacteria which can cause disease, but can also occur in other settings. This situation is not desirable,...
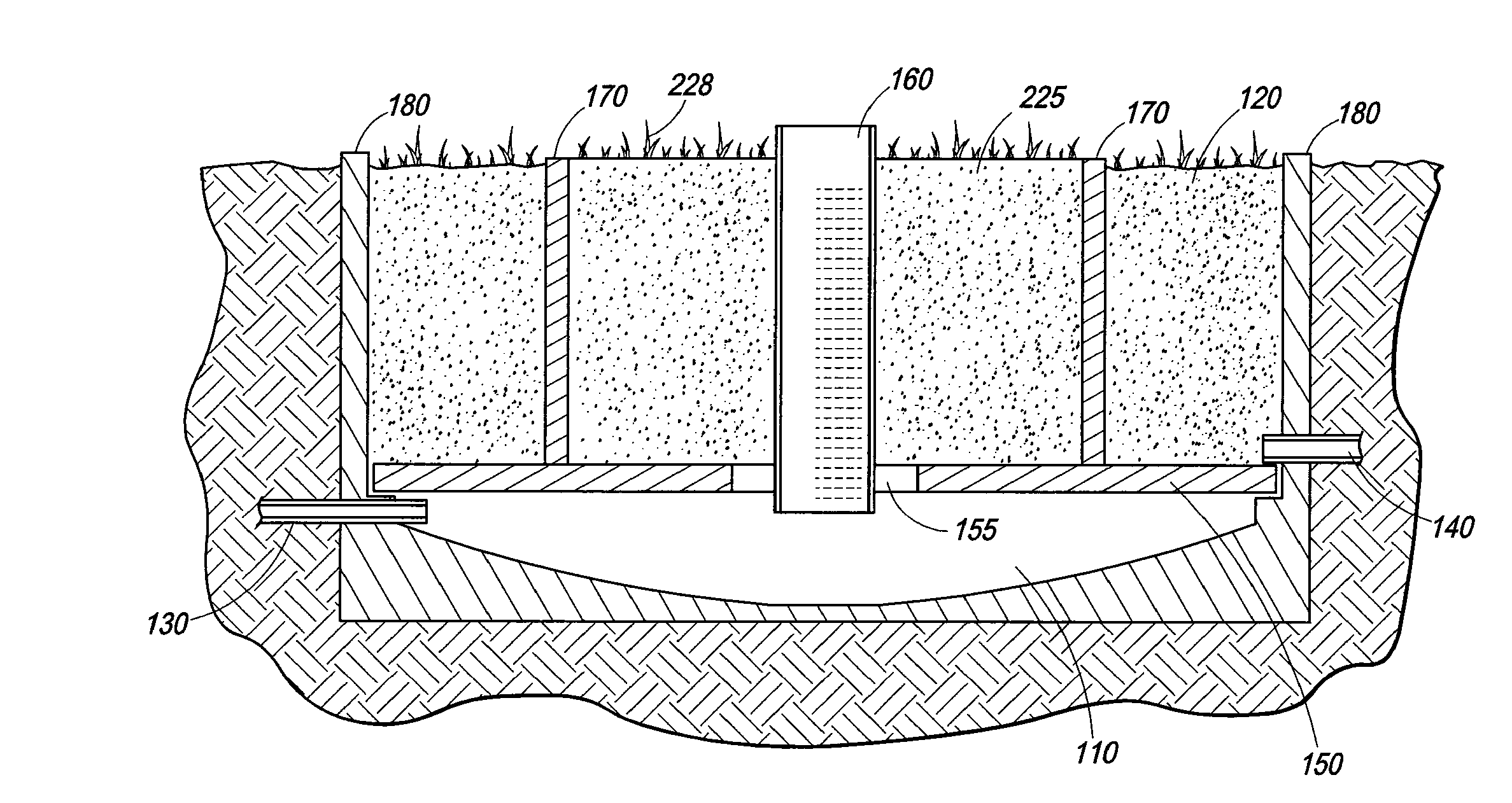
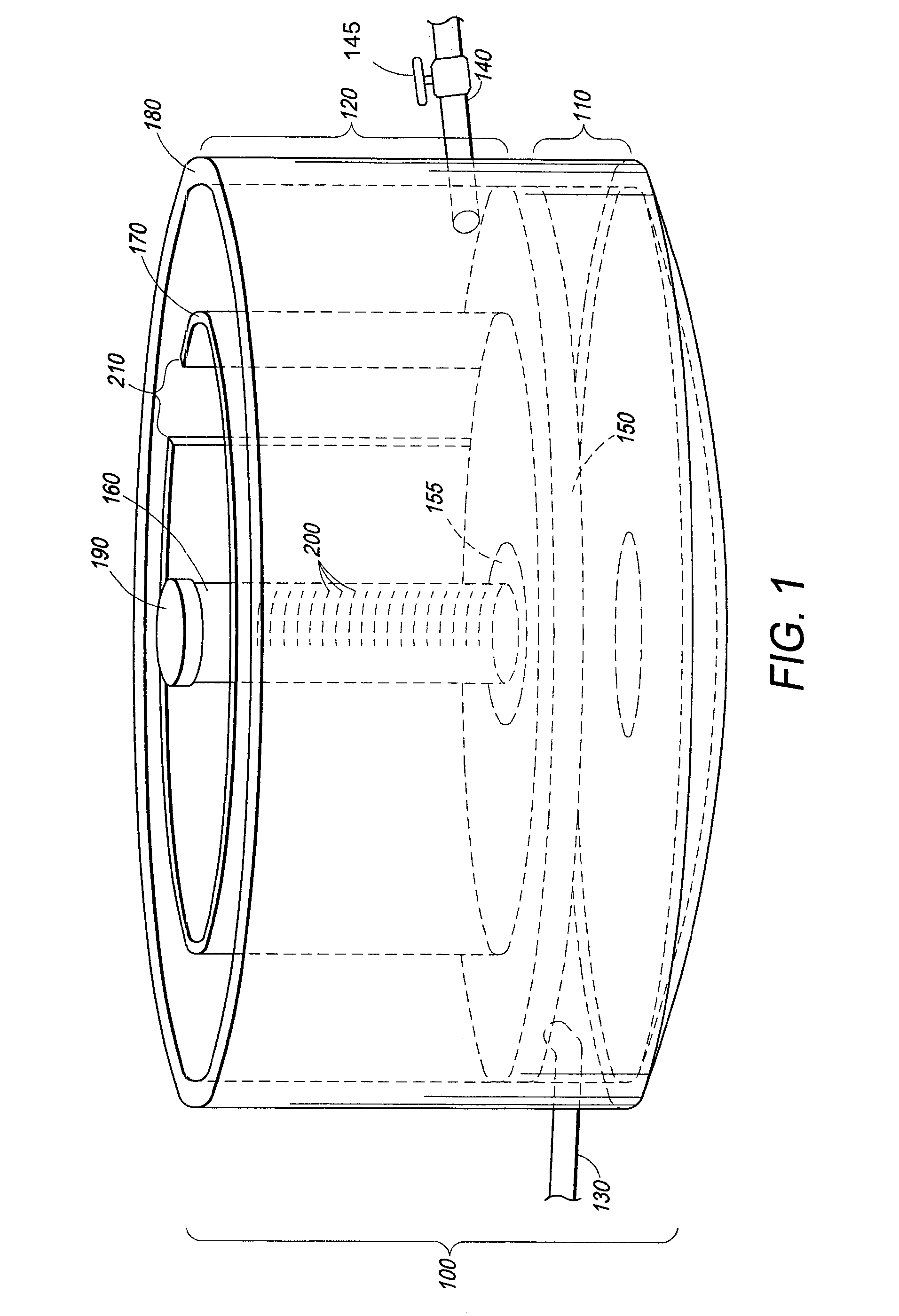
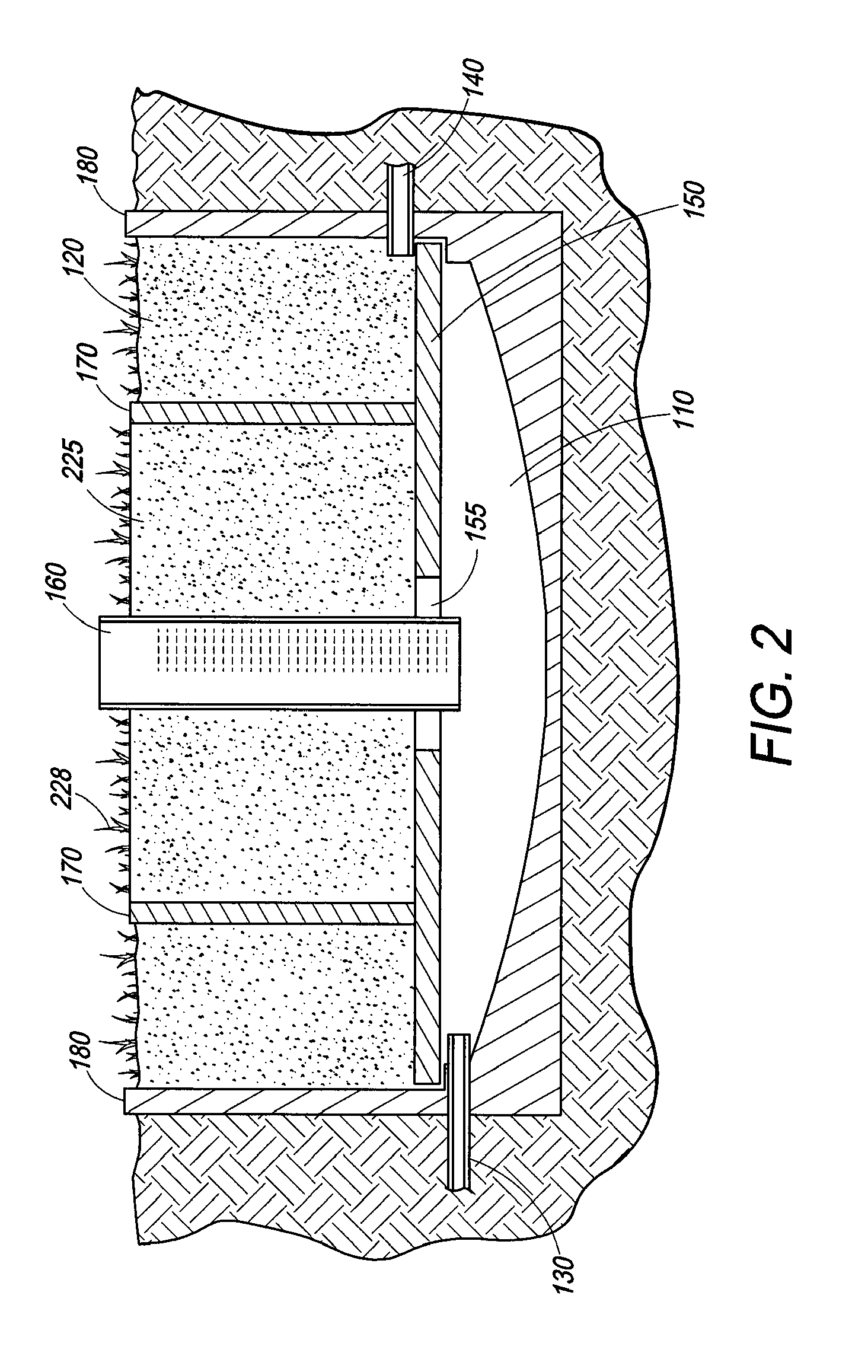
Wetland water treatment system
ActiveUS20080142438A1Easy maintenanceEasy constructionWater cleaningCentrifugal force sediment separationParticulatesWater treatment system
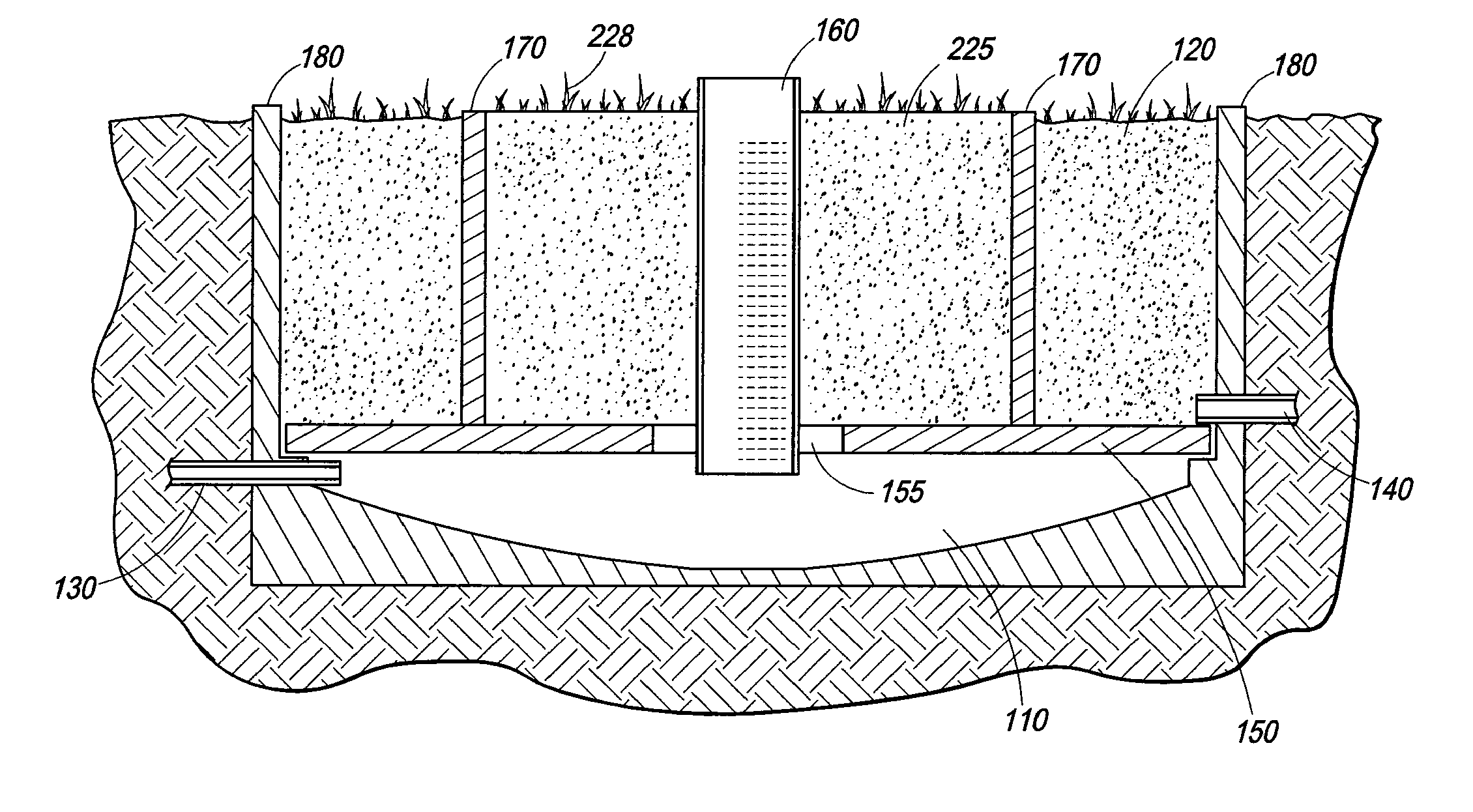
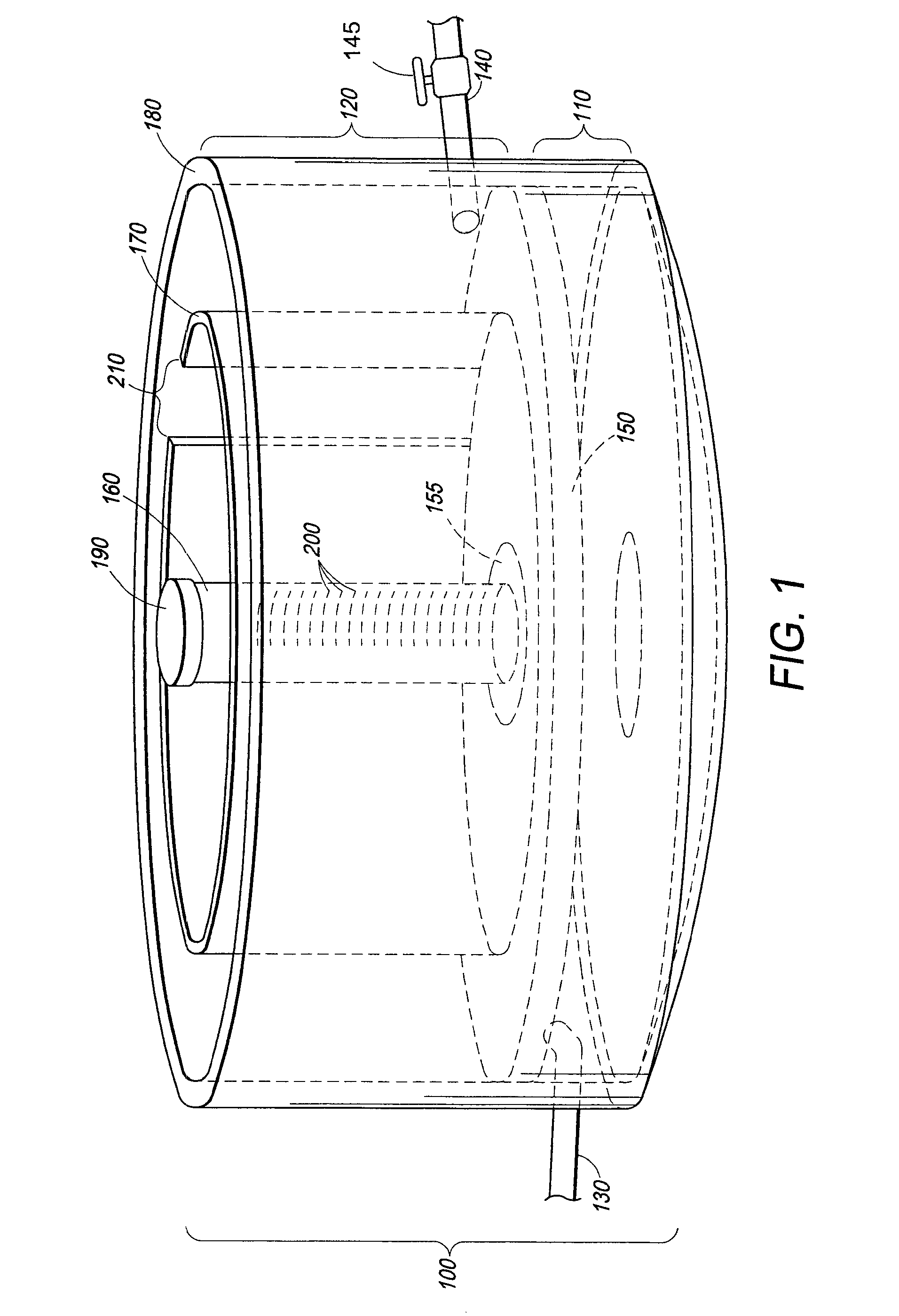
A self-contained wetlands treatment system to remove pollutants from water and treat stormwater runoff or other grey water. This is system and method wherein the water is passed through a wetland filtering and treatment system. This invention removes solids, metals (dissolved / particulate), nutrients (dissolved / particulate), oils, and bacterial contaminants from the water. The system and system housing can be fabricated, built, and assembled in a broad range of sizes and materials to accommodate and treat a broad range of influent flow rates.
Owner:MODULAR WETLAND SYST
Carbon nanotube filter
Monolithic, macroscopic, nanoporous nanotube filters are fabricated having radially aligned carbon nanotube walls. The freestanding filters have diameters and lengths up to several centimeters. A single-step filtering process was demonstrated in two important settings: the elimination of multiple components of heavy hydrocarbons from petroleum, a crucial step in post-distillation of crude oil, and the elimination of bacterial contaminants such as Escherichia coli or the nanometer-sized poliovirus from drinking water. All the filtration processes were repeated several times with completely reproducible results. These nanotube filters can be cleaned repeatedly after each filtration process to regain their full filtering efficiency.
Owner:BANARAS HINDU UNIVERSITY +1
Charged membrane
InactiveUS6849185B1Ion-exchanger regenerationSolid sorbent liquid separationBacterial contaminantsMicroporous membranes
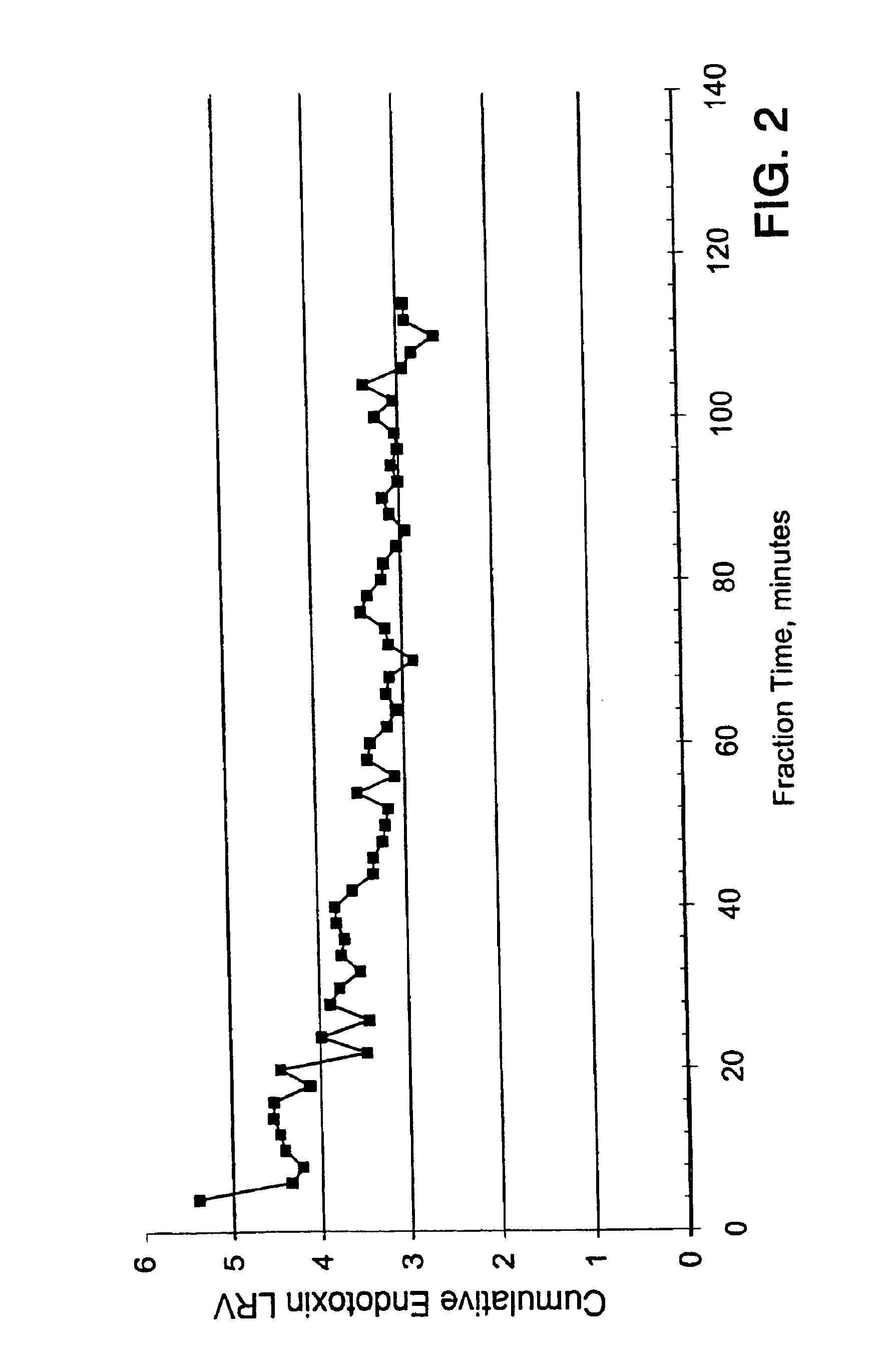
The present invention provides a hydrophilic charged microporous membrane comprising a porous hydrophobic substrate and a coating comprising a charge-providing agent. The present invention further provides a hydrophilic charged membrane comprising a porous hydrophobic substrate and a hydrophilic charge-providing agent distributed within the substrate. The present invention further provides a filter as well as a filter device comprising the inventive hydrophilic charged membrane. The present invention further provides a process for treating a fluid containing bacterial contaminants such as endotoxins comprising contacting the membrane with the fluid to provide a bacterial contaminant depleted fluid.
Owner:PALL CORP
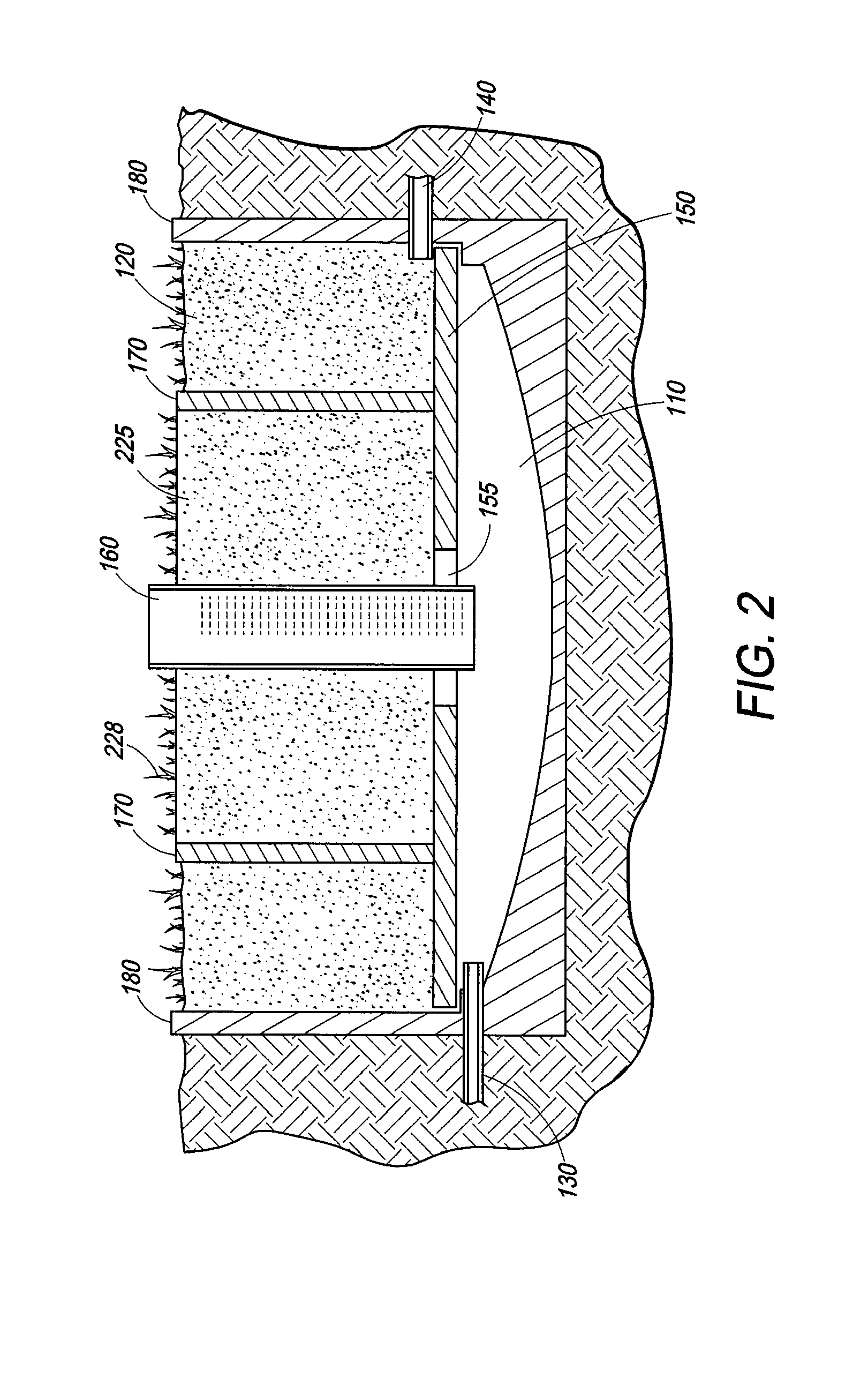
Wetland water treatment system
ActiveUS7674378B2Easy maintenanceEasy constructionWater cleaningCentrifugal force sediment separationParticulatesWater treatment system
A self-contained wetlands treatment system to remove pollutants from water and treat stormwater runoff or other grey water. This is system and method wherein the water is passed through a wetland filtering and treatment system. This invention removes solids, metals (dissolved / particulate), nutrients (dissolved / particulate), oils, and bacterial contaminants from the water. The system and system housing can be fabricated, built, and assembled in a broad range of sizes and materials to accommodate and treat a broad range of influent flow rates.
Owner:MODULAR WETLAND SYST
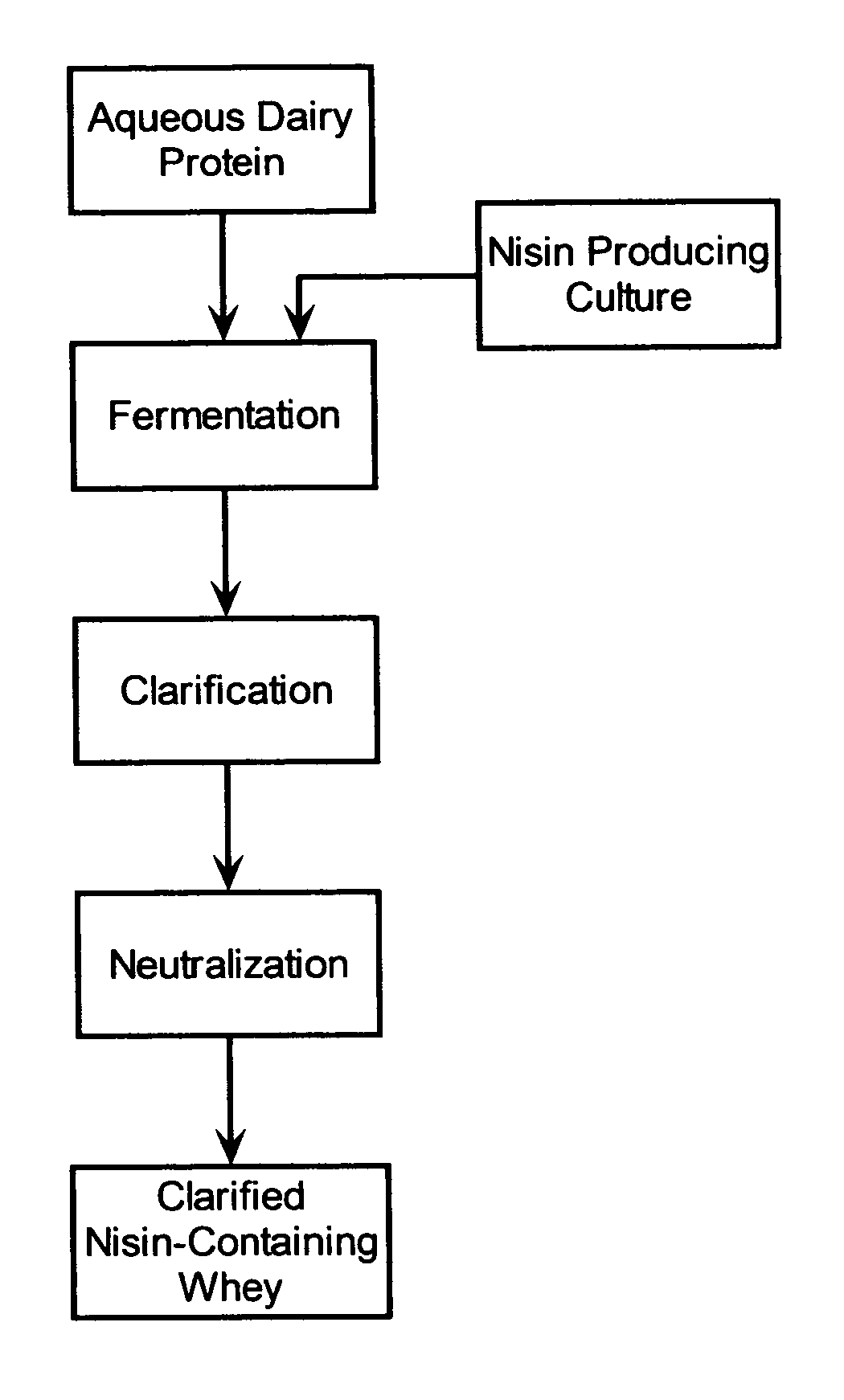
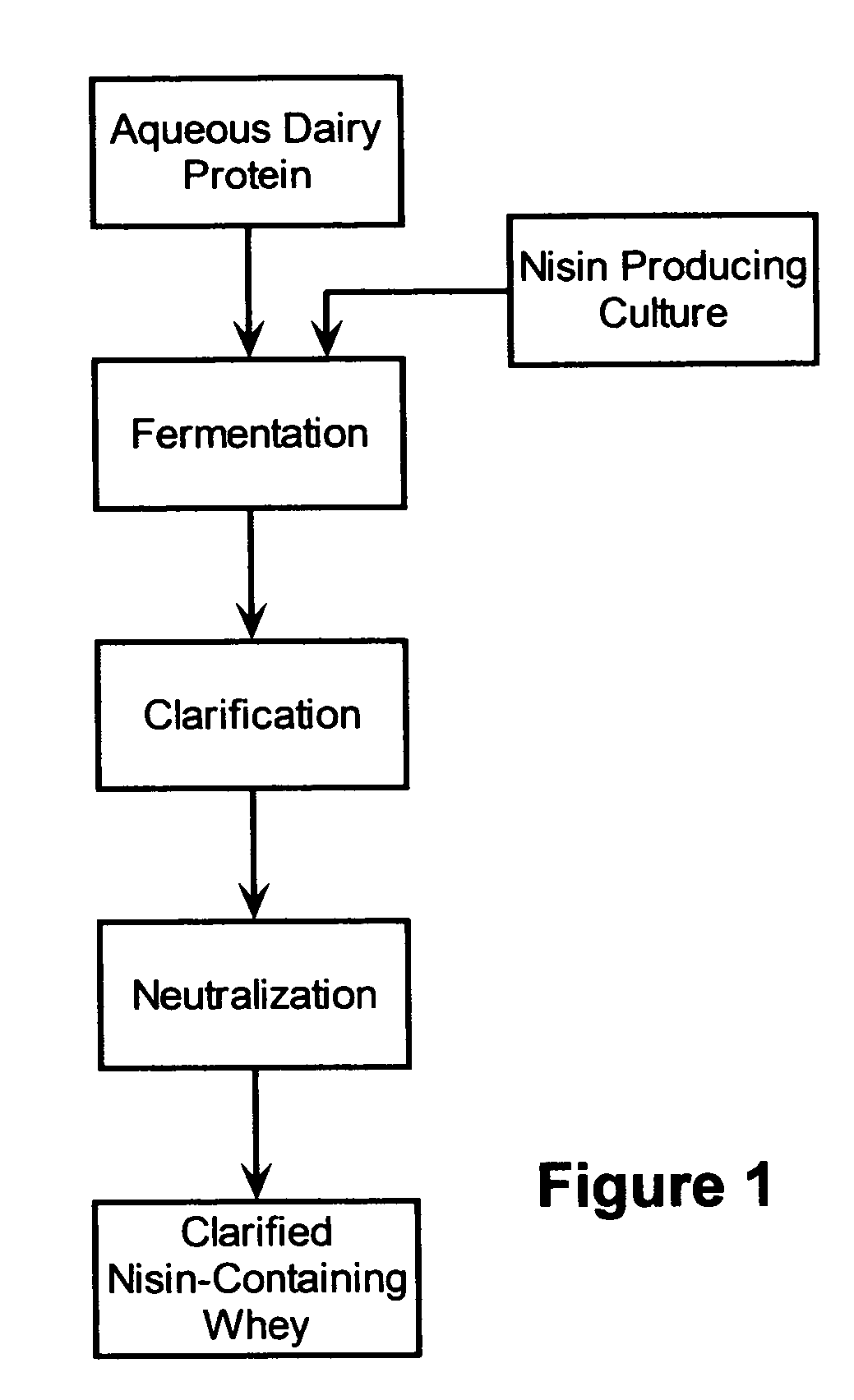
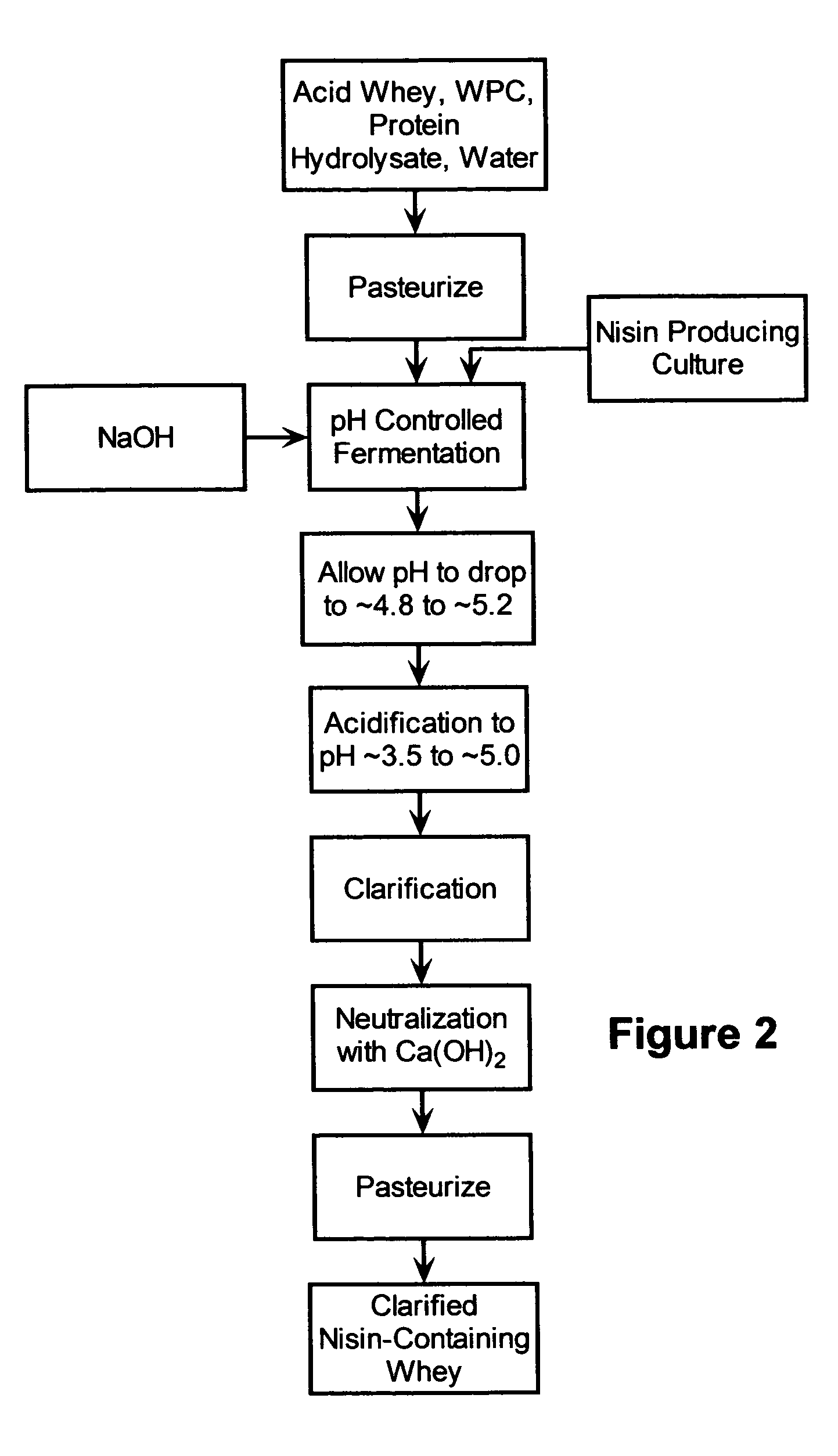
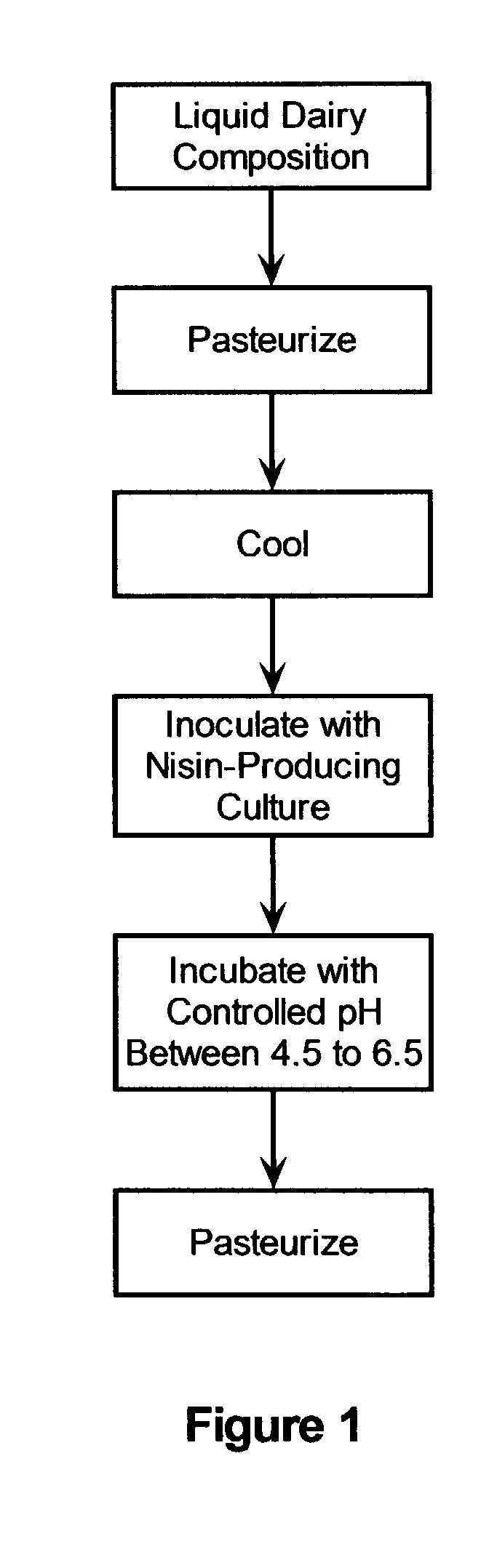
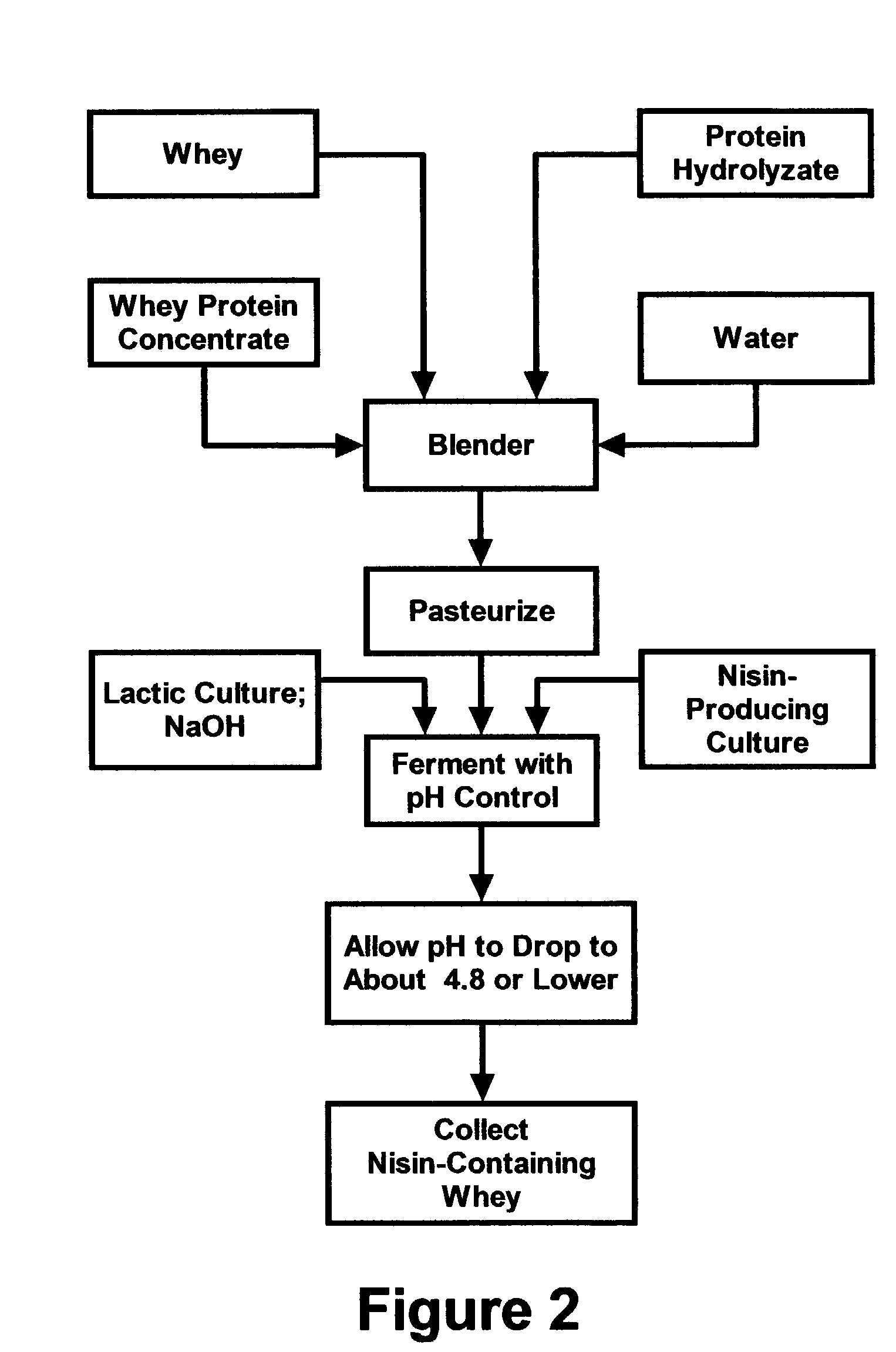
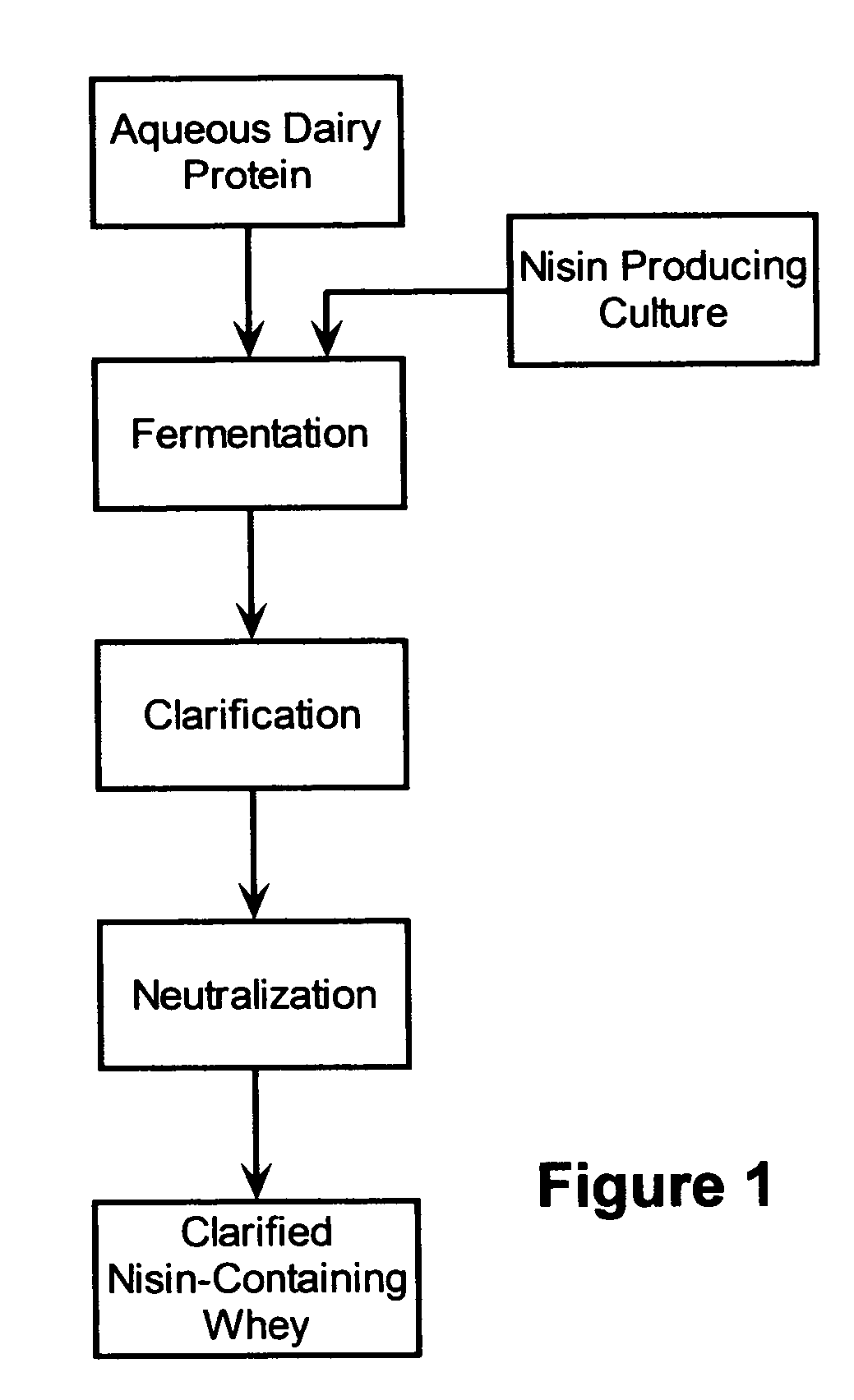
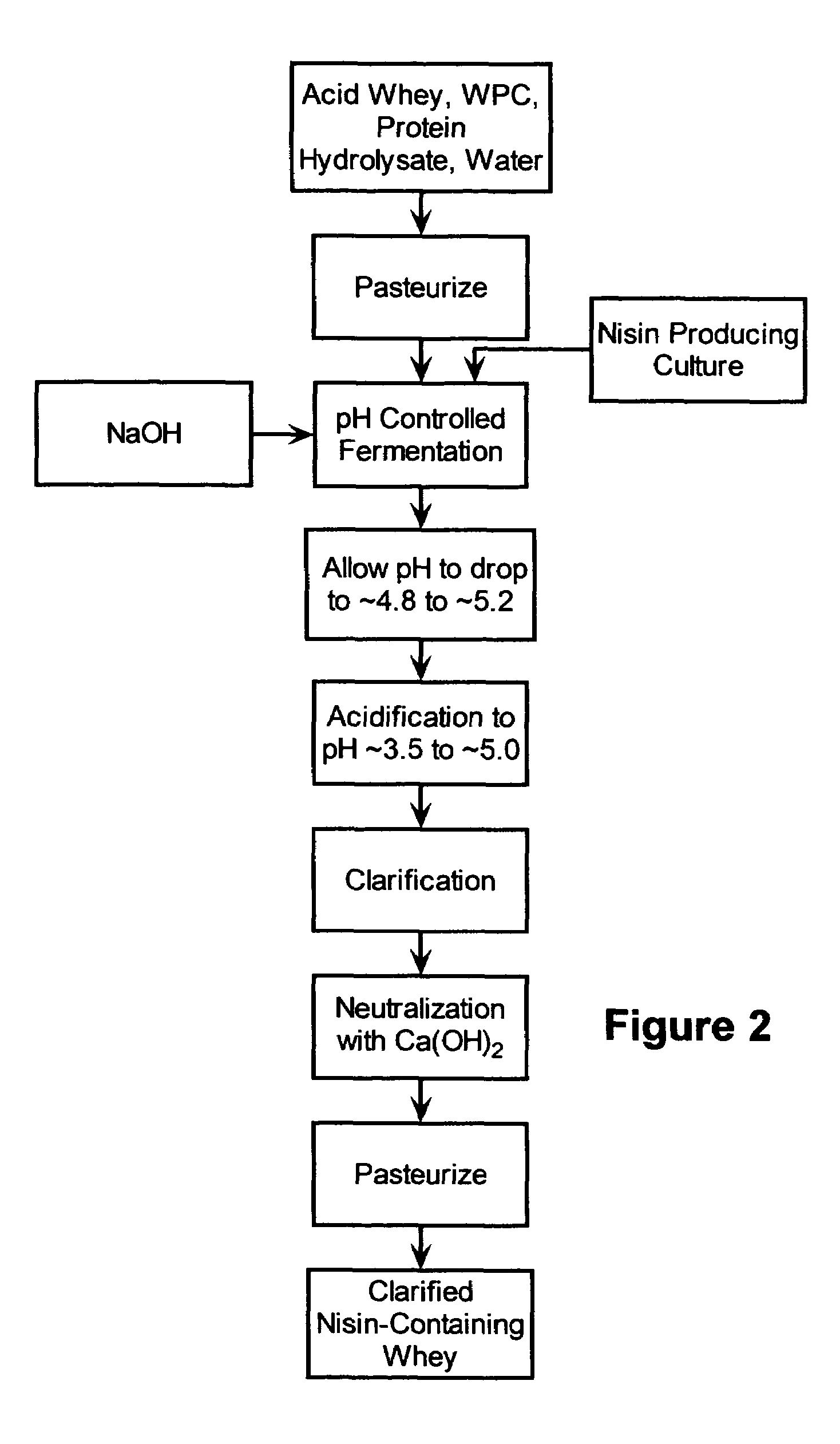
Stabilization of fresh mozzarella cheese using fermented whey
InactiveUS20050287272A1Reduce riskReduce detection limitMilk preparationDough treatmentMozzarella cheeseNisin
The invention is directed to a fermented and clarified nisin-containing whey and a method of making that can be used to produce a stabilized food product by adding, for example, to the pack water of fresh mozzarella cheese. The resulting stabilized food product retards or limits below detection levels the growth of toxins from pathogenic bacterial contaminants when the nisin-containing whey is added in amounts between about 10 to about 30% to the food product. The stabilized food product improves the safety of the food by retarding the growth of Listeria monocytogenes and improves the shelf life of the product by retarding the growth of gas forming bacteria such as bacteria from the Leuconostoc species.
Owner:KRAFT FOODS GRP BRANDS LLC
Smart FAIMS sensor
InactiveUS20080017790A1Time-of-flight spectrometersMaterial analysis by electric/magnetic meansUltraviolet lightsInhaled air
There is no mask on the market or registered with the USPTO that uses ultraviolet light to kill air-borne viral and bacterial contaminants. The invention will ensure that if an individual must perforce be exposed to contaminated air as a result of natural or manmade events, then that individual will be able to use the Ultraviolet Light Assisted Protective Breathing Mask (the “ULAP Breathing Mask”) to kill whatever known or unknown viruses or bacteria may be in the vicinity. The ULAP Breathing Mask kills these contaminants before inhaled air ever reaches the filters. The prototype weighs approximately three pounds, and so is easily portable and is practical.
Owner:OWLSTONE INC
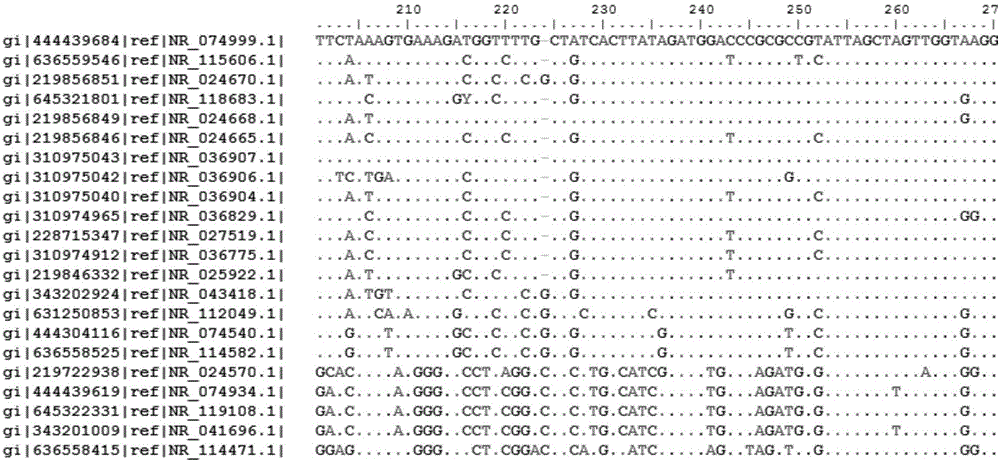
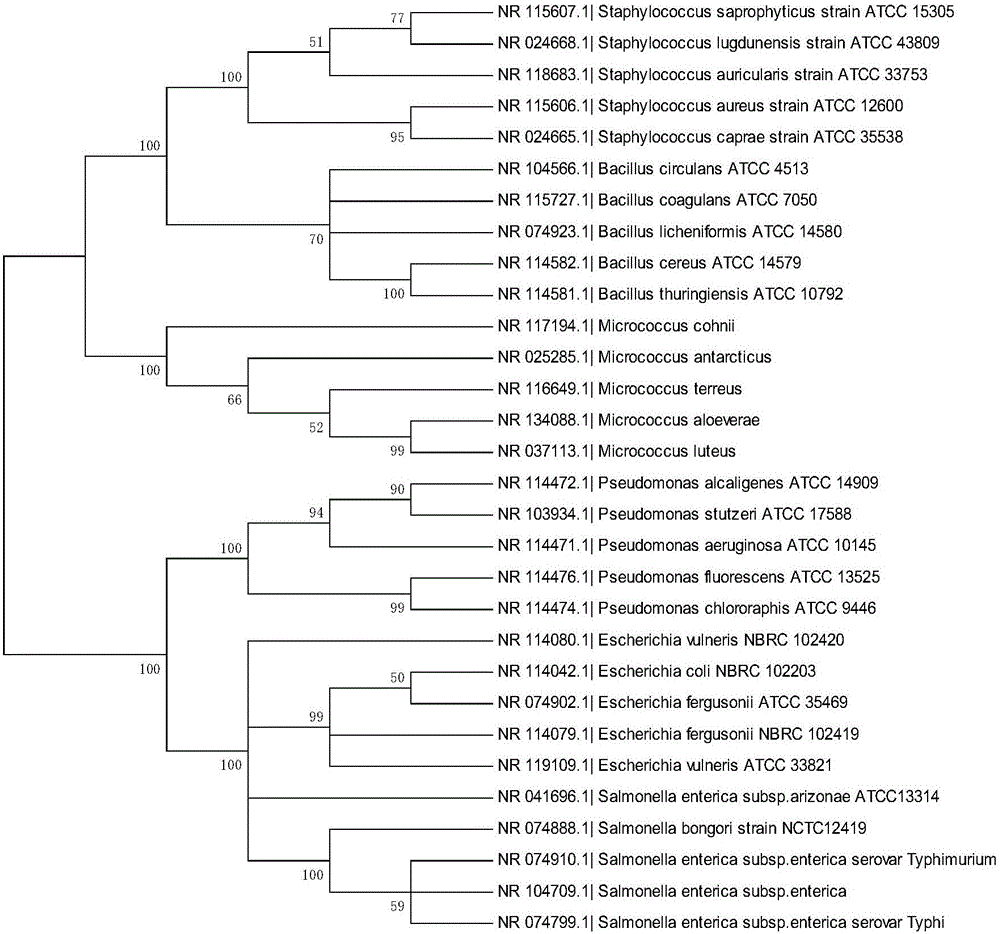
Bacterial nucleic acid sequencing identification method based on DNA bar code
InactiveCN106701914AClear scopeClear lengthMicrobiological testing/measurementSequence analysisDNA barcoding
The invention discloses a bacterial nucleic acid sequencing identification method based on a DNA bar code. Common bacterial contaminants in a medicine production environment can be effectively and accurately identified. The bacterial nucleic acid sequencing identification method comprises the steps that 1, a monoclonal bacterial strain is obtained through culture; 2, a genome DNA of the monoclonal bacterial strain obtained in the step 1 is extracted; 3, PCR is performed by using a general nucleic acid sequencing primer to amplify a specific sequence of nucleic acids; 4, a PCR product is extracted and purified; 5, a nucleic acid sequence of the PCR product obtained in the step 4 is determined; 6, a sequence analysis software is applied to perform sequence splicing, positioning is performed by using a general nucleic acid sequencing primer pair, a primer region is removed, and a DNA sequence of a corresponding fragment is obtained; 7, the DNA sequence of the corresponding fragment in the step is imported into a DNA bar code standard sequence core database or a GenBank database for sequence comparison, and bacteria are identified.
Owner:SHANGHAI INST FOR FOOD & DRUG CONTROL
Charged membrane
The present invention provides a hydrophilic charged microporous membrane comprising a porous hydrophobic substrate and a coating comprising a charge-providing agent. The present invention further provides a hydrophilic charged membrane comprising a porous hydrophobic substrate and a hydrophilic charge-providing agent distributed within the substrate. The present invention further provides a filter as well as a filter device comprising the inventive hydrophilic charged membrane. The present invention further provides a process for treating a fluid containing bacterial contaminants such as endotoxins comprising contacting the membrane with the fluid to provide a bacterial contaminant depleted fluid.
Owner:PALL CORP
Vacuum apparatus and method using ultraviolet radiation for sanitization
InactiveUS20060278088A1Air pressure further up inside the vacuum cleaner has been reducedReduce air pressureCombination devicesAuxillary pretreatmentFiltrationUltraviolet lights
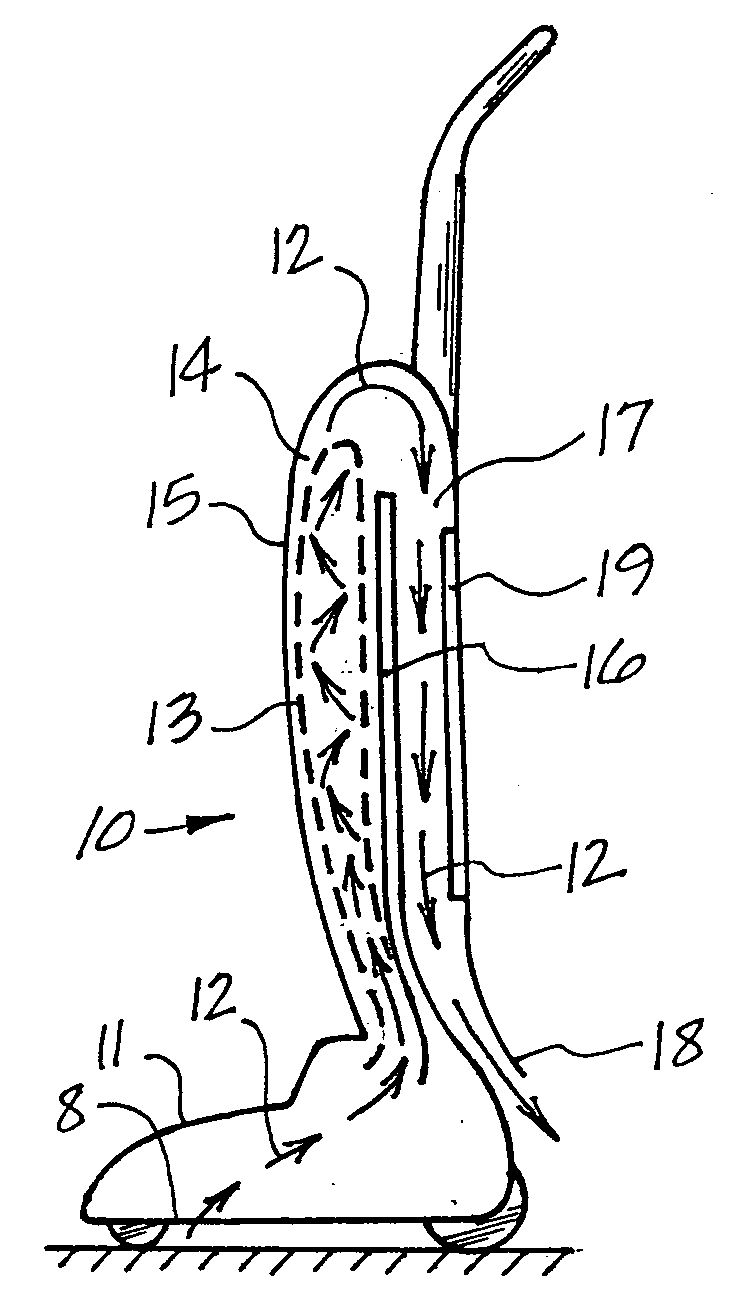
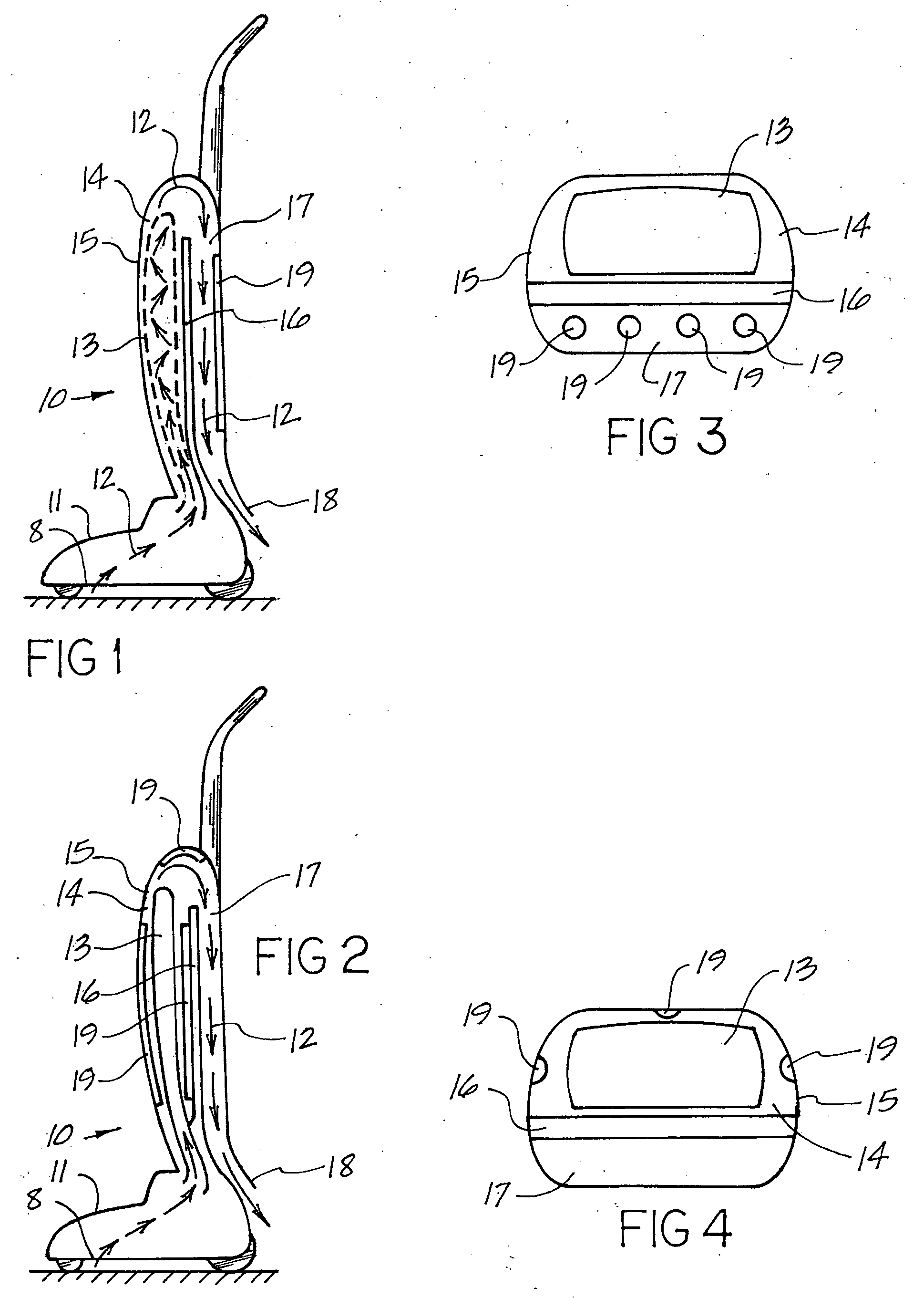
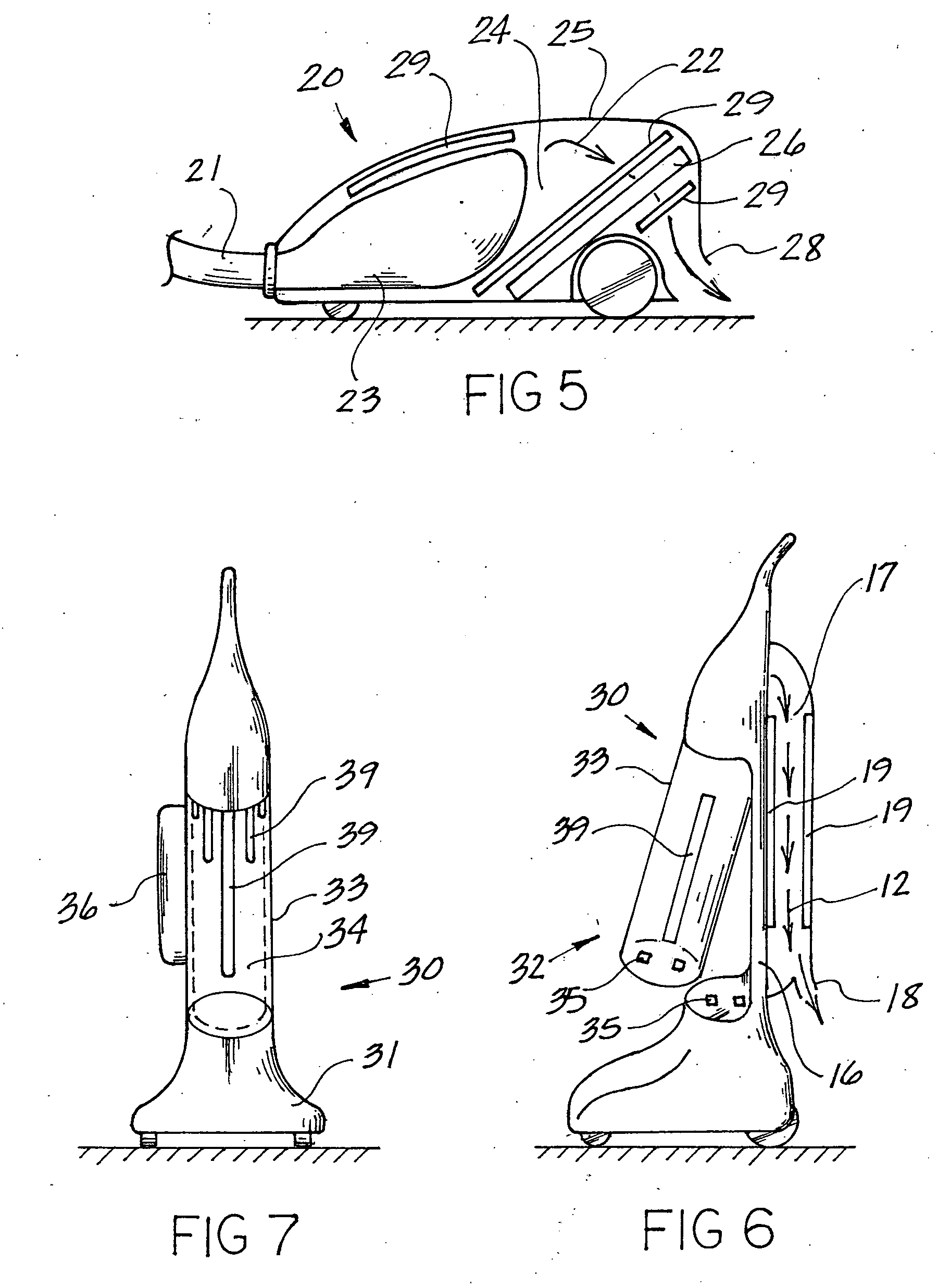
This invention deals specifically with the use of ultraviolet light emitters within a vacuum cleaner, where the lights are used to neutralize bacterial contamination. The air and debris entering into the vacuum is exposed to one or more ultraviolet light sources, with the resulting radiation causing the bacterial contaminants to be neutralized. The vacuum used may be an upright bag vacuum, an upright bagless vacuum, a floor type vacuum, or a shop type vacuum. Multiple chambers are provided, in which the lights are disposed in secondary chambers, in addition to any lights used at the point of initial filtration.
Owner:HELSEL DARIN R
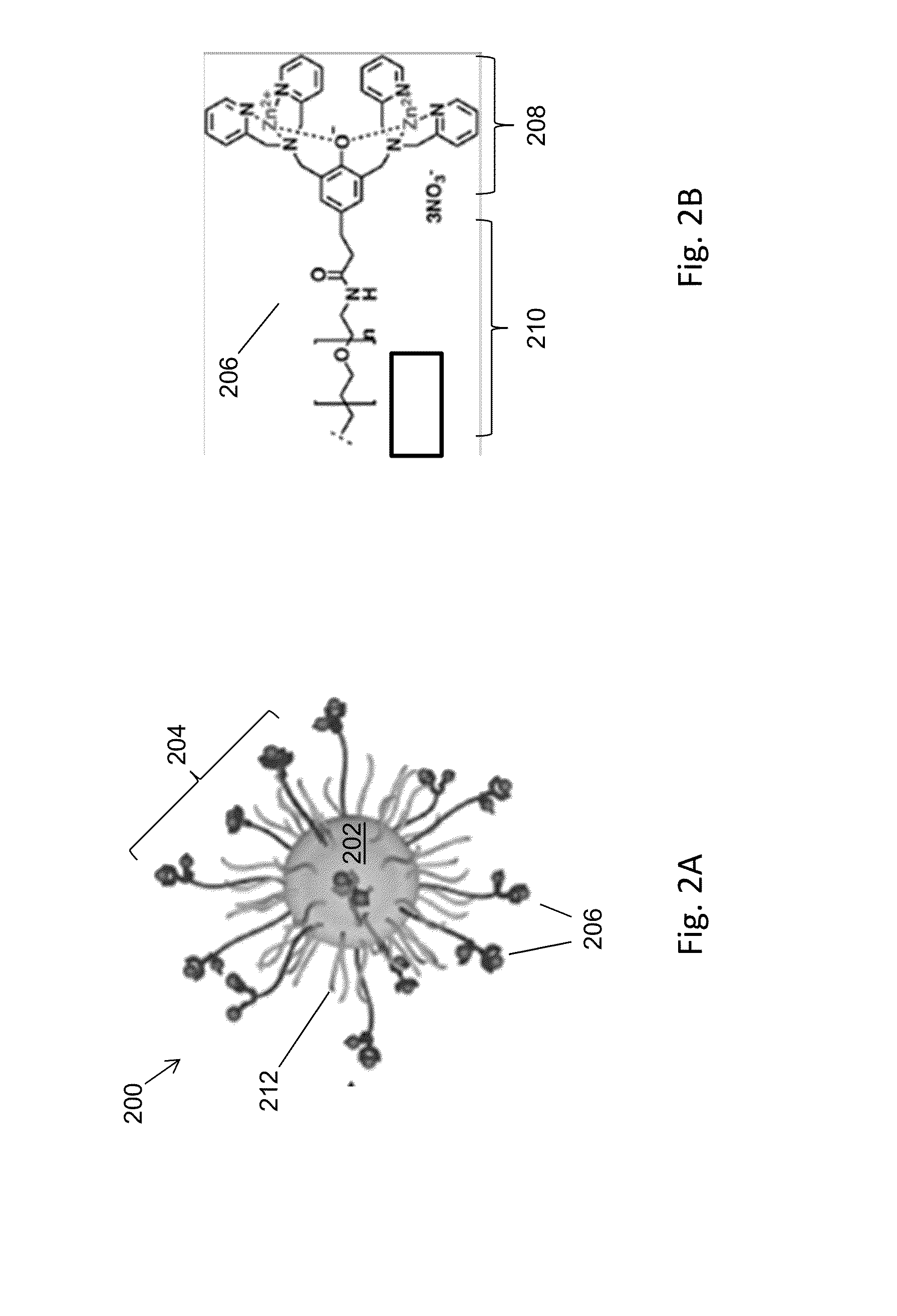
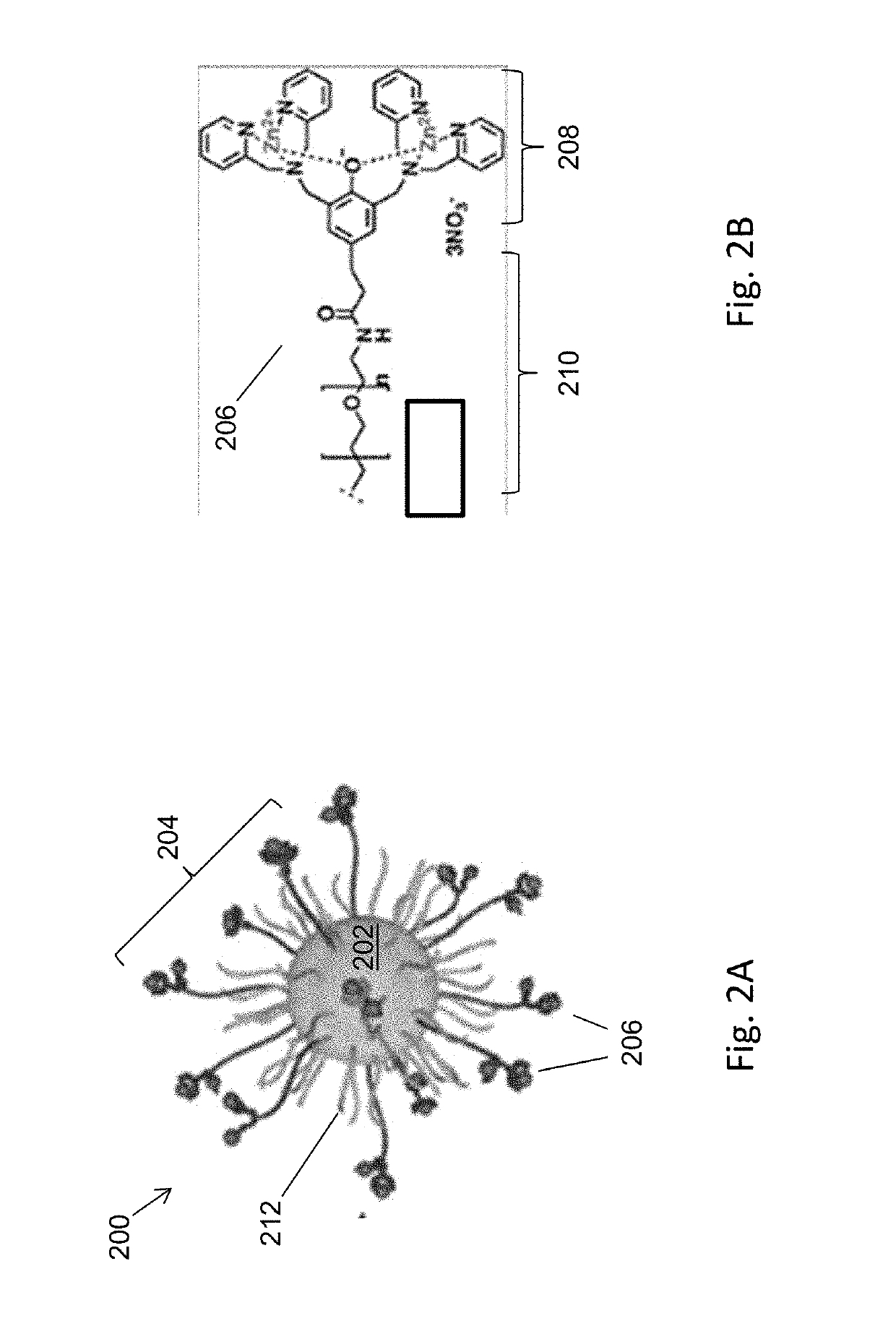
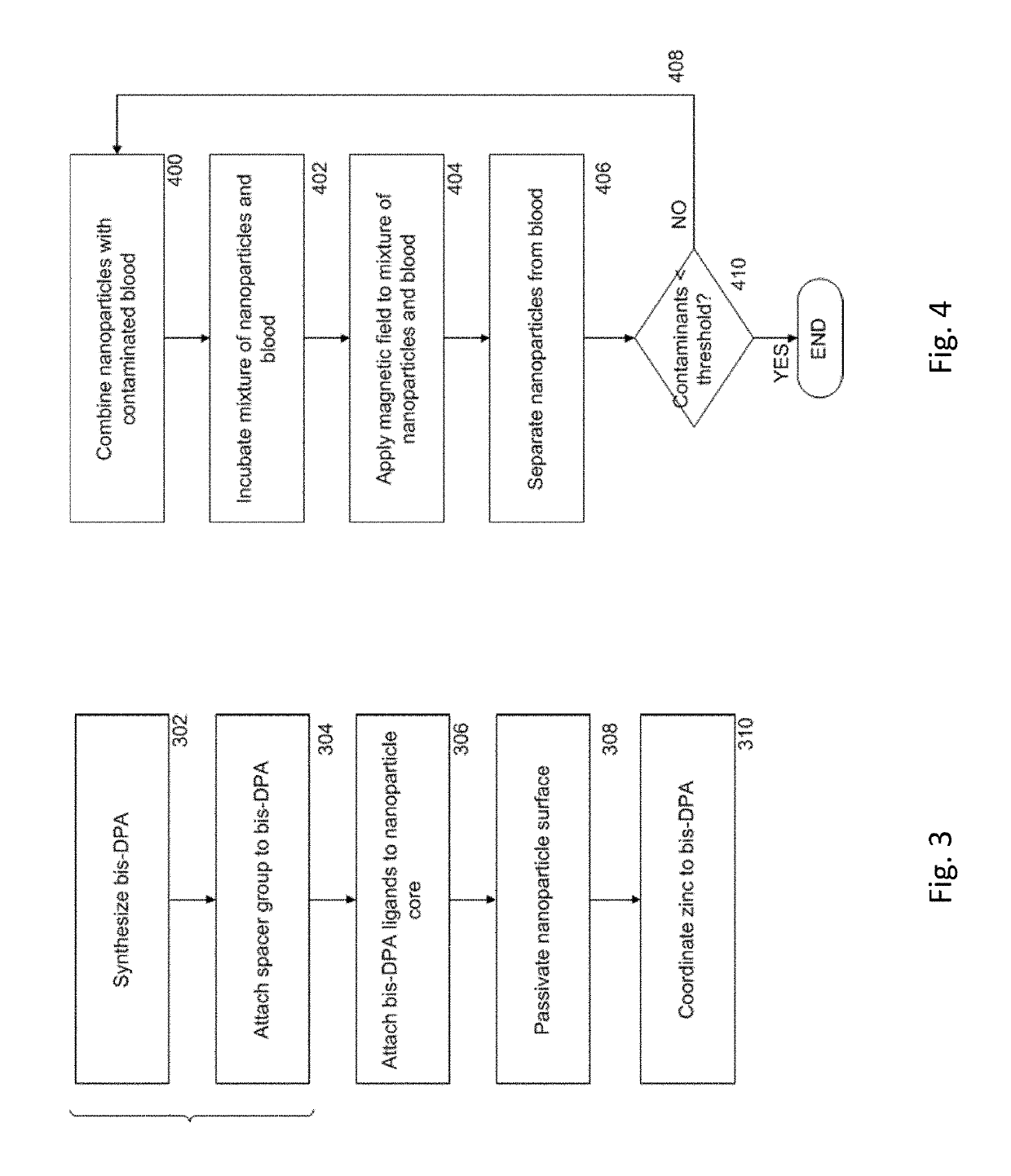
Magnetic separation using nanoparticles
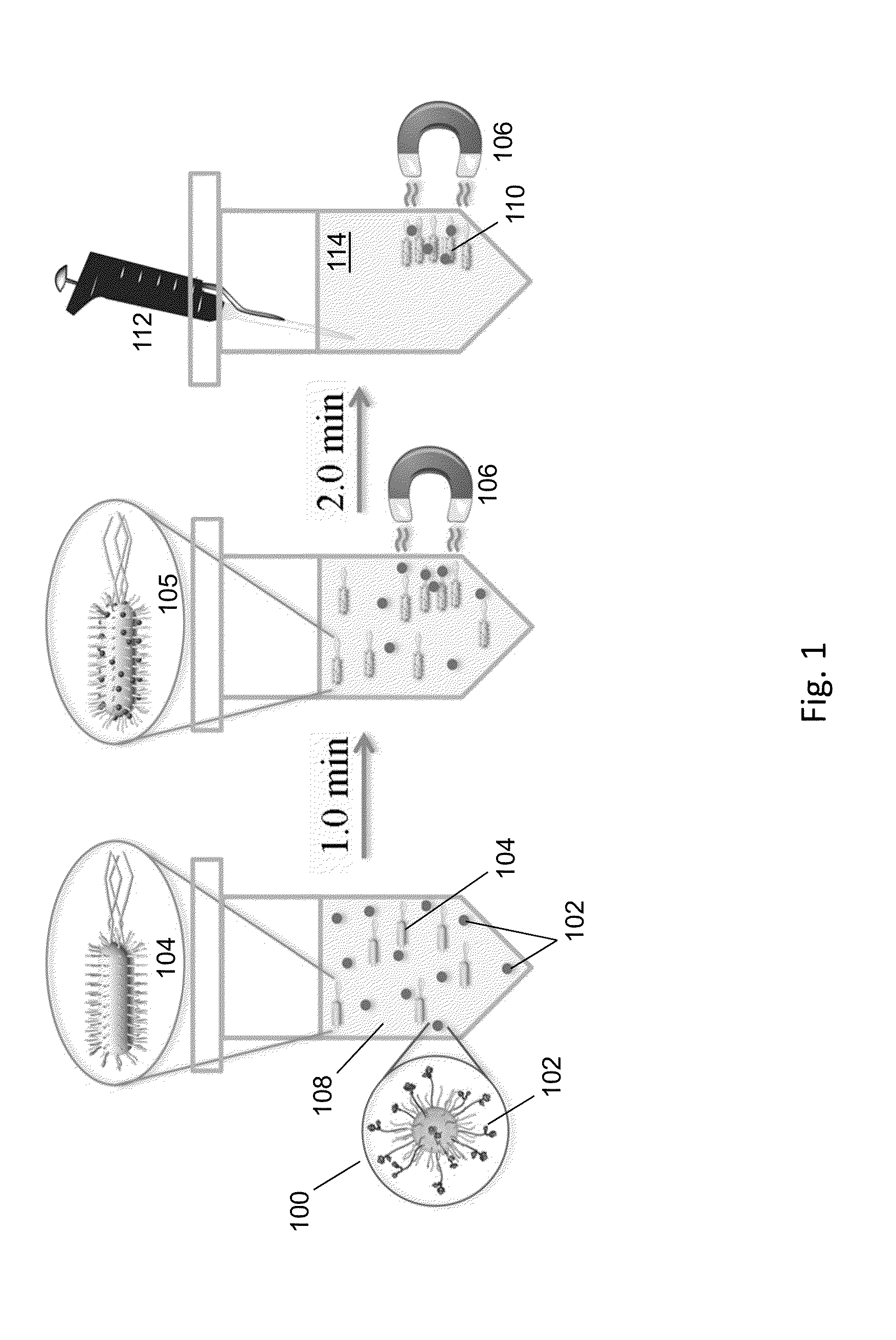
ActiveUS20140212335A1Efficient and selective separationFast bindingGaseous chemical processesLiquid-gas reaction of thin-film typeNanoparticleAlbumin solution
Nanoparticles as described herein are configured to bind to bacterial contaminants, such as Gram positive bacteria, Gram negative bacteria, and endotoxins. The nanoparticles include a core comprising a magnetic material; and a plurality of ligands attached to the core. The ligands include, for example, bis(dipicolylamine) (“DPA”) coordinated with a metal ion, e.g., Zn2+ or Cu2+, to form, e.g., bis-Zn-DPA or bis-Cu-DPA, which can bind to the bacterial contaminants. The nanoparticles can be included in compositions for use in methods and systems to separate bacterial contaminants from liquids, such as liquids, such as blood, e.g., whole or diluted blood, buffer solutions, albumin solutions, beverages for human and / or animal consumption, e.g., drinking water, liquid medications for humans and / or animals, or other liquids.
Owner:MASSACHUSETTS INST OF TECH +1
Stabilization of cooked and fully hydrated potato compositions
The present invention provides starchy food products, especially cooked and fully hydrated potato compositions, including fully hydrated mashed potato compositions and fully hydrated cut potato compositions, which are stabilized against the development of toxins from pathogenic bacterial contaminants. The stabilized compositions are attained by the incorporation of nisin-containing dairy composition derived from a nisin-producing culture. The invention also relates to methods of stabilizing starchy food products, especially cooked and fully hydrated potato compositions, including fully hydrated mashed potato compositions and fully hydrated cut potato compositions, against the development of toxins, wherein the method comprises incorporating nisin-containing dairy composition derived from a nisin-producing culture to the starchy food product. Since these compositions are cooked and fully hydrated prior to packaging, the ultimate consumer only needs to briefly heat the starchy food product in, for example, a conventional or microwave oven prior to consumption.
Owner:KRAFT FOODS GRP BRANDS LLC
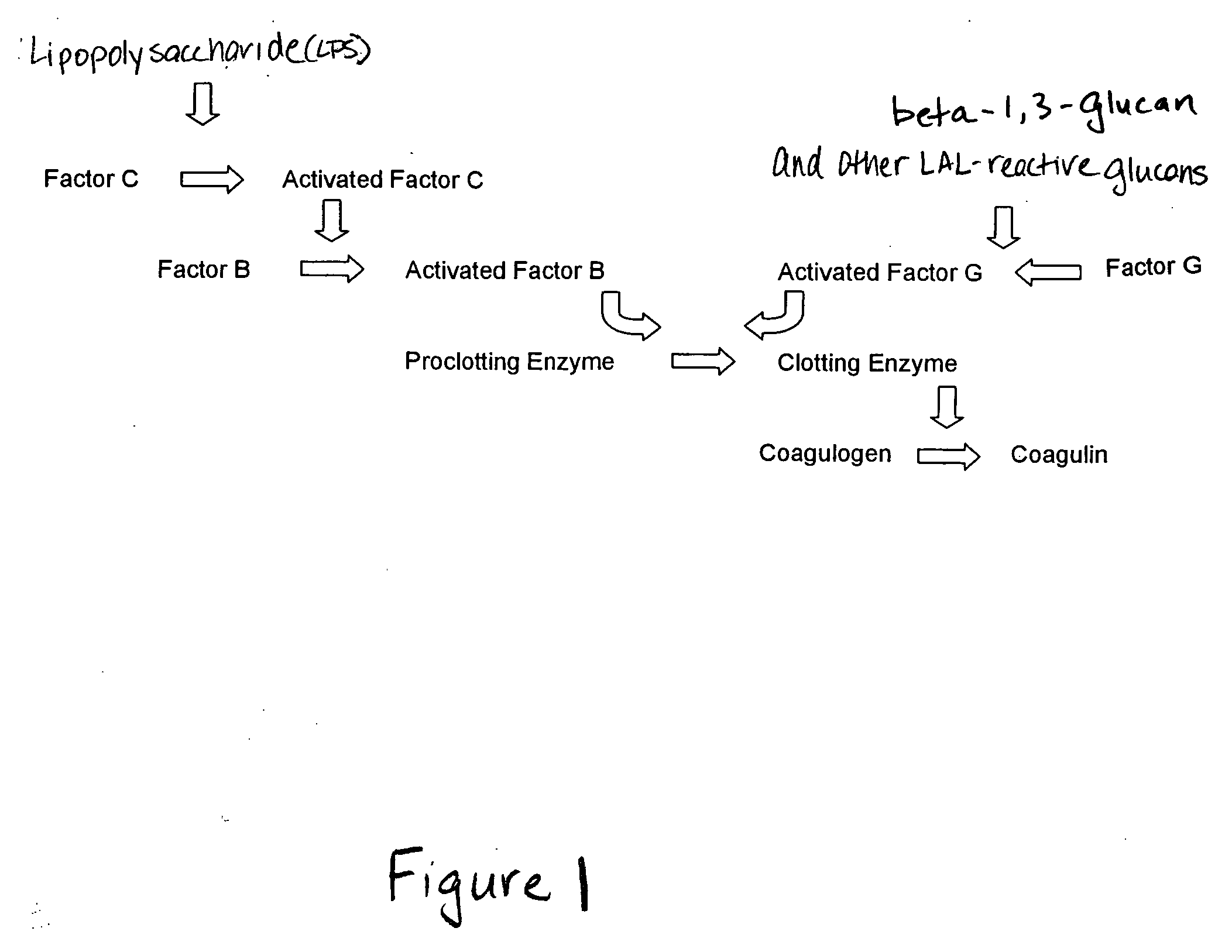
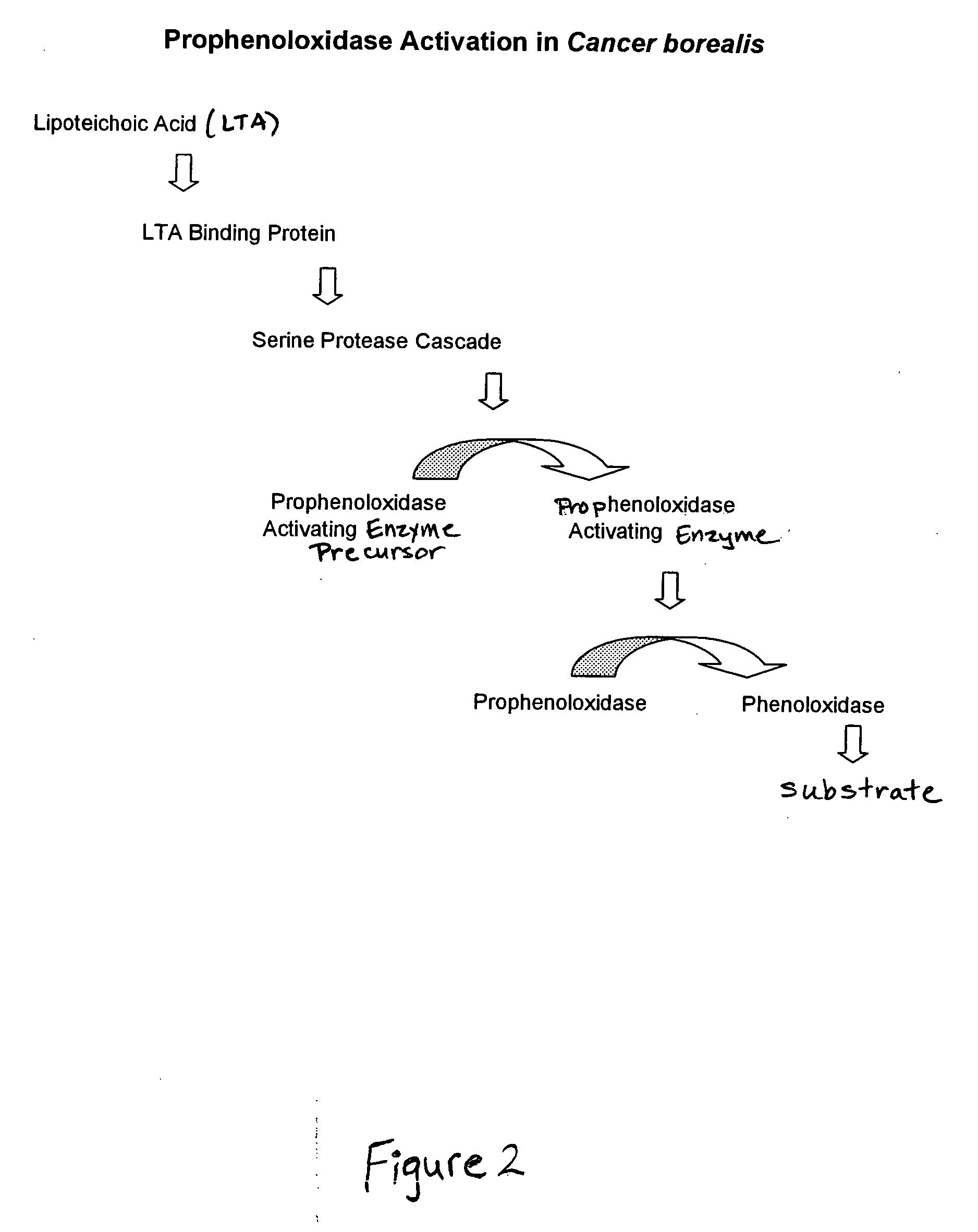
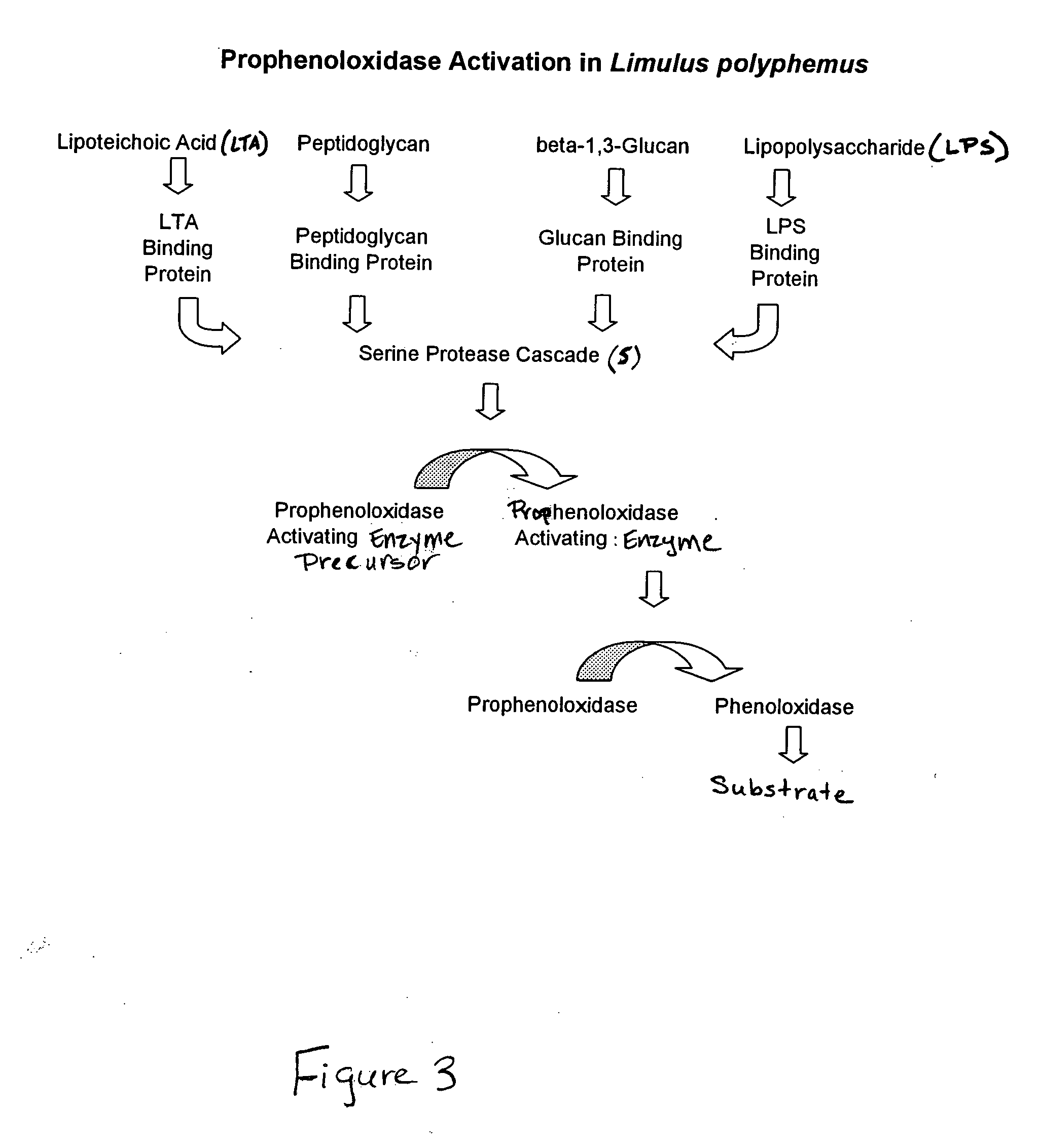
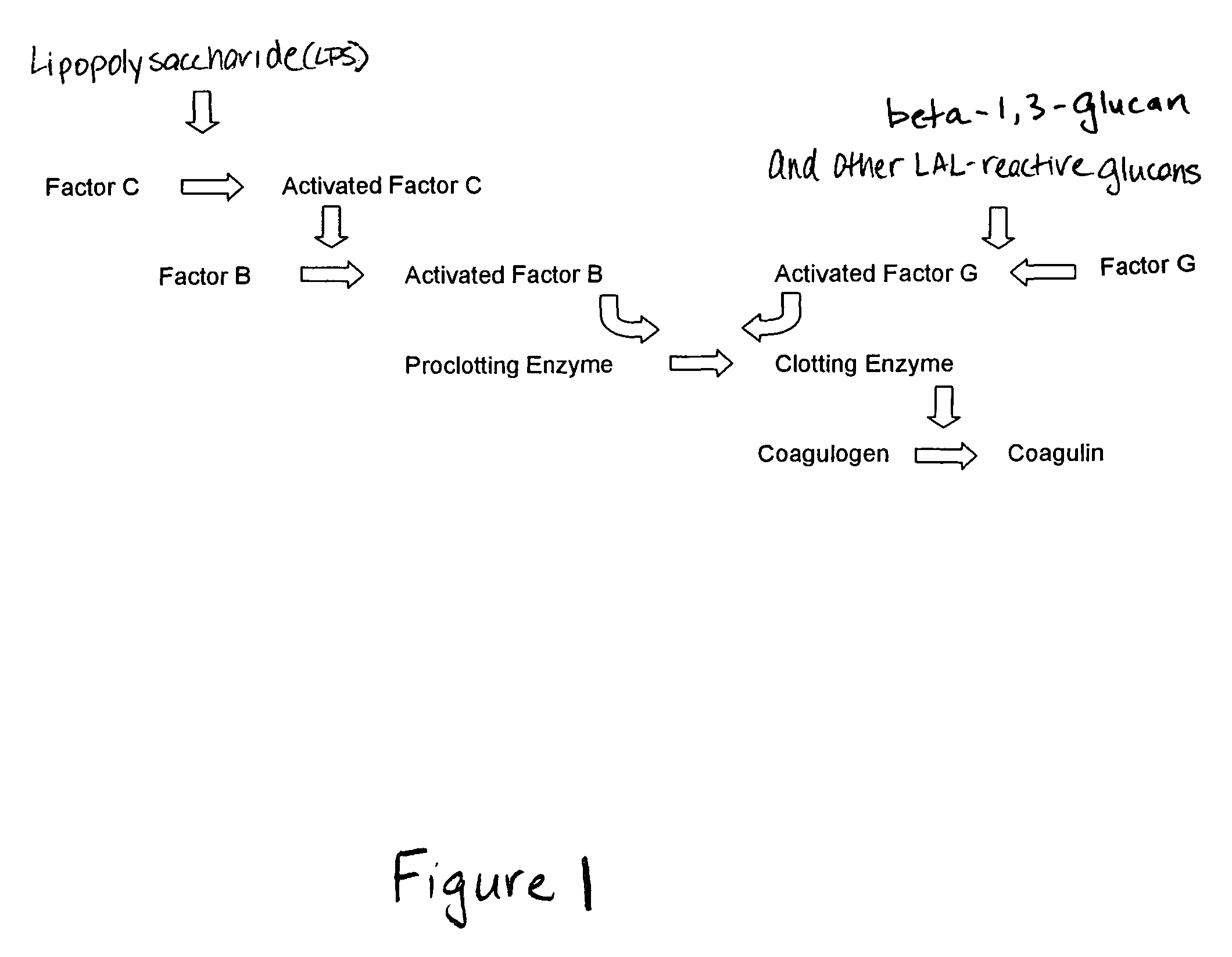
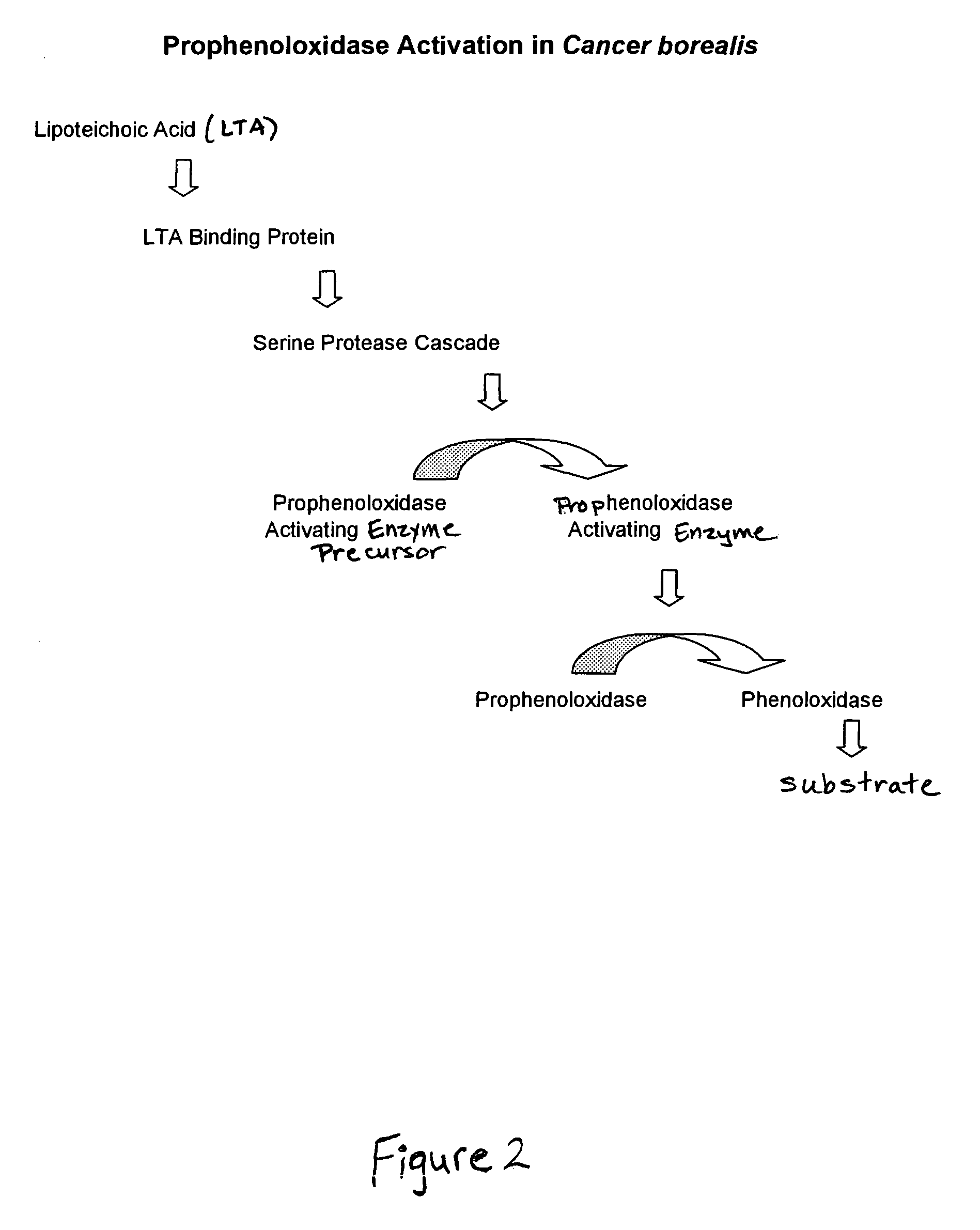
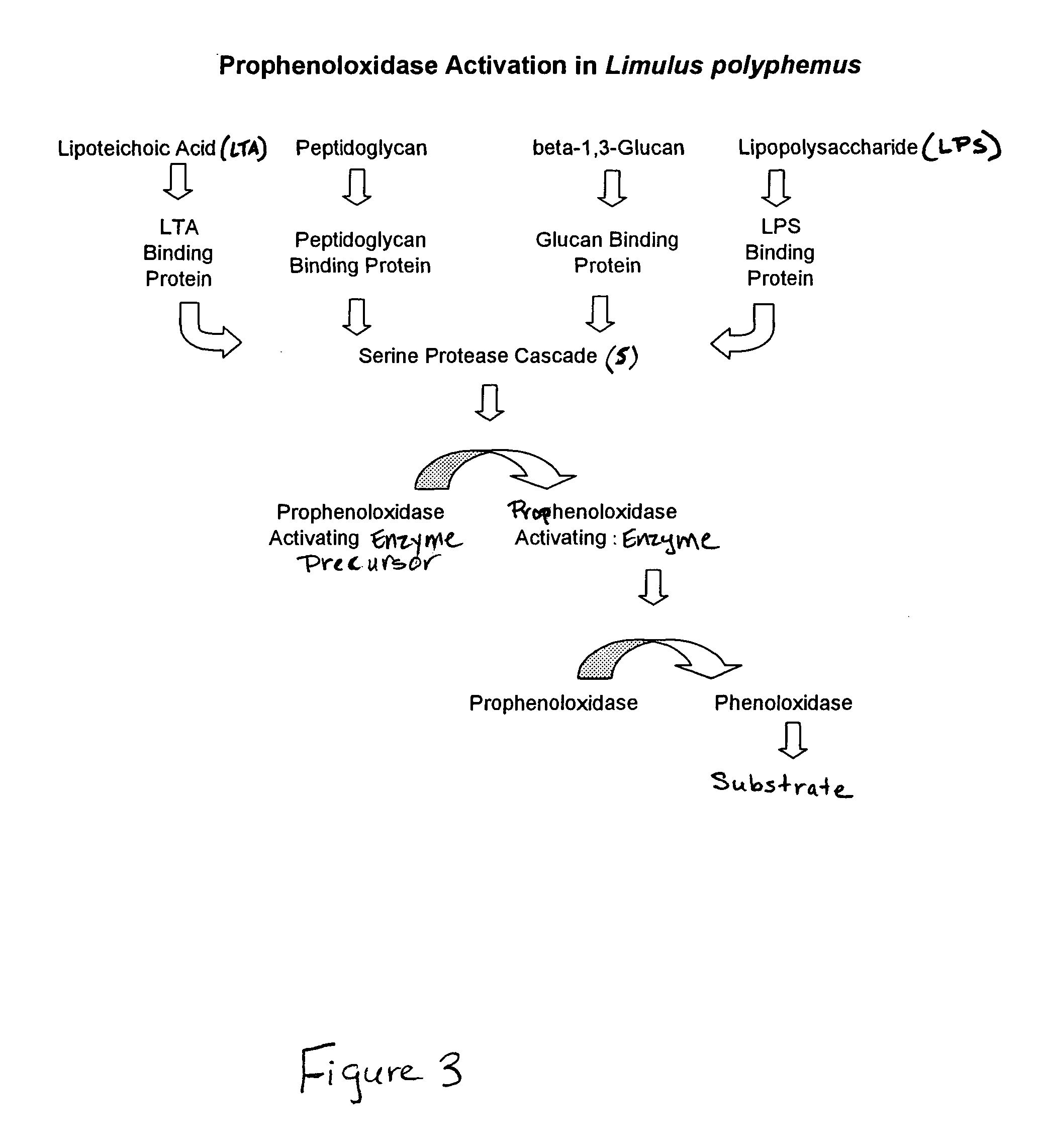
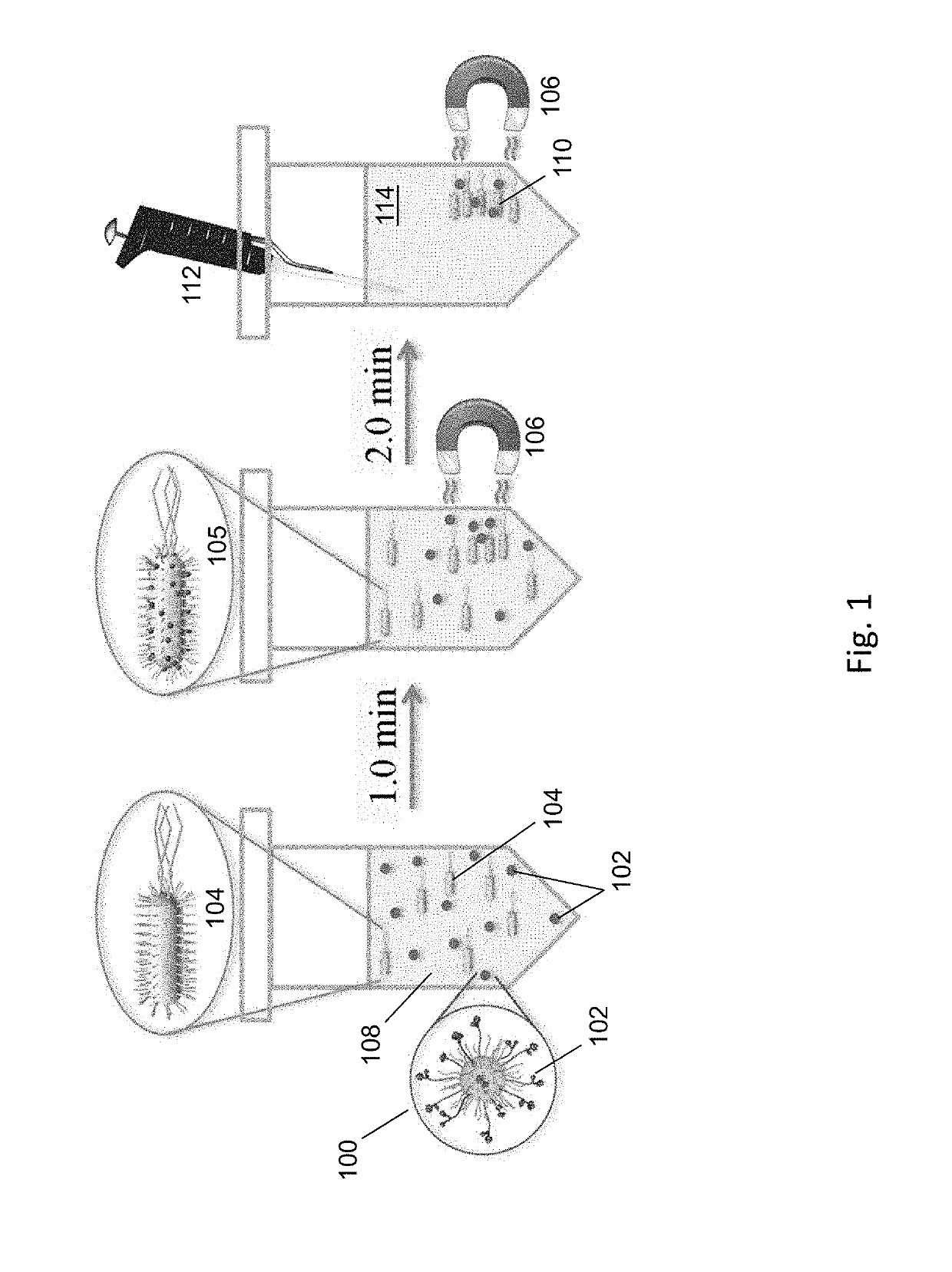
Methods and compositions for the detection and/or quantification of gram positive bacterial contaminants
InactiveUS20060183181A1Anthropod material medical ingredientsMicrobiological testing/measurementGramBacterial contaminants
The invention provides methods and compositions for the detection and / or quantification of a Gram positive bacterial contaminant in a sample. In particular, the invention provides hemocyte-based preparations, methods of making such hemocyte-based preparations, and methods of using such hemocyte-based preparations for the detection and / or quantification of the Gram positive bacterial contaminant.
Owner:CHARLES RIVER LAB INC
Stabilization of fresh mozzarella cheese using fermented whey
InactiveUS7323204B2Improve securityPromote growthMilk preparationDough treatmentBacteroidesMozzarella cheese
The invention is directed to a fermented and clarified nisin-containing whey and a method of making that can be used to produce a stabilized food product by adding, for example, to the pack water of fresh mozzarella cheese. The resulting stabilized food product retards or limits below detection levels the growth of toxins from pathogenic bacterial contaminants when the nisin-containing whey is added in amounts between about 10 to about 30% to the food product. The stabilized food product improves the safety of the food by retarding the growth of Listeria monocytogenes and improves the shelf life of the product by retarding the growth of gas forming bacteria such as bacteria from the Leuconostoc species.
Owner:KRAFT FOODS GRP BRANDS LLC
Underwater Pressure Arc Discharge System for Disinfection of Food and Food Products
InactiveUS20090324786A1Reduce bacterial contaminationMilk preservationDough treatmentElectricityFood borne
A method for reducing and / or eliminating bacterial contamination in a food product includes immersing the food product in the tank containing a fluid and discharging a pressure arc beneath the surface of the fluid wherein the pressure arc is effective in disrupting cell equilibrium and / or inactivating the bacteria. A system for use in the method includes an electrical input / output system, a reflector, the reflector reflecting an output charge between two oppositely charged electrodes, and a fluid filled tank. The method and system are effective in the reduction of food-borne bacterial contaminants particularly in or on poultry, red meat, and / or pork.
Owner:MCNAUGHTON JAMES L
Moisture permeable double-anti-bamboo charcoal composite facing material and process technique thereof
InactiveCN101109130ARich patternsClear textureOther manufacturing equipments/toolsDyeing processPolyesterYarn
The invention discloses an antibiotic bamboo-carbon compound shell fabric that can penetrate moisture and resist to ultra-violet rays as well as the processing process for the shell fabric, which pertains to the technical field of compound fabric of synthetic fiber and bamboo-carbon compound yarn. The warp uses two yarns, wherein, a warp A uses mid-twist S-twist long polyester yarn, while a warp B uses low-twist compound yarn of low-elasticity polyester fiber and high-elasticity polyurethane fiber yarn, the proportion of the warp A to B by weight is 50: 131. The weft uses two yarns, wherein, a weft A uses anti-biotic yarn of low twist, while a weft B uses compound yarn comprising bamboo-carbon fiber and Model fiber. The two wefts are cross-combined by 1:1, and the proportion of the weft A to B by mass is 166:177. The shell fabric from the invention can reflect or shield ultra-violet rays, convert the energy of ultra-violet rays, dissolve bacteria and contaminants, etc., so as to be antibiotic, absorb moisture, penetrate air and resist ultra-violet rays; in addition, the shell fabric is of rich patterns, clear textures, good elasticity, resistance to winkles and static, etc.
Owner:洪桂焕
Cutting board apparatus
ActiveUS20110031672A1Eliminate gapsGood return on investmentLamination ancillary operationsLaminationBacterial contaminantsMechanical engineering
The apparatus according to the present invention relates generally to a cutting board that can be used during food preparation. In particular the present invention relates to multi-layered cutting boards which are used for the preparation of food that are constructed to prevent bacterial contamination build-up between each stacked cutting layer of the cutting board. Prevention of bacterial contamination is achieved by sealing the perimeter surface of the cutting board using any method of sealing such as laser, heat and chemical processes. Sealing advantageously eliminates seams, crevices and gaps that naturally occur between stacked layers of the cutting board.
Owner:POLLI ANGELO MR
Modified basalt fibre applied to water quality purification
InactiveCN107262042ALarge specific surface areaUniform adsorptionOther chemical processesAlkali metal oxides/hydroxidesWater qualityOrganic matter
The invention relates to the technical field of research, development and manufacturing of basalt fibre, and discloses modified basalt fibre applied to water quality purification. The modified basalt fibre is prepared by adding 3 to 5 mass percent of a composite material and adopting a film pressing and forming technology on the basis of an original basalt stone material, so that the problems of low efficiency, high cost, complicated operation, difficulties in treating low-concentration metals and bacterial pollutants in the existing water quality purification treatment are solved; the modified basalt fibre has a good removal effect on low-concentration metal ions, organic matters and bacteria in water through physical-chemical adsorption; the treated water quality meets GB 5749-85 drinking water standards; the removal rate reaches 99.9 percent; therefore, the modified basalt fibre has a good application prospect.
Owner:安徽梦谷纤维材料科技有限公司
Apparatus and method for resuscitating and revitalizing hydrocarbon fuels
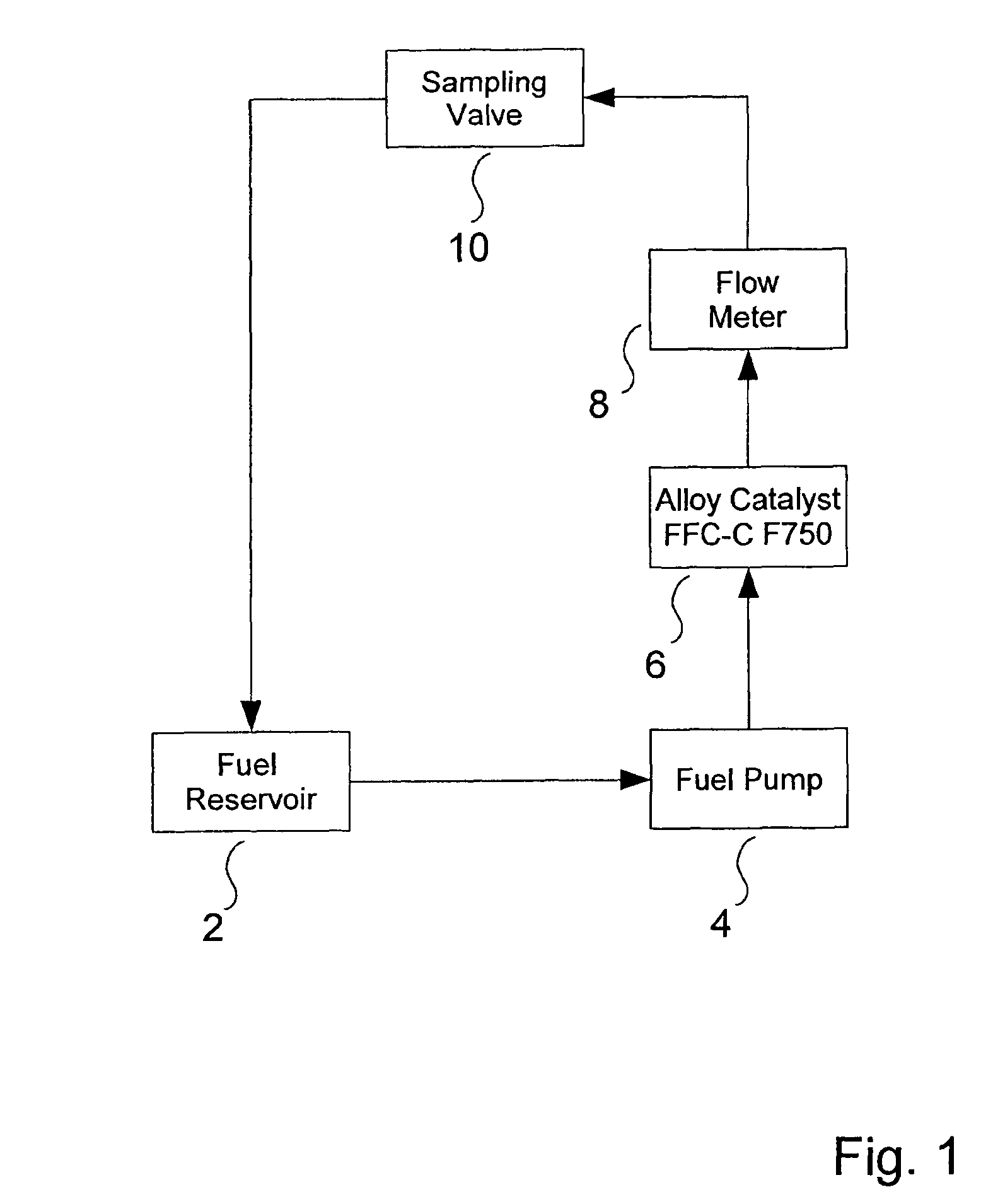
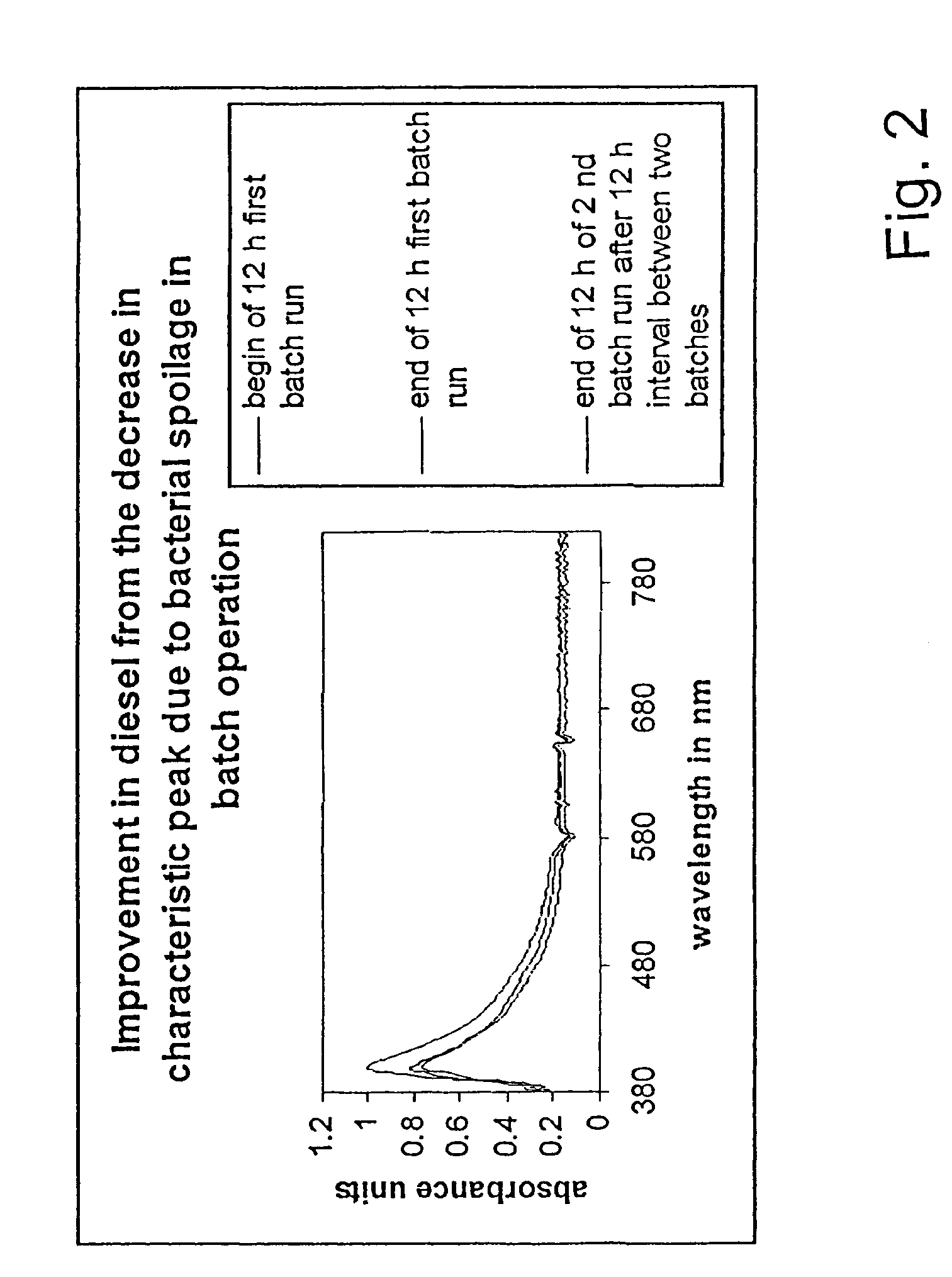
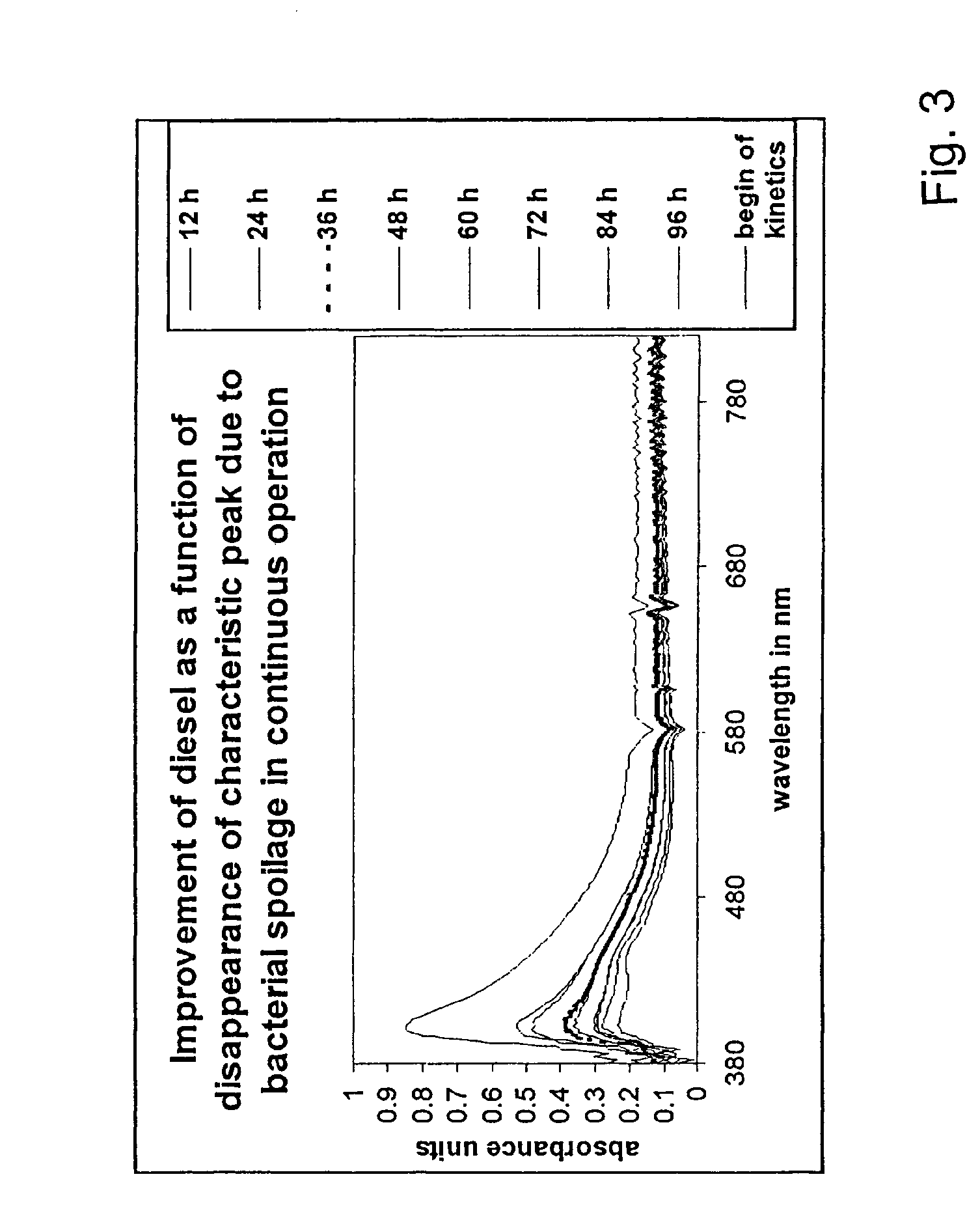
ActiveUS8298405B2Improve combustion efficiencyRefining with non-metalsLiquid fuel feeder/distributionBiodieselCombustion
The invention provides a metal alloy fuel catalyst for decontaminating a hydrocarbon fuel, including diesel and bio-diesel fuel, of a bacterial contamination and for improving fuel combustion. The metal alloy fuel catalysts preferably includes about 70% Sn, about 22% Sb, about 4% Bi, and about 4% Pb, although other formulations are possible. The fuel catalyst can take the form of an in-line component in a fuel system or be coated within a fuel storage container.
Owner:ADVANCED POWER SYST INT
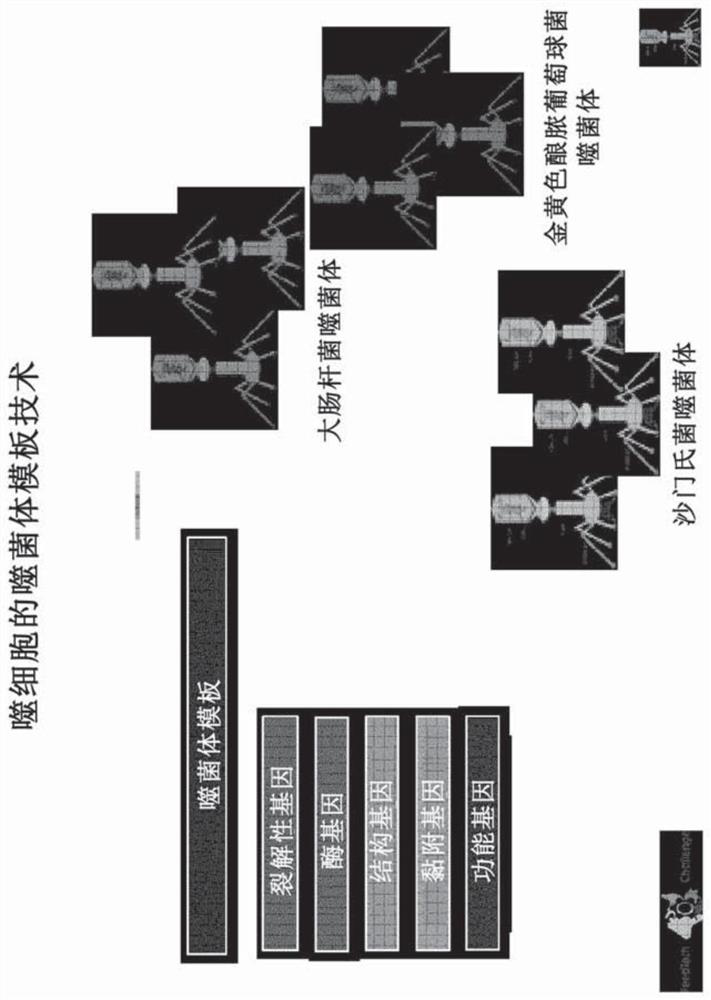
Genetically engineered bacteriophage
There is disclosed a method of engineering bacteriophages comprising: identifying a bacteriophage with only one attachment gene; isolating said bacteriophage; removing said attachment gene from the genome of said bacteriophage; and inserting a non-natural attachment gene into the genome of said bacteriophage wherein said non-natural attachment gene is specific for attaching to a selected bacteria.There is also disclosed a mutant bacteriophage comprising a heterologous nucleic acid sequence encoding a first specific attachment gene, the first specific attachment gene being different than an inactivated attachment gene and being specific for a selected bacteria. In another embodiment, there is disclosed a method of eliminating a microbial contaminant, the method comprising: obtaining one ormore lytic enzymes produced by a mutant bacteriophage; applying the one or more lytic enzymes to a bacterial contaminant, without prior infection of the bacterial contaminant with a bacteriophage, toeliminate the bacterial contaminant.
Owner:噬菌体技术公司
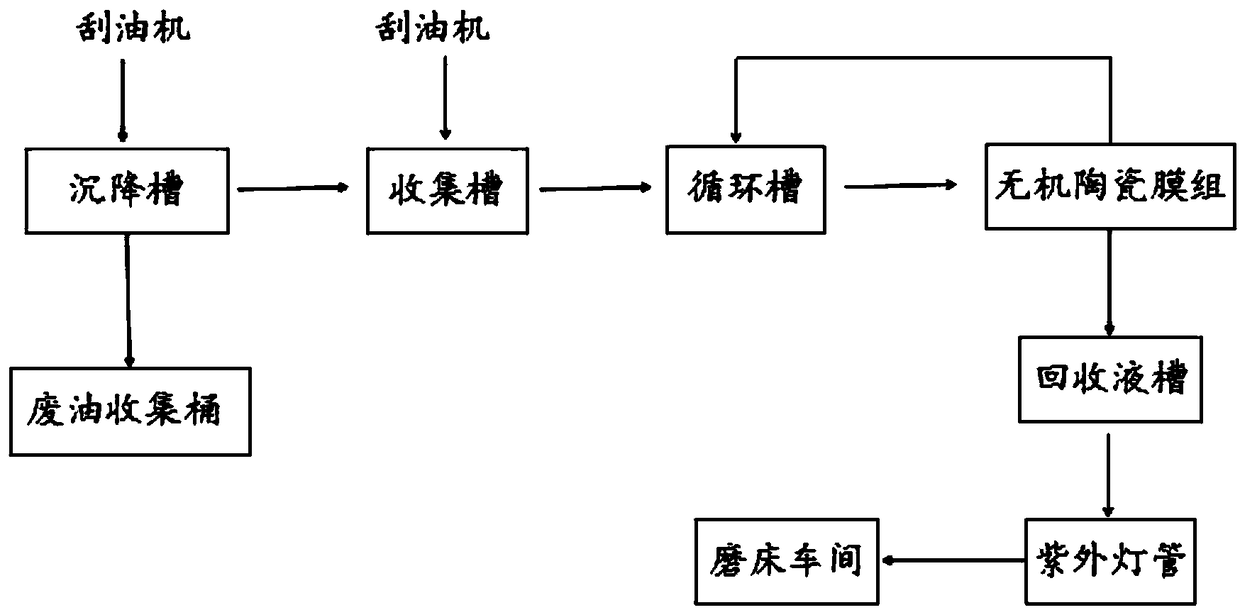
Method for recovering cutting fluid by ceramic membrane filtration
InactiveCN108083525AAchieve purification effectAchieve recyclingFatty/oily/floating substances removal devicesWater/sewage treatment by irradiationPreservativeLiquid tank
The invention discloses a method for recovering cutting fluid by ceramic membrane filtration. The method comprises the following steps: (1) preliminary oil removal treatment is performed; (2) secondary oil removal treatment is performed; (3) the cutting fluid flows through an inorganic ceramic membrane group and is filtered and purified; (4) the cutting fluid filtered by the inorganic ceramic membrane group enters a recovery liquid tank, flows through an ultraviolet lamp tube for sterilization and then is supplied to a grinding machine workshop. The cutting fluid is filtered and recovered withan inorganic ceramic membrane technology and can be purified and recovered, pollutant oil, bacteria and impurities in the cutting fluid are removed, and main raw materials such as a surfactant, a preservative, a bactericide and the like of the cutting fluid are retained, so that the cutting fluid is effectively recycled, and the recovery efficiency is high; The method is simple and easy to operate and is suitable for large-scale operation; the recovered cutting fluid is sterilized by the ultraviolet lamp tube, bacterial contamination in the cutting fluid can be removed, and the cutting fluidis purified.
Owner:安徽名创新材料科技有限公司
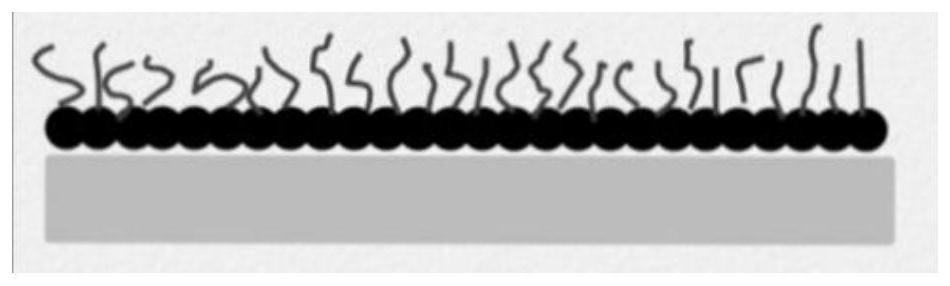
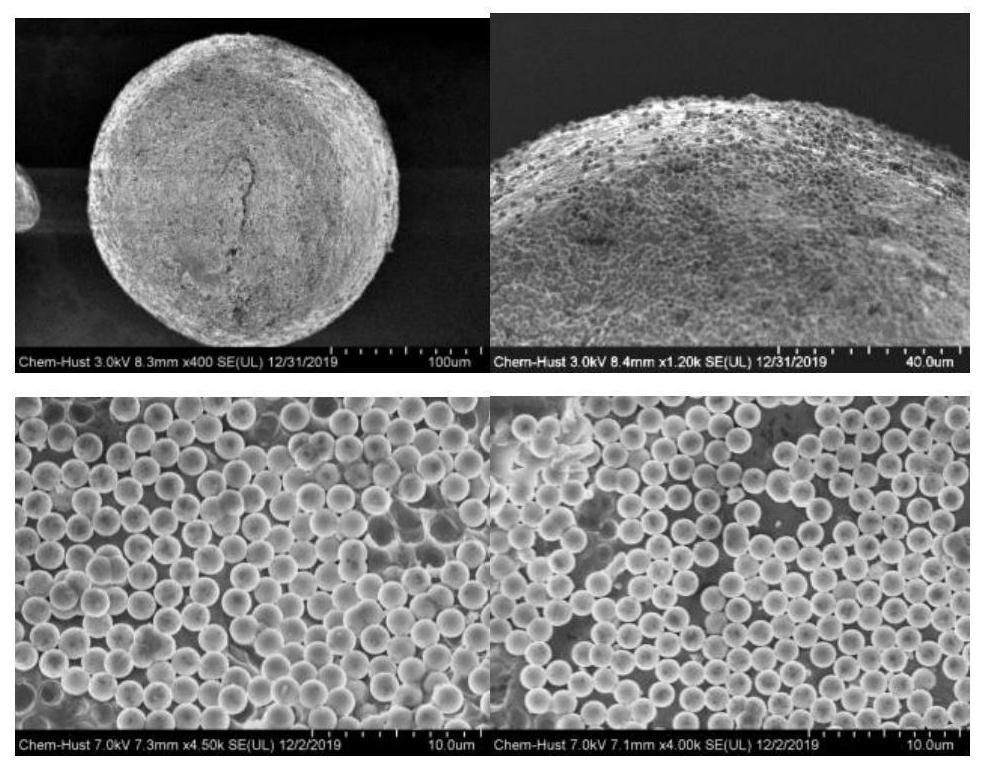
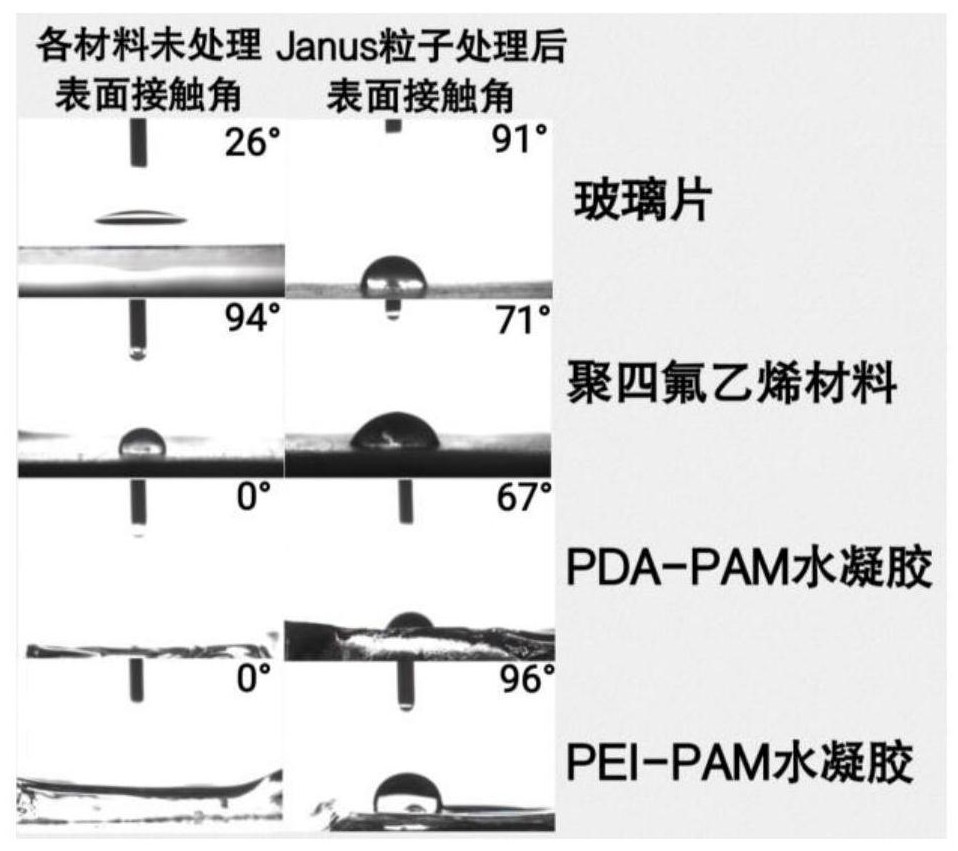
Surface-hydrophobic hydrogel as well as preparation and application thereof
ActiveCN113698654ADelay water lossResolve attachmentCoatingsBandagesPolymer scienceBacterial contaminants
The invention belongs to the field of polymer soft materials, and relates to surface-hydrophobic hydrogel as well as preparation and application thereof. The surface of a hydrogel main body material is modified with amphiphilic Janus particles, the hydrophilic ends of the amphiphilic Janus particles interact with the hydrogel main body, and the hydrophobic ends of the Janus particles form a hydrophobic surface on the surface of the hydrogel main body material, so that the surface-hydrophobic hydrogel is obtained. The hydrophobic surface of the hydrogel can prevent the adsorption of bacteria and pollutants and delay the evaporation of water in the hydrogel, so that the long-term stable storage of the hydrogel is facilitated. The surface-hydrophobic hydrogel has potential application value in the fields of antifouling and self-cleaning, wound dressings and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Dental water disinfector
InactiveUS20140017122A1Eliminate viable bacterialLavatory sanitoryNature of treatment waterWater useMedicine
The present invention generally relates to water disinfection systems for use in dental operatory settings. In particular, a dental water disinfector is described, wherein the dental water disinfector is configured to purify water used during a dental procedure. In preferred embodiments of the present invention, the dental water disinfector is utilized to eliminate bacterial contaminants present in water supplies utilized by dental drills and irrigation equipment.
Owner:UNION DENTAL HLDG +1
Universal liquid perfusion lubricating coating and preparation method thereof
InactiveCN111500183AUniversalStain/soil resistant fibresBiochemical fibre treatmentSilicic acidSolid substrate
The invention discloses a universal liquid perfusion lubricating coating and a preparation method thereof, and belongs to the field of biomimetic material chemistry. The coating is composed of a dopamine activated layer, a silicon dioxide nanoparticle layer, a fluorinated layer and a lubricating oil layer. The preparation method comprises the following steps: firstly, dopamine is oxidized and self-polymerized to form the polydopamine activated layer under the assistance of polyethyleneimine, then the silicon dioxide nanoparticle layer is formed through a polycondensation reaction of tetramethyl silicate, the fluorinated layer is obtained through further treatment of fluorine-containing silane, lubricating oil is poured to form the lubricating oil layer, and the liquid pouring lubricating coating with universality is obtained. A polluted liquid can slide off from the surface at a small inclination angle, the adhesion amount to proteins and bacterial pollutants is small, the anti-pollution characteristic and the anti-bioadhesion characteristic are excellent, and the coating is feasible for most solid substrates and has universality.
Owner:TIANJIN POLYTECHNIC UNIV
Produce conveying and sizing equipment
ActiveUS10618740B2Improve cleanabilityIncreased durabilityControl devices for conveyorsMechanical conveyorsProcess engineeringSprocket
The present invention includes conveying and sorting systems utilizing a plurality of continuous rails which support a plurality of sliding pucks that are urged forward using a plurality of sprocket wheels, and which utilize magnetically operated activation components for causing produce to be selectively dropped from cups carried by the pucks into sorting bins. The structures for the conveying and actuating components of the systems have smooth external surfaces reducing potential contamination by bacteria, contaminants and debris, thereby making them easily accessible for cleaning, and allowing for longer continuous operation times and higher efficiency.
Owner:REED LORIN
Thermotropic air cleaner
InactiveCN101732750AQuality improvementIncrease indoor oxygen contentGaseous substancesDeodrantsIndoor air qualityToxic material
The invention provides a thermotropic air cleaner, belonging to daily electronic equipment field. The thermotropic air cleaner comprises a cleaner framework relevant to application, an airflow generation assembly and a cleansing electric heating element on which an electric heating element is arranged, wherein, the airflow generation assembly is arranged in the cleaner framework and is used for generating airflow, and the cleansing electric heating element is arranged on the cleaner framework and is positioned at least one side of the front side and the back side of the airflow generation assembly. The system is used for providing an element capable of cleansing air quality by the cleansing electric heating element through utilizing the airflow generation assembly to generate airflow on the basis of electric heating cleansing related to the invention. The cleaner can clean bacterial pollutant and toxic material in indoor air, add indoor oxygen content and improve indoor air quality.
Owner:SHANGHAI LIANGKE ELECTRONICS TECH
Methods for the detection and/or quantification of gram positive bacterial contaminants
InactiveUS7968280B2Anthropod material medical ingredientsMicrobiological testing/measurementGramBacterial contaminants
The invention provides methods and compositions for the detection and / or quantification of a Gram positive bacterial contaminant in a sample. In particular, the invention provides hemocyte-based preparations, methods of making such hemocyte-based preparations, and methods of using such hemocyte-based preparations for the detection and / or quantification of the Gram positive bacterial contaminant.
Owner:CHARLES RIVER LAB INC
Tissue pathogen inactivation/removal process
ActiveUS7585461B2Prevents and reduces undesirable damageReduce the temperatureBone implantPreparing sample for investigationLipid formationActive agent
Bone such as cortical or cancellous bone or connective tissue are centrifuged as a solution is applied to the tissue in a continuous process. The centrifuge creates forces, which cause the flowing solution to penetrate substantially all of the cavities of the tissue. The solution, which may be alcohol, a detergent, an oxidizer, or a surfactant, with or without water, flushes the cavities of the tissue and removes and inactivates viral and bacterial contaminants. In a second embodiment, bone or connective tissue is centrifuged in a batch process to remove contaminants from cavities in the tissue by first spinning the tissue dry and then submerging the tissue in a viral / bacteria cleansing solution and centrifuging the submerged tissue and solution at a sufficiently high speed and radius from the spin axis to create substantially high G forces on the tissue and solution to force the solution into and through the tissue cavities. The solution with the flushed contaminants is then removed from the vicinity of the tissue. In a further embodiment, an enzyme is centrifuged with the tissue to digest lipids and proteins which are then removed with a flushing solution which may also inactivate viral and bacterial contaminants. In yet a further embodiment, the centrifuge can be used to infuse biologically and / or structurally useful materials into the tissue. Further, the lipids and proteins may be removed by a chemically active agent such as oxo anions.
Owner:WARSAW ORTHOPEDIC INC
Magnetic separation using nanoparticles
ActiveUS10350320B2Efficient and selective separationFast bindingLavatory sanitoryDeodrantsNanoparticleAlbumin solution
Nanoparticles as described herein are configured to bind to bacterial contaminants, such as Gram positive bacteria, Gram negative bacteria, and endotoxins. The nanoparticles include a core comprising a magnetic material; and a plurality of ligands attached to the core. The ligands include, for example, bis(dipicolylamine) (“DPA”) coordinated with a metal ion, e.g., Zn2+ or Cu2+, to form, e.g., bis-Zn-DPA or bis-Cu-DPA, which can bind to the bacterial contaminants. The nanoparticles can be included in compositions for use in methods and systems to separate bacterial contaminants from liquids, such as liquids, such as blood, e.g., whole or diluted blood, buffer solutions, albumin solutions, beverages for human and / or animal consumption, e.g., drinking water, liquid medications for humans and / or animals, or other liquids.
Owner:MASSACHUSETTS INST OF TECH +1
Closestool with function of automatically opening and closing closestool cover
InactiveCN111705882AAvoid pollutionHigh degree of automationFlushing devicesBathroom coversStructural engineeringDiaphragm seal
The invention relates to a closestool with the automatic closestool cover opening and closing function, which comprises a closestool body, mounting boxes are symmetrically connected to the lower end of the side wall of the closestool body, and a driving mechanism is arranged on each mounting box. According to the invention, the diaphragm is controlled through the cooperation of the driving mechanism and the magnetic block; when a user needs to stand on the pedal during defecation, the pedal is turned on; the closestool body is automatically opened to be used, when a user leaves the pedal afterdefecation, the diaphragm seals the closestool body again, water is flushed at the moment, bacterial pollutants in the closestool body can be prevented from polluting a toilet, and the closestool ishigh in automation degree, convenient to use and high in practicability.
Owner:陈兴华
ATP-bioluminescence immunoassay
InactiveUS8518658B1Wide applicationWeak matrixMicrobiological testing/measurementMaterial analysisSalmonella entericaBacteroides
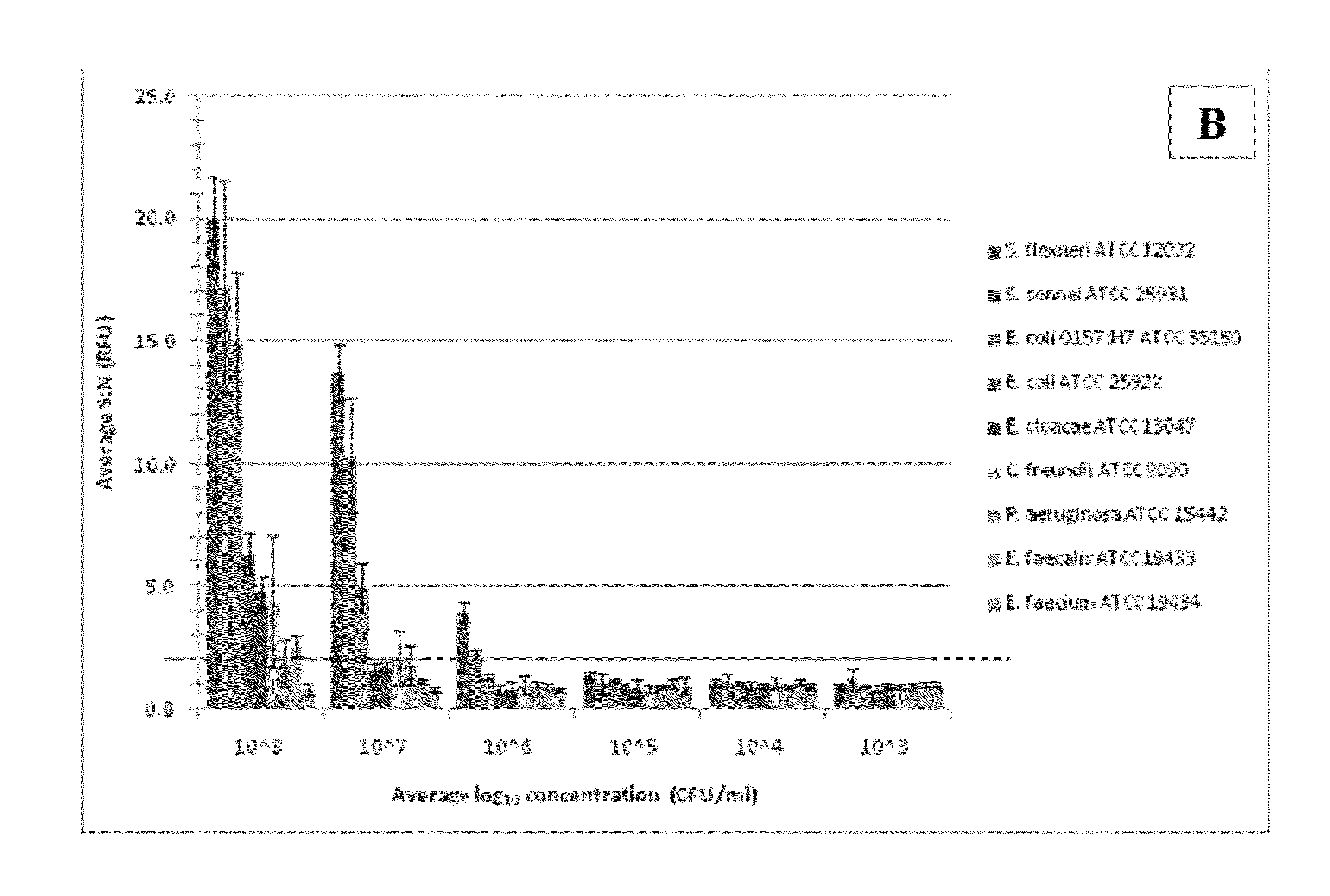
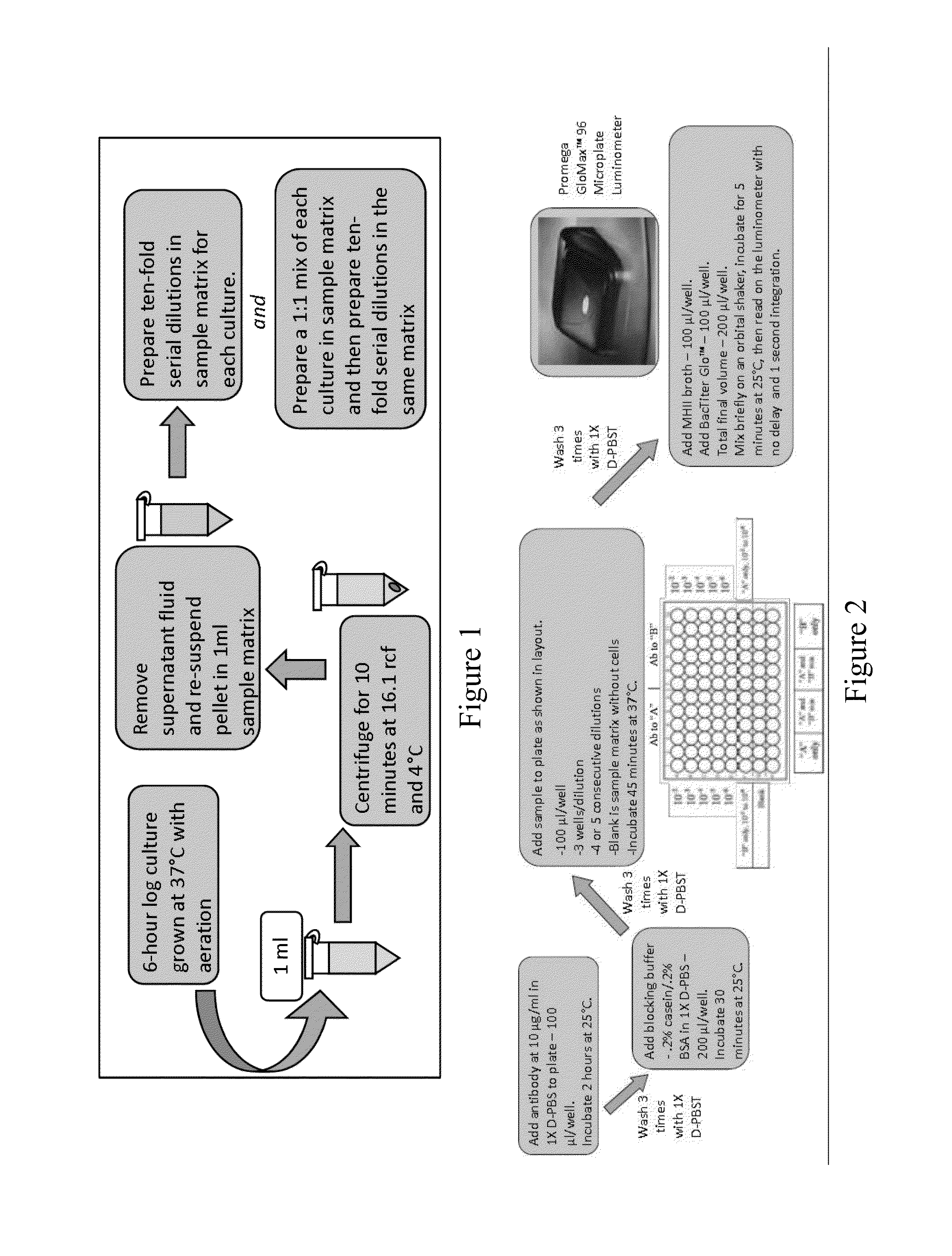
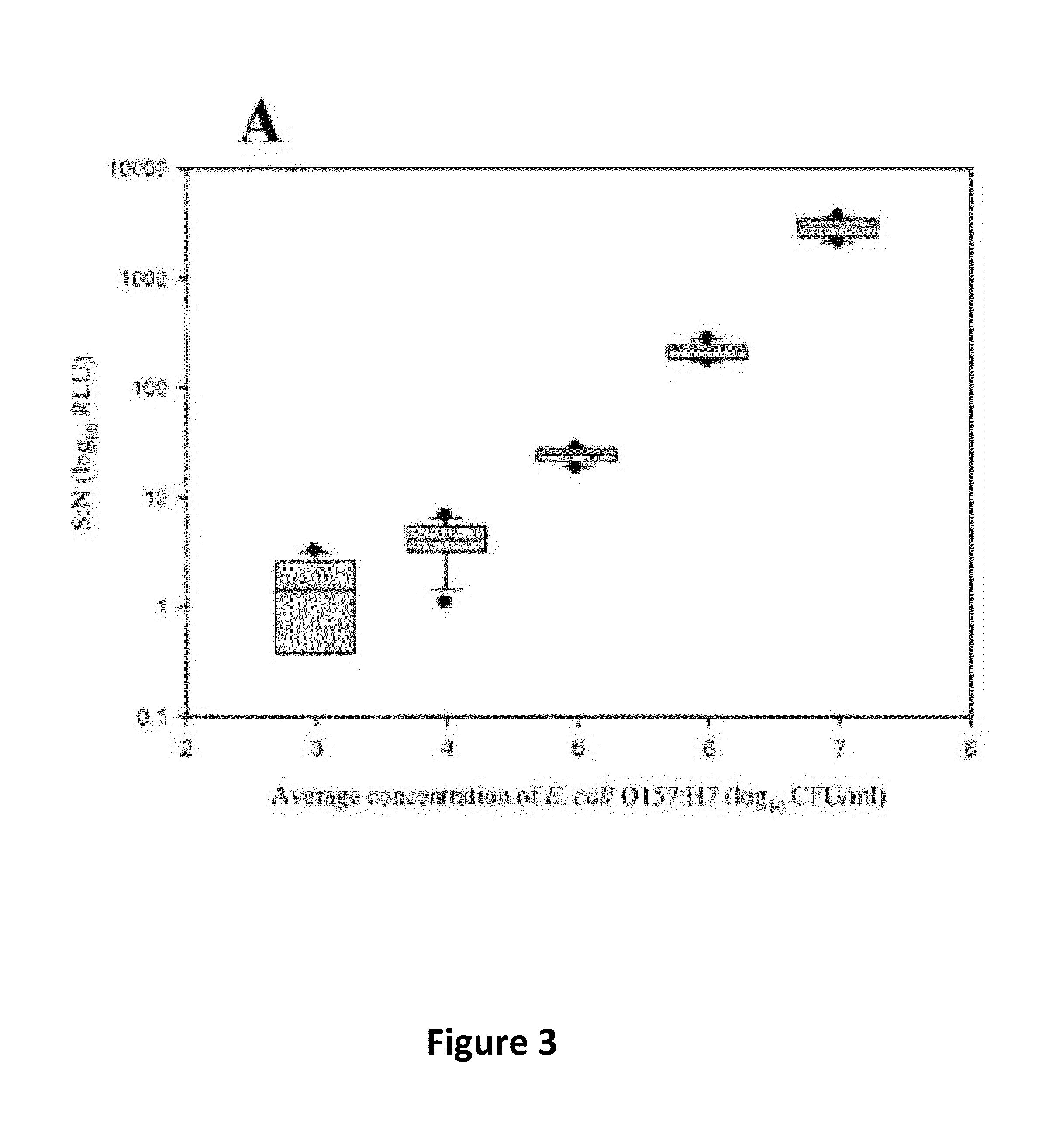
Disclosed is a method and associated device for the rapid identification of viable bacterial contaminants in food products. The method detects viable microbes by using a combined ATP-bioluminescence immunoassay. Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium were selected as target organisms in various matrices including ground beef homogenate, apple juice, milk, and phosphate-buffered saline. Specific antibodies were immobilized on the surface of well plates in which the sample matrices were incubated. The plates were washed, and the wells were incubated with BacTiter-Glo reagent in Mueller-Hinton II broth. Bioluminescent output was measured with a luminometer and signal-to-noise ratios were calculated. The LOD was not affected by the presence of non-target cells. A strong linear correlation was observed between the number of cells and luminescent output over 4 orders of magnitude. This method provides a means of simultaneously detecting and identifying viable pathogens in complex matrices.
Owner:UNIV OF SOUTH FLORIDA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com