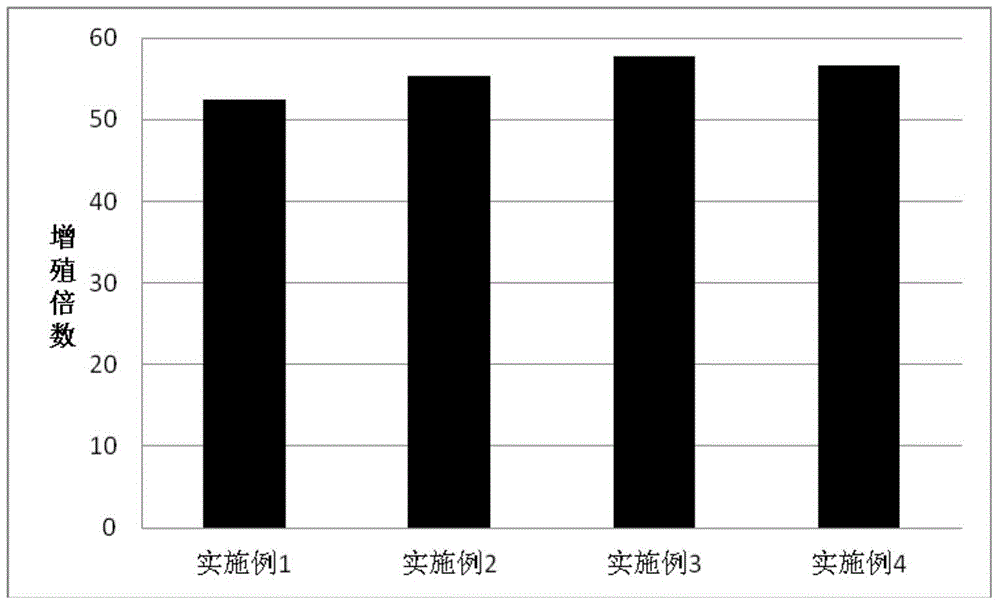
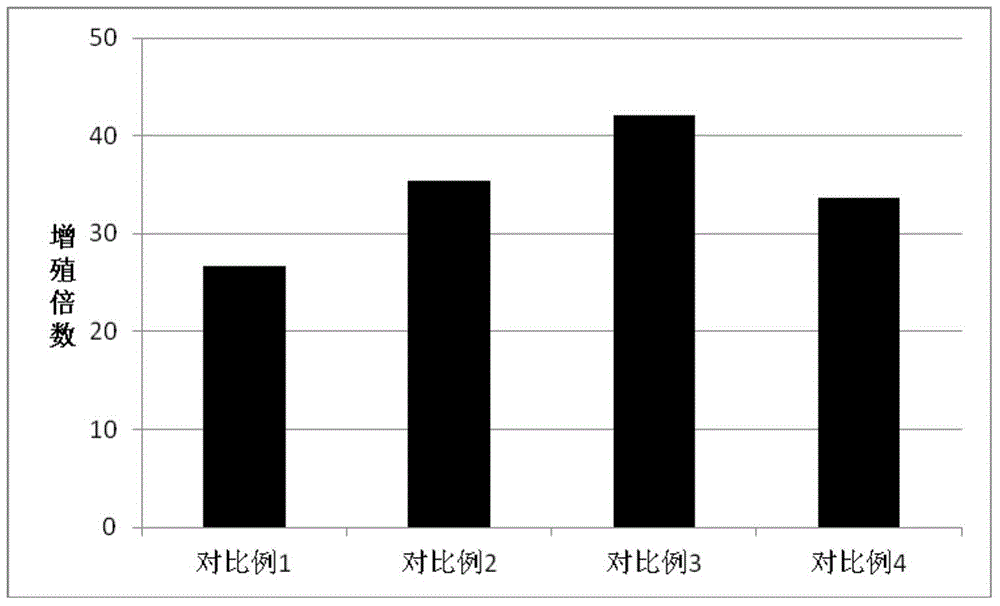
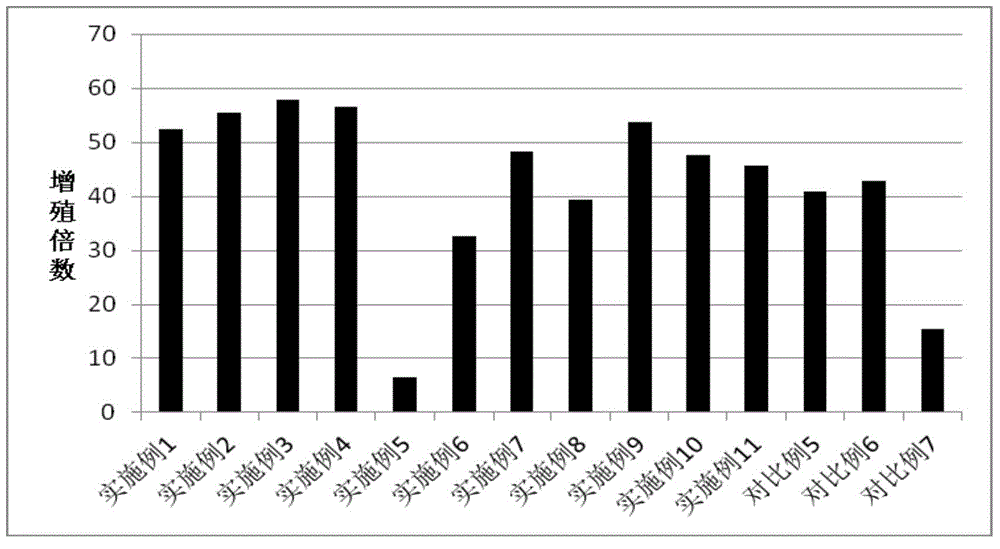
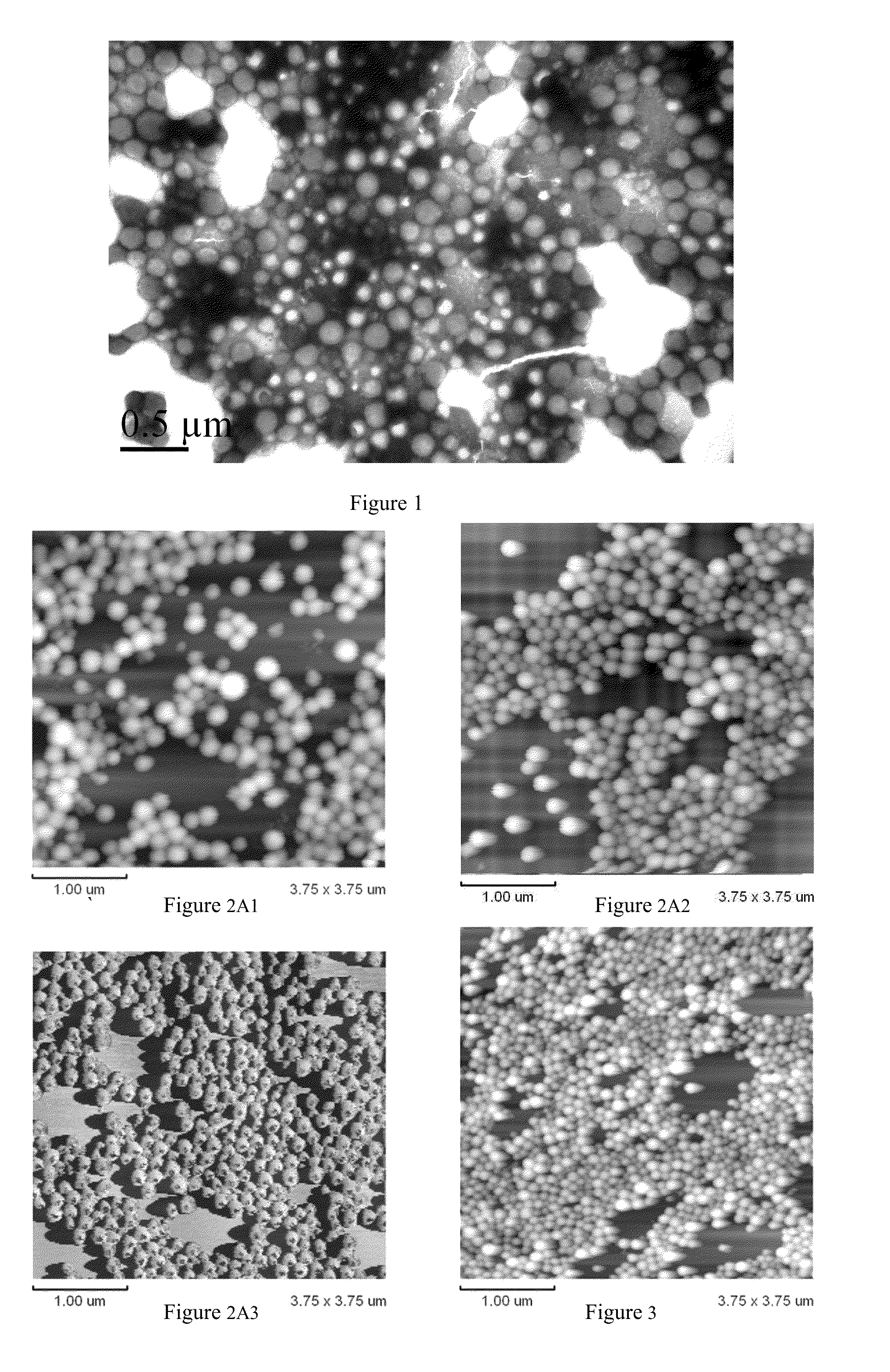
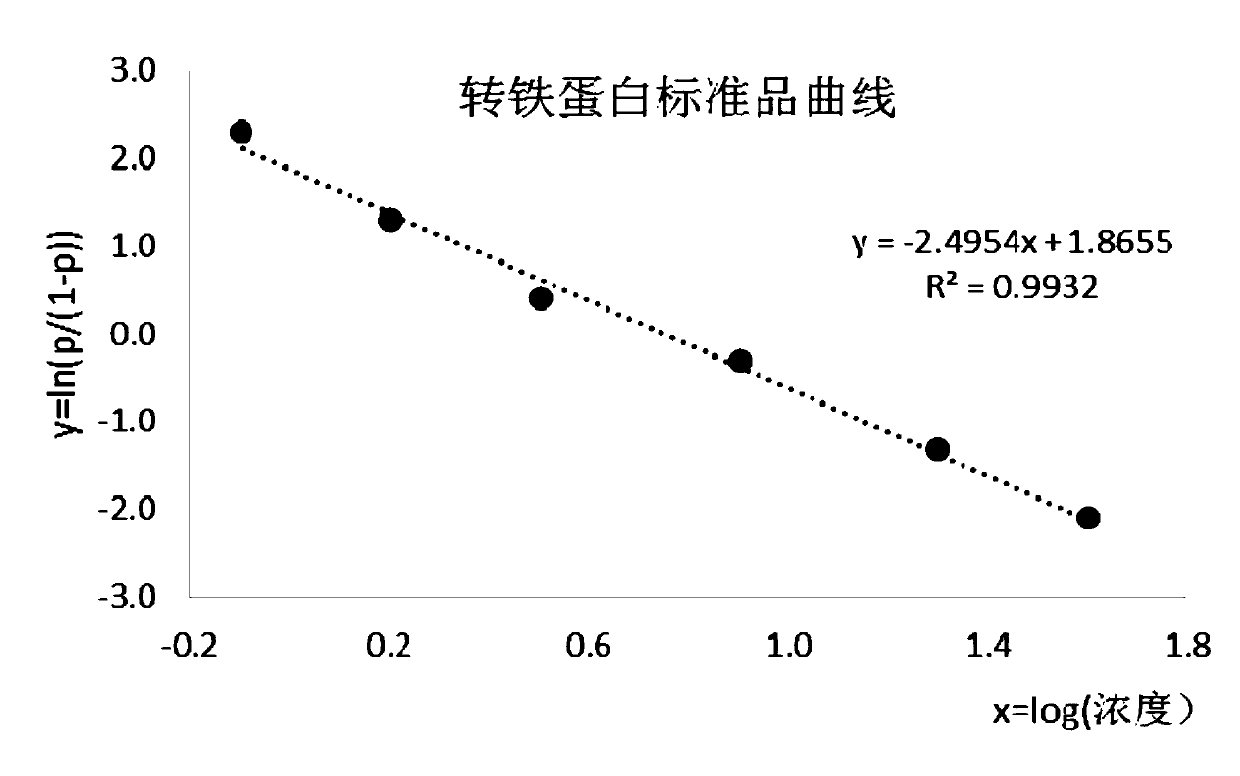
Patents
Literature
85 results about "Albumin solution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Paclitaxel-based antitumor formulation
InactiveUS20070020337A1Simplifies plantFinal yieldPowder deliveryBiocideNanoparticle ProductionAlbumin solution
Antitumor formulation based on nanoparticles of paclitaxel and human serum albumin as obtained by the addition of a biocompatible acid to an aqueous albumin solution before this is mixed with paclitaxel during the nanoparticle production process, the injectable solutions of this formulation having a pH between 5.4 and 5.8 and having stability and inalterability with time.
Owner:ABRAXIS BIOSCI LLC
Method for preparing macromolecule resin type bilirubin sorbent
InactiveCN1557538AImprove mechanical propertiesAvoid the danger of blood pressure dropsOther chemical processesCross-linkSorbent
The present invention features that the bilirubin adsorbent is synthesized by selecting well biocompatible polystyrene-divinylbenzene copolymer as carrier, PHEMA as coating agent, epichlorohydrin for activating the functional hydroxy radical of PHEMA, and glutaraldehyde as cross-linking agent for cross-linking functional cyclodextrin molecule. The bilirubin adsorbent has biocompatibility and adsorption capacity superior to active carbon and cationic resin adsorbing material, and has no adsorbent molecule falling resulting in immunological dysfunction. Animal experiment shows that the novel bilirubin adsorbent may be used to eliminate bilirubin from blood, blood plasma and albumin solution effectively.
Owner:杭州科锐净化工程有限公司
Plasma head for tissue welding
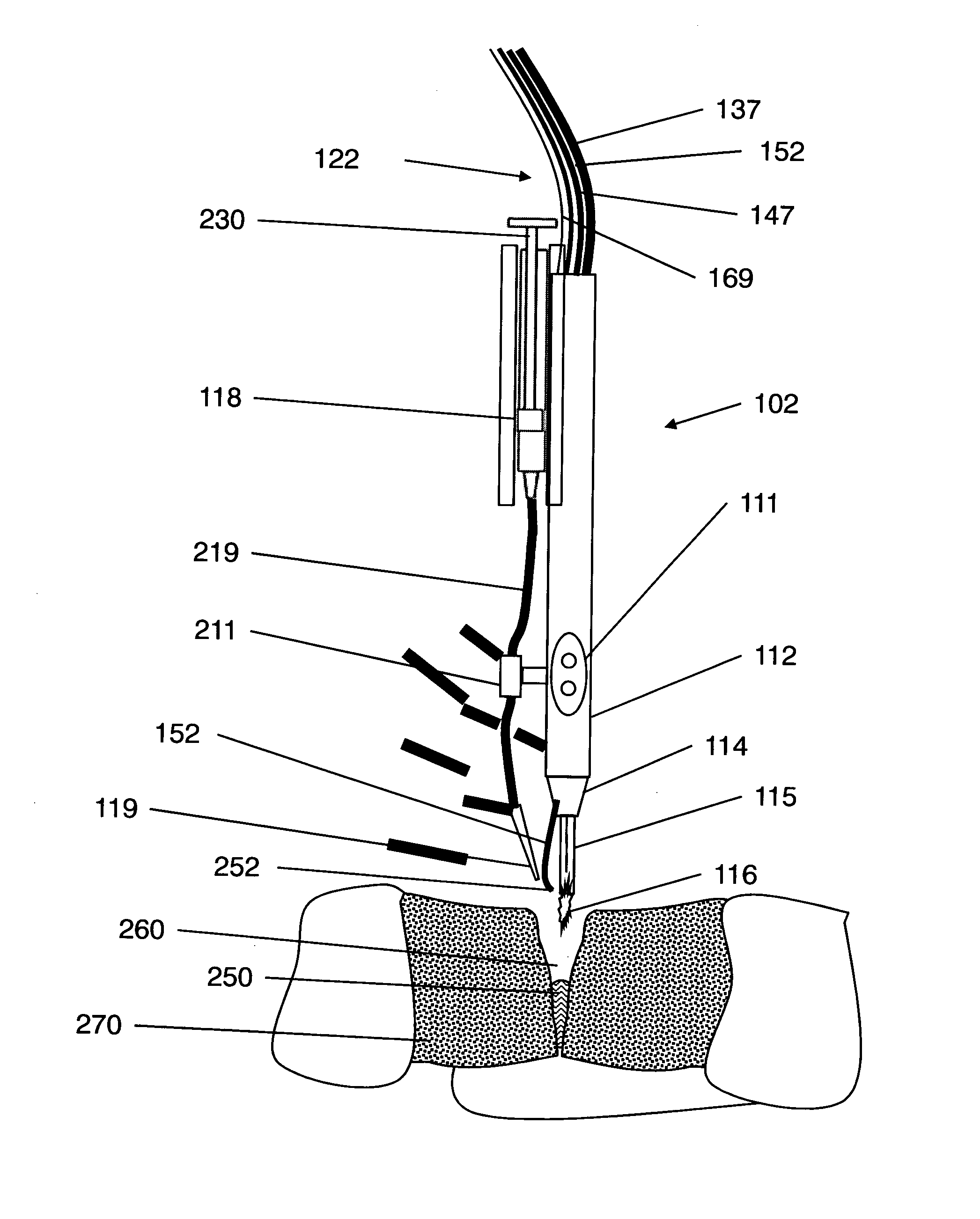
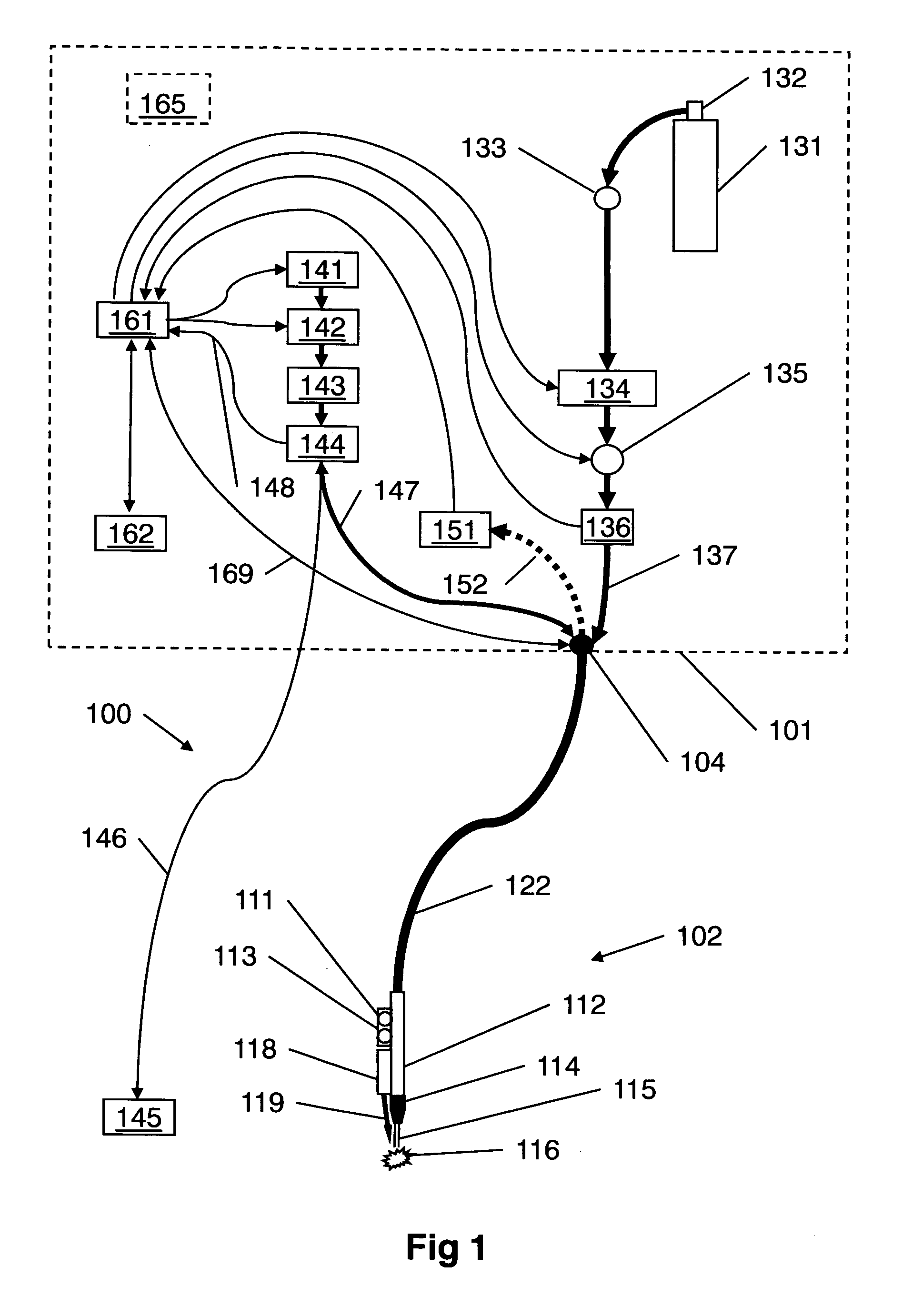
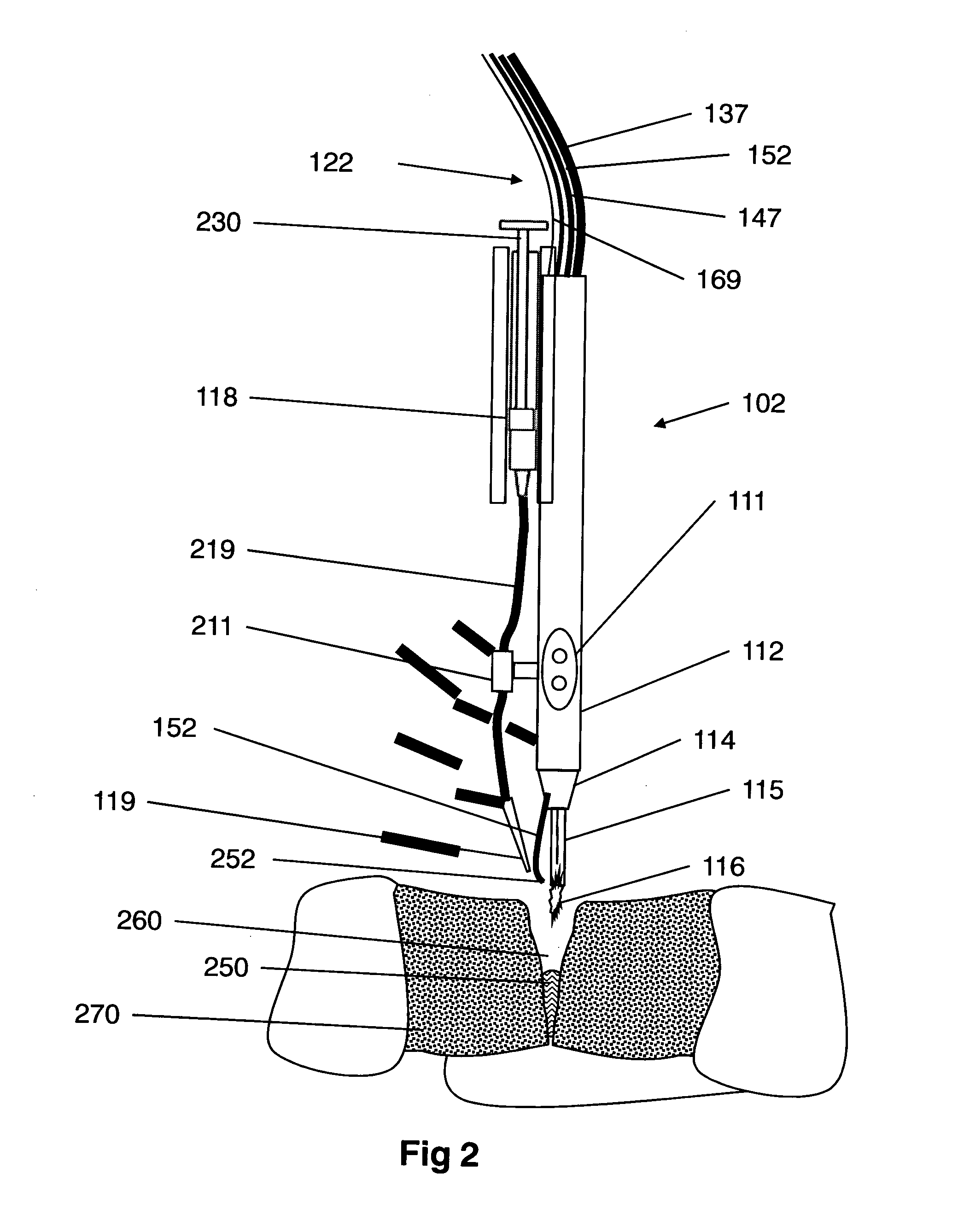
InactiveUS9060750B2Easy to adjustIncreasing RF power/frequencyDiagnosticsSurgical instruments for heatingAlbumin solutionHand held
A compact medical device for tissue welding is provided. The hand-held plasma heads are configured for deep cuts and long cuts. A bio-compatible liquid capable of solidifying in response to application of plasma, such as an albumin solution, is applied to the wound. Plasma created from a gas such as helium is then applied to said bio-compatible liquid to solidify it and seal the wound. An additional polymerizing gas may also be applied. A feedback mechanism may maintain the temperature of said plasma. A wiper fort removal of excess liquid may also be provided.
Owner:IONMED
Plastic container containing albumin solution
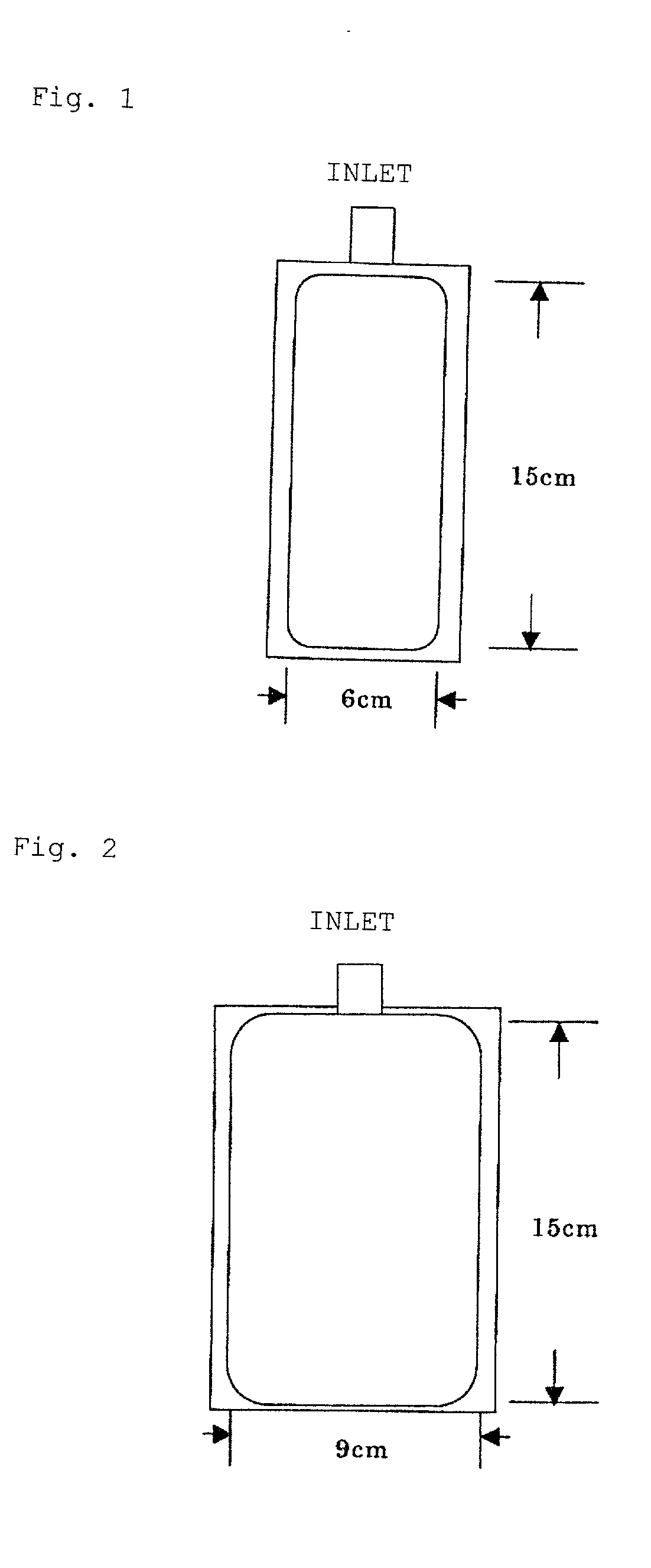
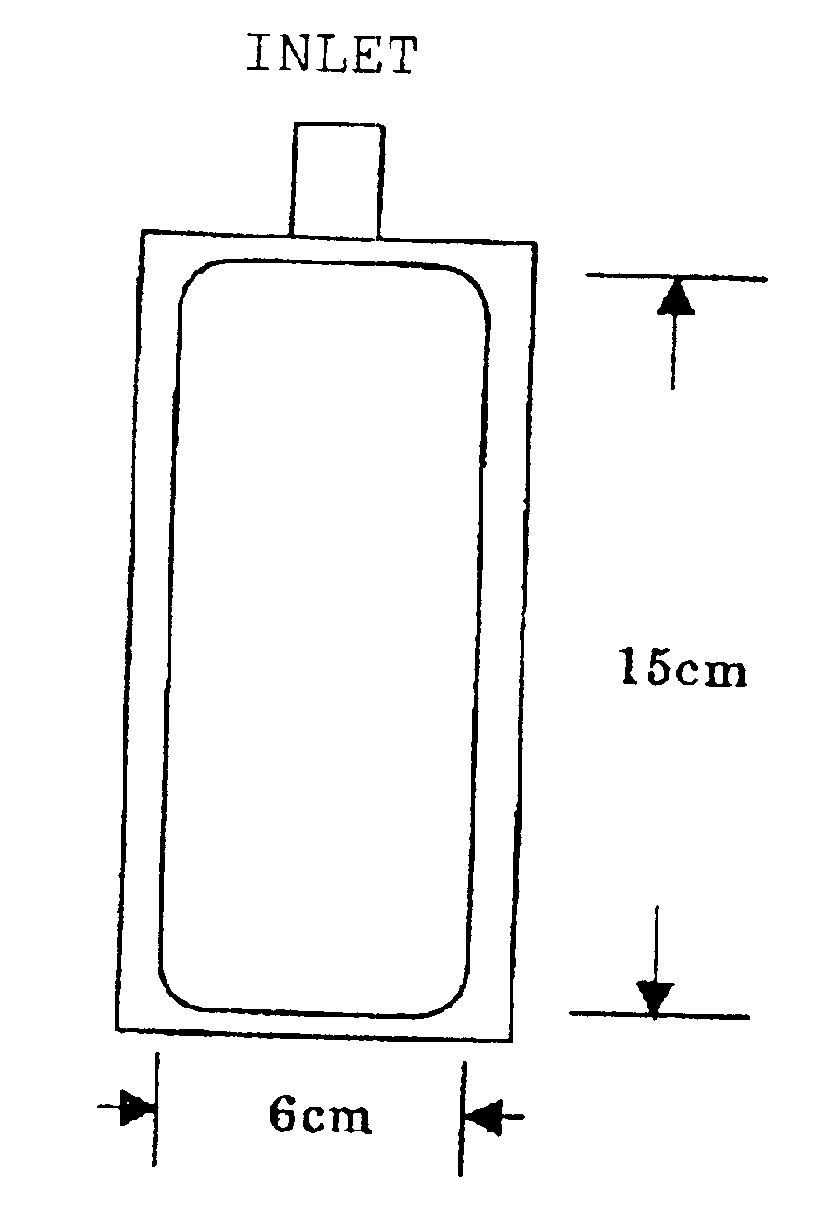
InactiveUS20020192411A1Maintain performanceImprove discharge performancePeptide/protein ingredientsSurgical needlesWater vaporWater vapor permeability
The present invention provides a plastic container containing albumin solution and a packaged plastic container containing an albumin solution. The plastic container containing an albumin solution having an albumin concentration of 1 to 500 mg / ml, has at least one inlet / outlet for a liquid, and has a water vapor permeability of 1.5 g / m2 / day.1013.25 hPa or less when the vapor permeability is measured at a pressure of 1013.25 hPa per surface area of 1 m2 for 24 hours at a temperature of 25° C. and at a relative humidity of 60%. The plastic container containing an albumin solution can be packaged with an outer packaging material to provide the packaged plastic container.
Owner:NIPRO CORP
Magnetic separation using nanoparticles
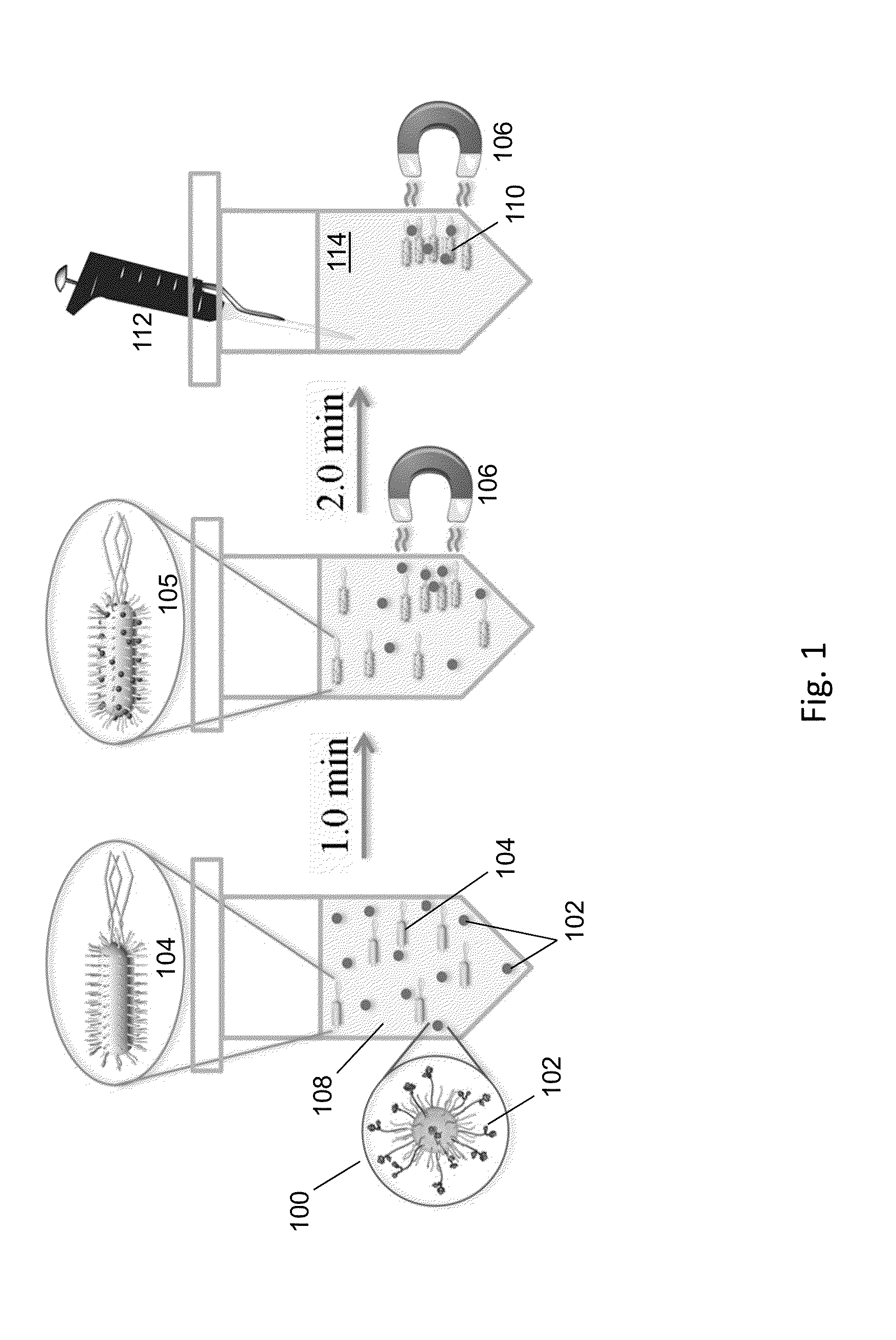
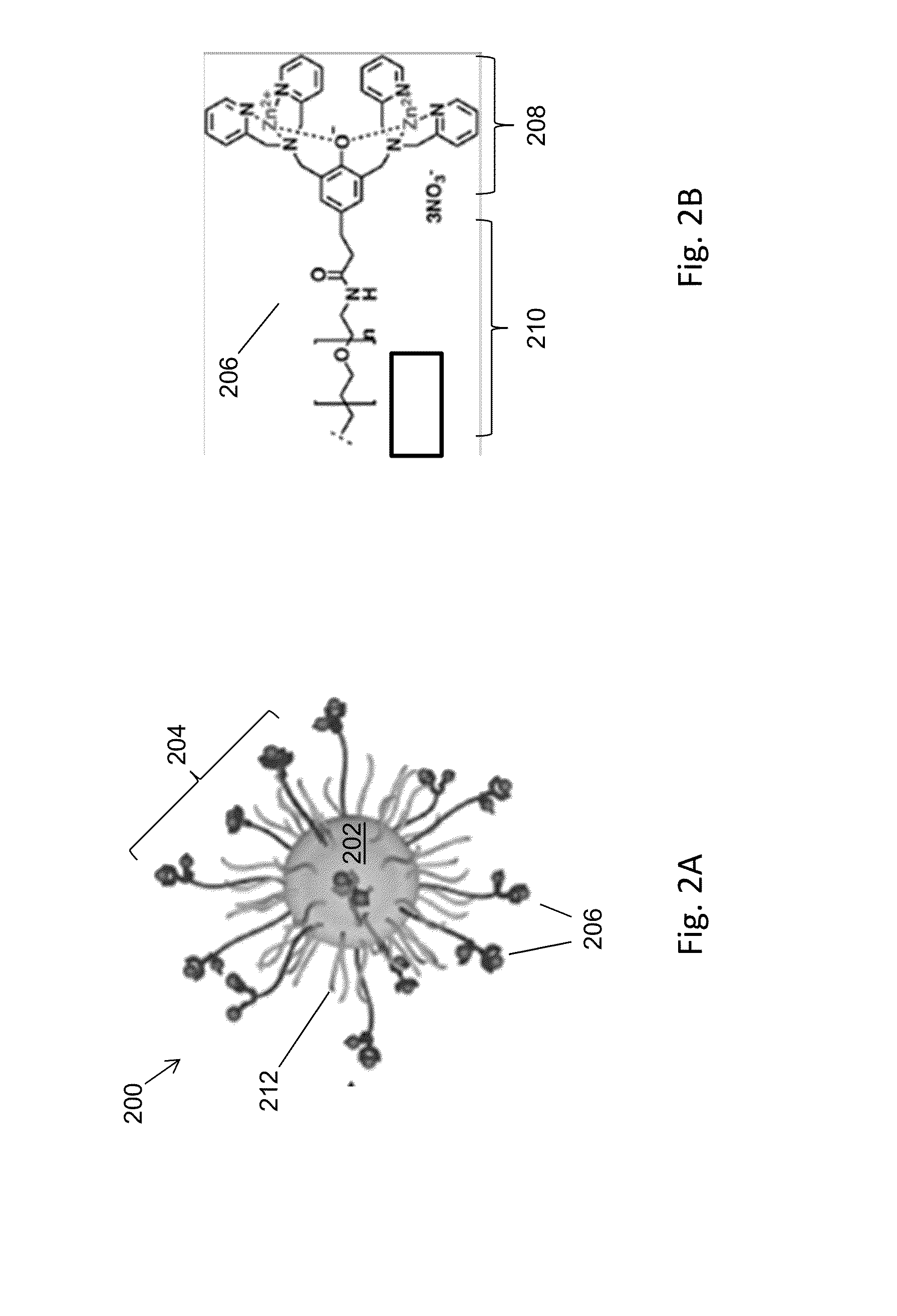
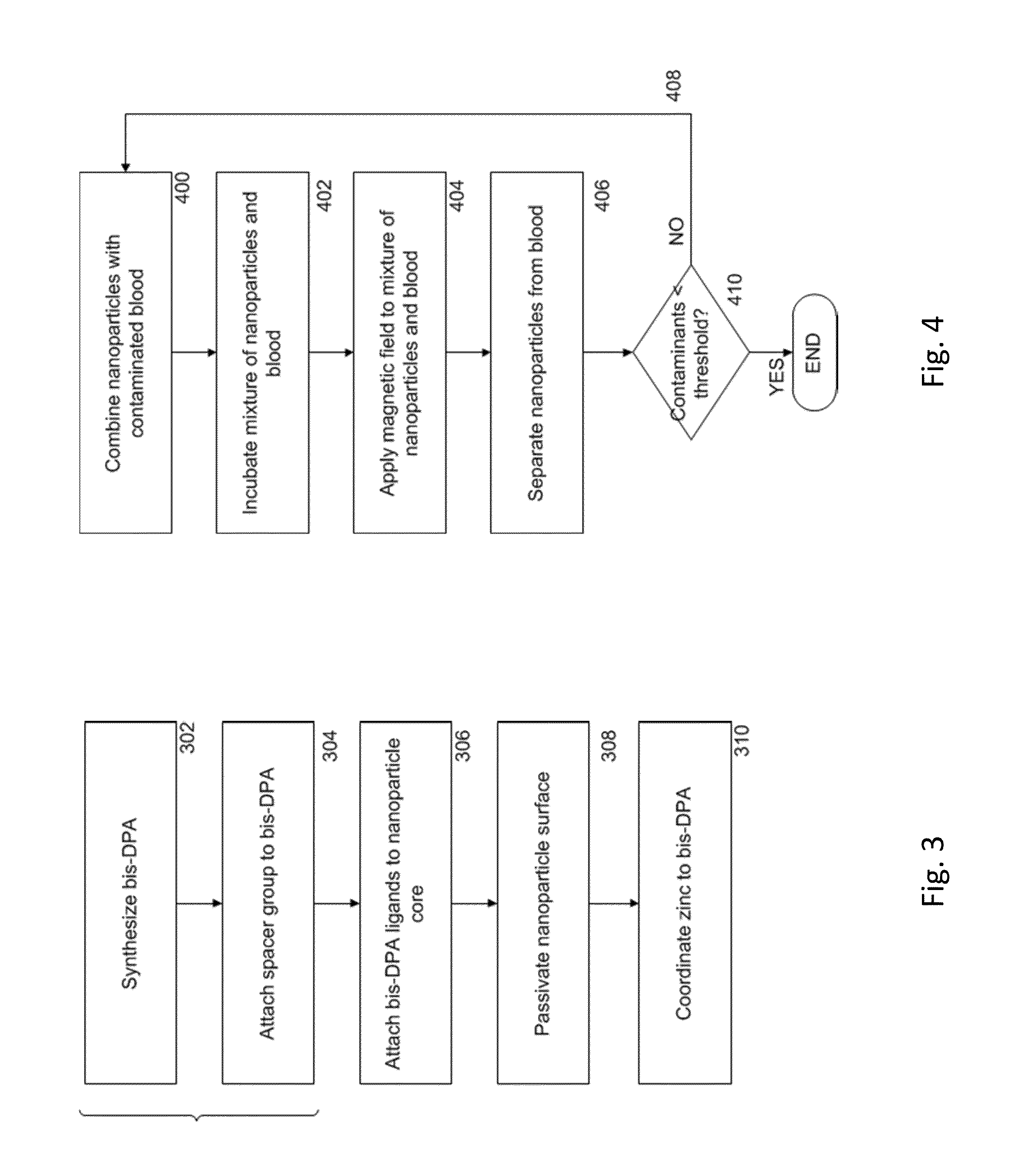
ActiveUS20140212335A1Efficient and selective separationFast bindingGaseous chemical processesLiquid-gas reaction of thin-film typeNanoparticleAlbumin solution
Nanoparticles as described herein are configured to bind to bacterial contaminants, such as Gram positive bacteria, Gram negative bacteria, and endotoxins. The nanoparticles include a core comprising a magnetic material; and a plurality of ligands attached to the core. The ligands include, for example, bis(dipicolylamine) (“DPA”) coordinated with a metal ion, e.g., Zn2+ or Cu2+, to form, e.g., bis-Zn-DPA or bis-Cu-DPA, which can bind to the bacterial contaminants. The nanoparticles can be included in compositions for use in methods and systems to separate bacterial contaminants from liquids, such as liquids, such as blood, e.g., whole or diluted blood, buffer solutions, albumin solutions, beverages for human and / or animal consumption, e.g., drinking water, liquid medications for humans and / or animals, or other liquids.
Owner:MASSACHUSETTS INST OF TECH +1
Serum-free medium and preparation method thereof
InactiveCN104911147AComposition is stableGood functional consistencyBlood/immune system cellsAlbumin solutionCulture mediums
The invention relates to a serum-free medium and a preparation method thereof. The preparation method of the serum-free medium includes: loading fatty acid on FAF (fatty-acid-free) albumin, and then adding an obtained albumin solution into a basic culture medium. The serum-free medium obtained by using the preparation method of the serum-free medium is stable in component and good in functional consistency, and no difference can be caused due to difference of species origins or processing batches of the albumin.
Owner:英普乐孚生物技术(上海)有限公司
Arsenic Compound Solution and Albumin Nanoparticle and Lyophilized Preparation Entrapping Arsenic Compound Prepared Using Same
ActiveUS20140370119A1Clear structureGood effectBiocidePeptide/protein ingredientsAlbumin solutionCompound (substance)
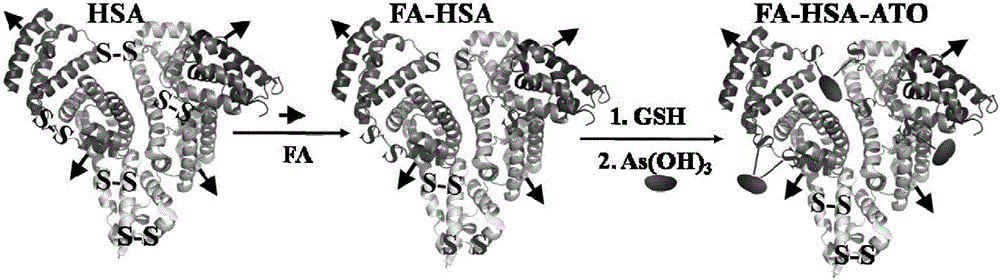
An arsenic compound solution, and an albumin nanoparticle and a lyophilized preparation prepared using same and entrapping an arsenic compound. The arsenic compound solution is prepared using the following method: adding As2O3 powder in sterile deionized water to obtain a suspension, 6 to 25 mg of As2O3 powder being added in every mL of sterile deionized water; dripping an NaOH solution to the obtained suspension until the powder is fully dissolved and adjusting the pH of the solution to 7.5 to 9, and making the concentration of the obtained arsenic compound solution be 5 to 20 mg / mL in terms of the added As2O3. The albumin nanoparticle is prepared by mixing an albumin solution and the arsenic solution and the arsenic solution with the mass ratio of the added As2O3 in arsenic compound solution to the albumin ranging from 1:5 to 1:20 and using a method of solvent removing and physical curing.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Bovine serum albumin extraction method and bovine serum albumin
The invention discloses a bovine serum albumin extraction method which comprises the following steps: first concentration and liquid exchange: conducting ultrafiltration concentration on a protein dissolution solution through a first ultrafiltration membrane to obtain a protein concentrate; first purification: conducting chromatographic purification collection on the protein concentrate through a weak anion exchange medium to obtain a crude albumin solution; second concentration and liquid exchange: conducting ultrafiltration concentration on the crude albumin solution through a second ultrafiltration membrane to obtain a crude albumin concentrate; second purification: conducting chromatographic purification collection on the crude albumin concentrate through a strong cation exchange medium to obtain an albumin solution. According to the extraction method, through the steps of two times of ultrafiltration concentration and two times of chromatographic purification, impurities in blood plasma can be removed to the maximum extent, and the purity of the bovine serum albumin is improved. Besides, the invention also provides bovine serum albumin obtained through the method.
Owner:CHENGDU YUANRUI BIOTECH CO LTD
Fluorescence immunochromatographic kit for quantitative joint detection of four urinary micro proteins and preparation method thereof
The invention discloses a fluorescence immunochromatographic kit for quantitative joint detection of four urinary micro proteins and a preparation method thereof, which solves the problems that the detection of urinary micro proteins in clinical detection cannot be simultaneously carried out. A fluorescence immunochromatographic kit for quantitative joint detection of four urinary micro proteins comprises an immunochromatographic strip coated with an albumin capture antibody, an alpha 1-microglobulin capture antibody, a transferrin capture antibody, a beta 2-microglobulin capture antibody anda protein A, and a fluorescent microsphere labeled antigen solution including a fluorescent microsphere labeled albumin solution, a fluorescent microsphere labeled alpha 1-microglobulin solution, a fluorescent microsphere labeled transferrin solution and a fluorescent microsphere labeled beta 2-microglobulin solution; and fluorescent microspheres are also labeled with IgG immunoglobulin. The invention has the advantages of rapid detection and the like.
Owner:杭州康知生物科技有限公司
Non-programmable-controlled cooling cryopreservation method and protection agent for hematopoietic stem cells at minus 80DEG C
InactiveCN103548814AImprove survival rateSimple recipeDead animal preservationHydroxyethyl starchChemistry
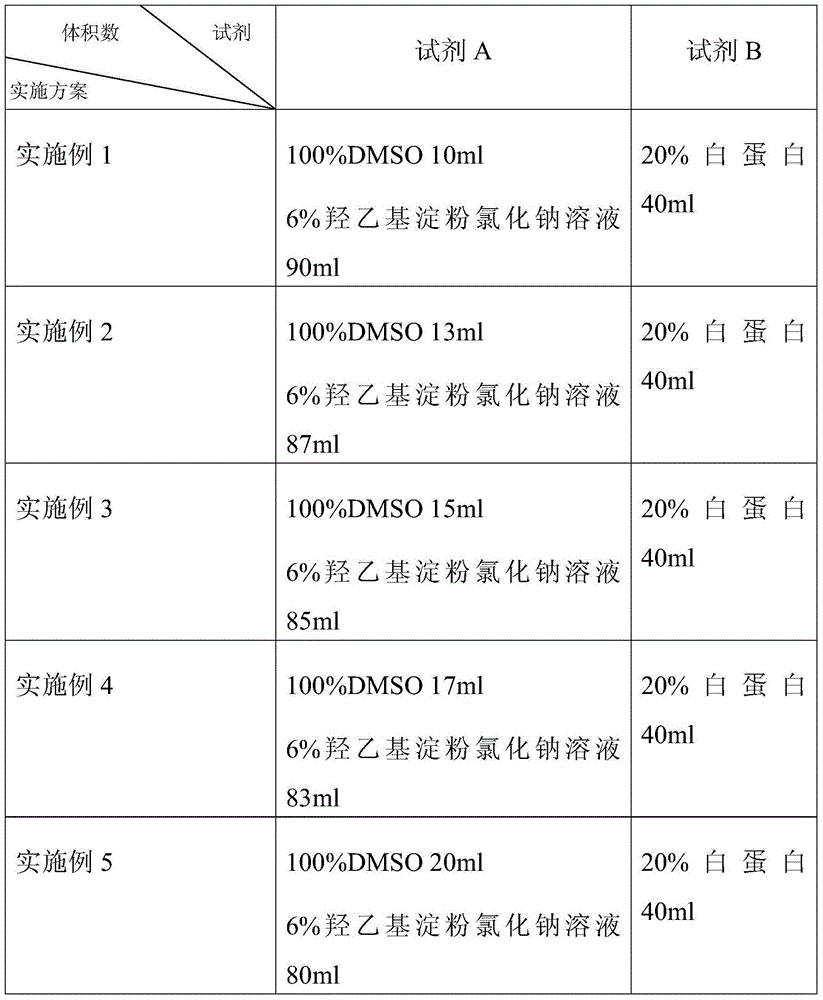
The invention relates to a non-programmable-controlled cooling cryopreservation method and a protection agent for hematopoietic stem cells at minus 80DEG C. The protection agent consists of 5 parts by volume of reagent A and 2 parts by volume of reagent B, wherein the reagent A is a dimethyl sulfoxide and hydroxyethyl starch solution, wherein the mass percents of the dimethyl sulfoxide and hydroxyethyl starch are 10 to 20 percent and 5.4 to 6 percent respectively, the reagent B is an albumin solution, and the mass percent of the albumin is 20 to 40 percent. The formula of the protection agent is simple, and raw materials are cheap; by adopting the preservation method, the hematopoietic stem cells can be simply and conveniently stored under the minus 80-DEG C non-programmable-controlled cooling condition, the survival rate of the stored cells is higher than 90 percent, and the method is suitable for being clinically popularized and applied and used for preserving the clinic hematopoietic stem cells at the low temperature of minus 80DEG C for a short time.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
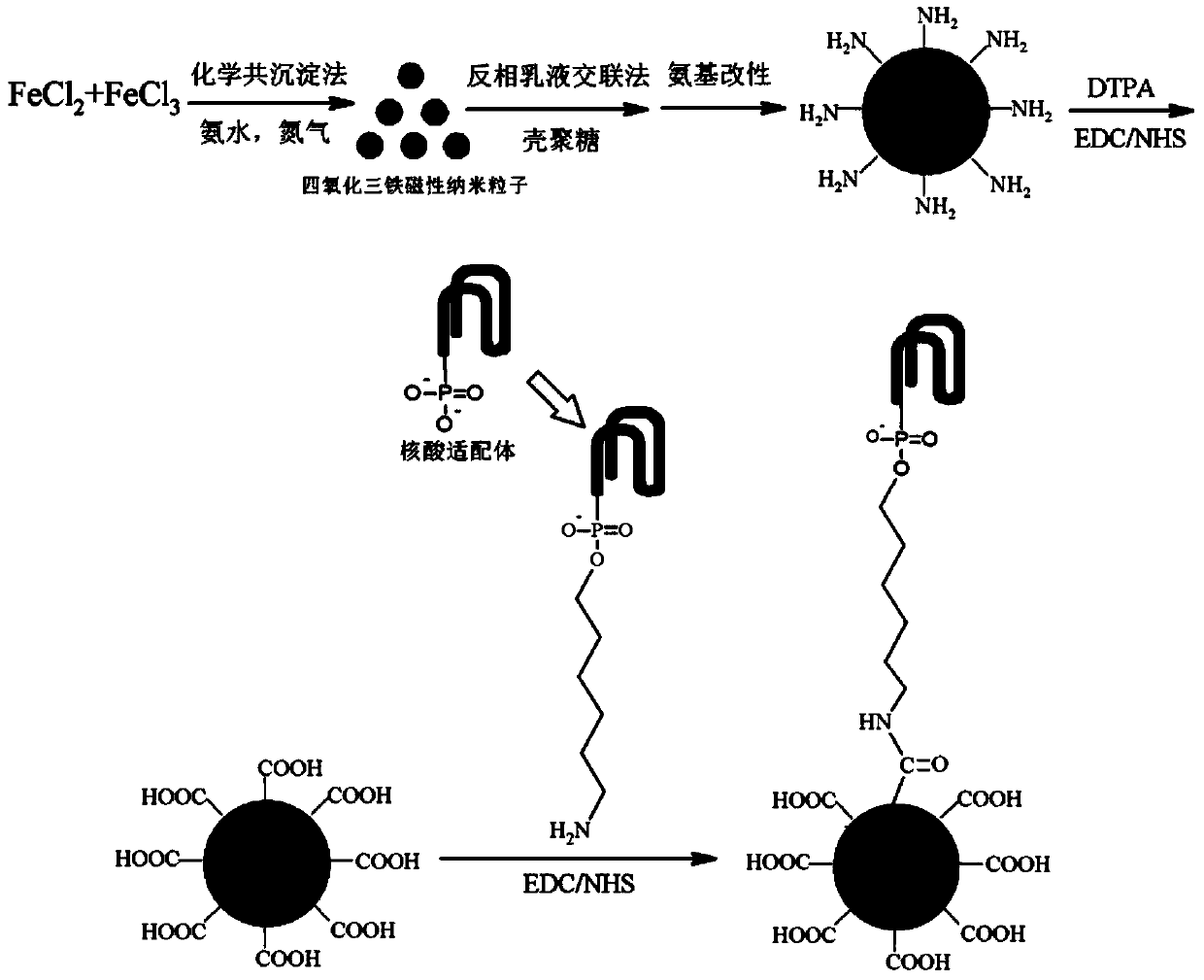
Single-stranded DNA nucleic acid modified chitosan magnetic microsphere preparation method
ActiveCN103990443AGood modification effectGood biocompatibilityOther chemical processesCross-linkMicrosphere
The present invention discloses a single-stranded DNA nucleic acid modified chitosan magnetic microsphere preparation method, which comprises: (1) adopting a chemical co-precipitation method to obtain Fe3O4 magnetic nanoparticles; (2) adopting an inverse emulsion cross-linking method to obtain CS / Fe3O4 magnetic microspheres; (3) adding the CS / Fe3O4 magnetic microspheres to APTES to obtain -NH2 modified CS / Fe3O4 magnetic microspheres; (4) adding DTPA to obtain -COOH modified CS / Fe3O4 magnetic microspheres under effects of EDC and NHS; and (5) adding EDC and other solutions, adding a bovine albumin solution, and shaking to obtain the adenosine aptamer modified CS / Fe3O4 magnetic microspheres. According to the present invention, the disadvantages of easy shedding, instability and the like of the conventional physical adsorption method are overcome, and the immobilization amount of the aptamer is significantly increased.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Preparation method and using method of hydrophilic adhesive glass slide
InactiveCN104502619AGood compatibilityPromotes even spreadingMaterial analysisSilanesAlbumin solution
The invention discloses a preparation method and a using method of a hydrophilic adhesive glass slide. The preparation method comprises the following steps: pre-processing the glass slide by using amino silane solutions with the volume concentration of 0.8-8 percent or polylysine solutions with the volume concentration of 0.1-1 percent; washing; airing; soaking the aired glass slide into albumin solutions with the mass concentration of 0.5-5 percent; taking out; washing; and airing to obtain the hydrophilic adhesive glass slide. The hydrophilic adhesive glass slide has hydrophilic groups on the surface, and is compatible with biological macromolecules and high in adhesion property; waterborne reagents are easily and uniformly spread on the surface of the glass slide, so that the defect that a common adhesive glass slide is always unevenly stained when used on an immunohistochemistry autostainer are overcome.
Owner:沈洪武
Preparation method of novel albumin nano medicament under mild condition of 37 DEG C
InactiveCN109771656AImprove stabilityGood storage stabilityMacromolecular non-active ingredientsHalf-lifeFreeze-drying
The invention belongs to the technical field of biomedical engineering nano medicaments and discloses a preparation method of a novel albumin nano medicament under a mild condition of 37 DEG C. The preparation method comprises the following steps of: 1) preparing an albumin aqueous solution; 2) preparing a reducing agent solution, adding the reducing agent solution into the albumin aqueous solution, adding a protein denaturing agent solution, and reacting for 0.5-6 hours at the temperature of between 30 and 80 DEG C to obtain a reduced albumin solution; 3) dialyzing or ultra-filtering the reduced albumin solution, and removing unreacted reducing agent and protein denaturing agent to obtain a reduced albumin nano solution; 4) mixing the reduced albumin nano solution with an acidic buffer solution to enable the pH value to be between 2.8 and 5.0, placing the mixture in an environment of 37 DEG C, stirring to obtain albumin nano particles, dialyzing, and freeze-drying to obtain the finalalbumin nano medicament. The albumin nanoparticles have very good serum stability and storage stability, stably exists in a body circulation system, increases the half-life of the medicament, and havereduction sensitivity characteristics.
Owner:四川载荧生物科技有限公司
Folic acid quantitative detection kit
InactiveCN108169498AReduce manufacturing costStrong specificityColor/spectral properties measurementsBiological testingFolic acid bindingLatex particle
The invention provides a folic acid quantitative detection kit suitable for all kinds of fully automatic biochemical analyzer, wherein the kit includes a reagent R1, a reagent R2, a calibration product and a quality control product. The reagent R1 includes a folic acid binding albumin solution, and the reagent R2 includes a folic acid antibody latex particle suspension. The folic acid quantitativedetection kit can quickly quantitatively detect the content of folic acid in whole blood, erythrocytes and serum in ten minutes, has the advantages of high specificity, high sensitivity and simple operation. In addition, the kit has reasonable preparation cost, and can be used in batches in clinic.
Owner:XINCHANG COUNTY MEIDI BIOLOGICAL TECH
Stabilized albumin preparaions
InactiveCN1638788ALittle change in appearanceImprove stabilityPeptide/protein ingredientsDigestive systemSide effectMedium chain fatty acid
This invention provides albumin preparations with safety and without any risk of side effects, which are free from viruses or contaminating proteins and can be stably stored over a long time while showing neither changes in appearance nor decrease in content. <??>There are provided a stabilized albumin preparation produced by uniformly mixing a medium-chain fatty acid or a salt thereof and a sulfur-containing amino acid or a derivative thereof with an aqueous albumin solution (e.g., a buffer such as phosphate buffer which can be administered as pharmaceutical preparations, injection water, or a physiological saline) and dissolving them therein, and then processing the mixture solution into a formulation suitable for parenteral administration such as an intravenous fluid preparation or an injectable solution, and a stabilization method for an albumin preparation.
Owner:NIPRO CORP
Albumin-purification method comprising a nanofiltration step, solution, and composition for therapeutic use containing the same
InactiveUS20070161122A1Efficiently optimisedOptimising durationFactor VIIPeptide/protein ingredientsPurification methodsAdditive ingredient
The invention relates to an albumin-purification method comprising a step consisting in subjecting an aqueous albumin solution, with a concentration of between 15 g / l and 80 g / l and a pH of not less than 7, to nanofiltration in a temperature range of between 15 DEG C. and 55 DEG C. The invention also relates to: a virally-safe aqueous albumin solution which can be obtained using the invention method and in which the sites for the transport and binding of the active therapeutic ingredients of the albumin are available; and an albumin composition for therapeutic use, which is obtained by adapting the albumin solution that is intended for clinical use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Method for polishing albumin
The present invention provides a method of polishing albumin to remove contaminants comprising passing an albumin enriched solution through a hydrophobic charge-induction chromatographic resin and recovering the albumin solution which passes through the resin. Also provided is a polished albumin solution prepared by said method.
Owner:CSL LTD
Stable cabazitaxel and albumin composition, and preparation method thereof
ActiveCN106852911AEasy to solveOvercome severe allergic deficienciesOrganic active ingredientsPowder deliveryOrganic solventWater insoluble
The invention provides a stable aseptic lyophilized cabazitaxel composition, and a preparation method thereof. The prescription and the preparation technology of the above preparation are screened, so the cabazitaxel and albumin composition prepared from a low concentration of an albumin solution has the characteristics of good stability and good safety, and also has the advantages of no use of water-insoluble chloroform / dichloromethane or other highly toxic organic solvents in the preparation process, no addition of a lyophilization protection agent or a protein stabilizer in a lyophilizing step, reduction of the medicine production cost, and guaranteeing of the safety of clinic medication.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
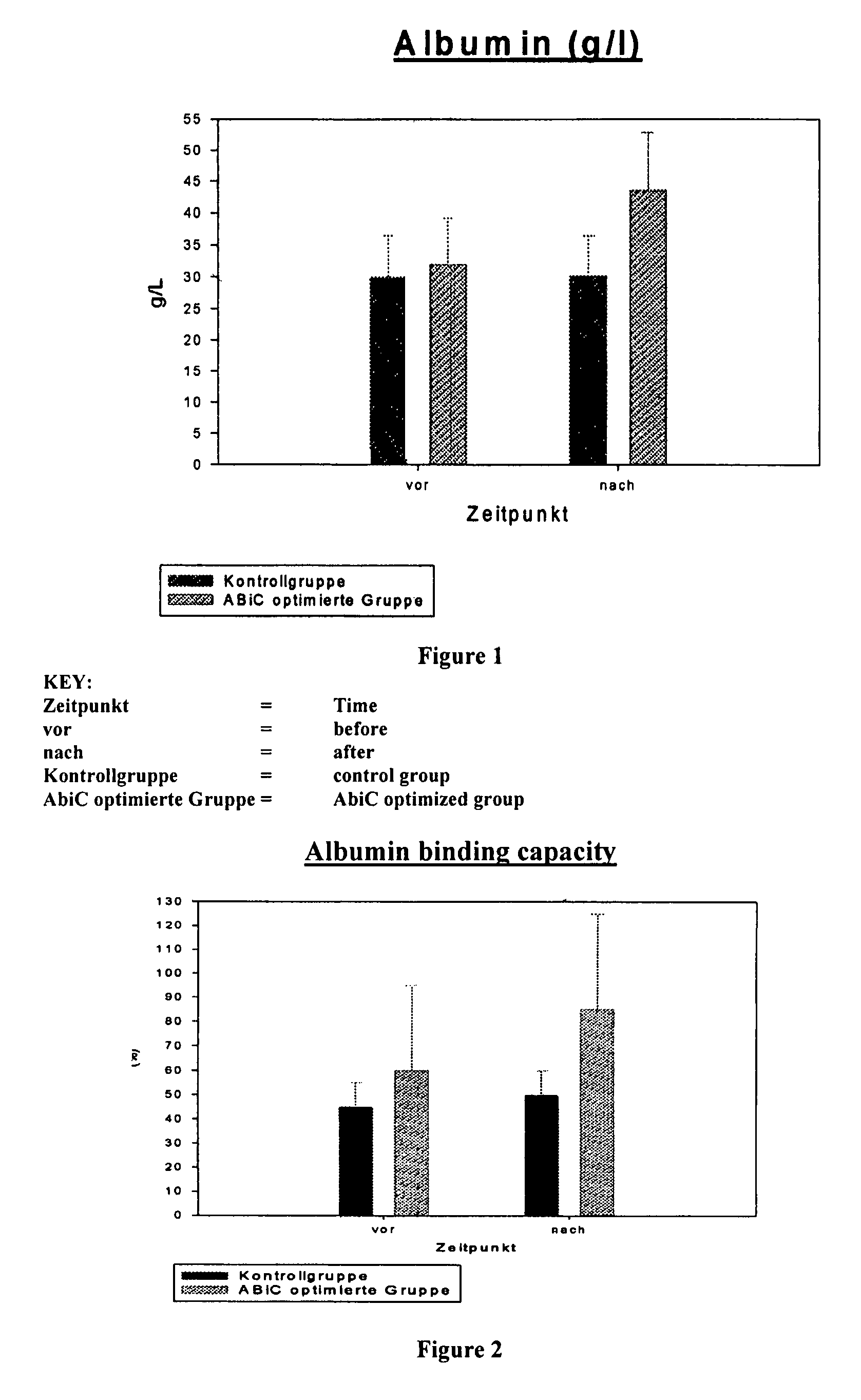
Stabilizer molecule-depleted albumin solution
ActiveUS8236927B2Without damaging structureSimple and fast and inexpensivePeptide/protein ingredientsPharmaceutical delivery mechanismBinding siteAlbumin solution
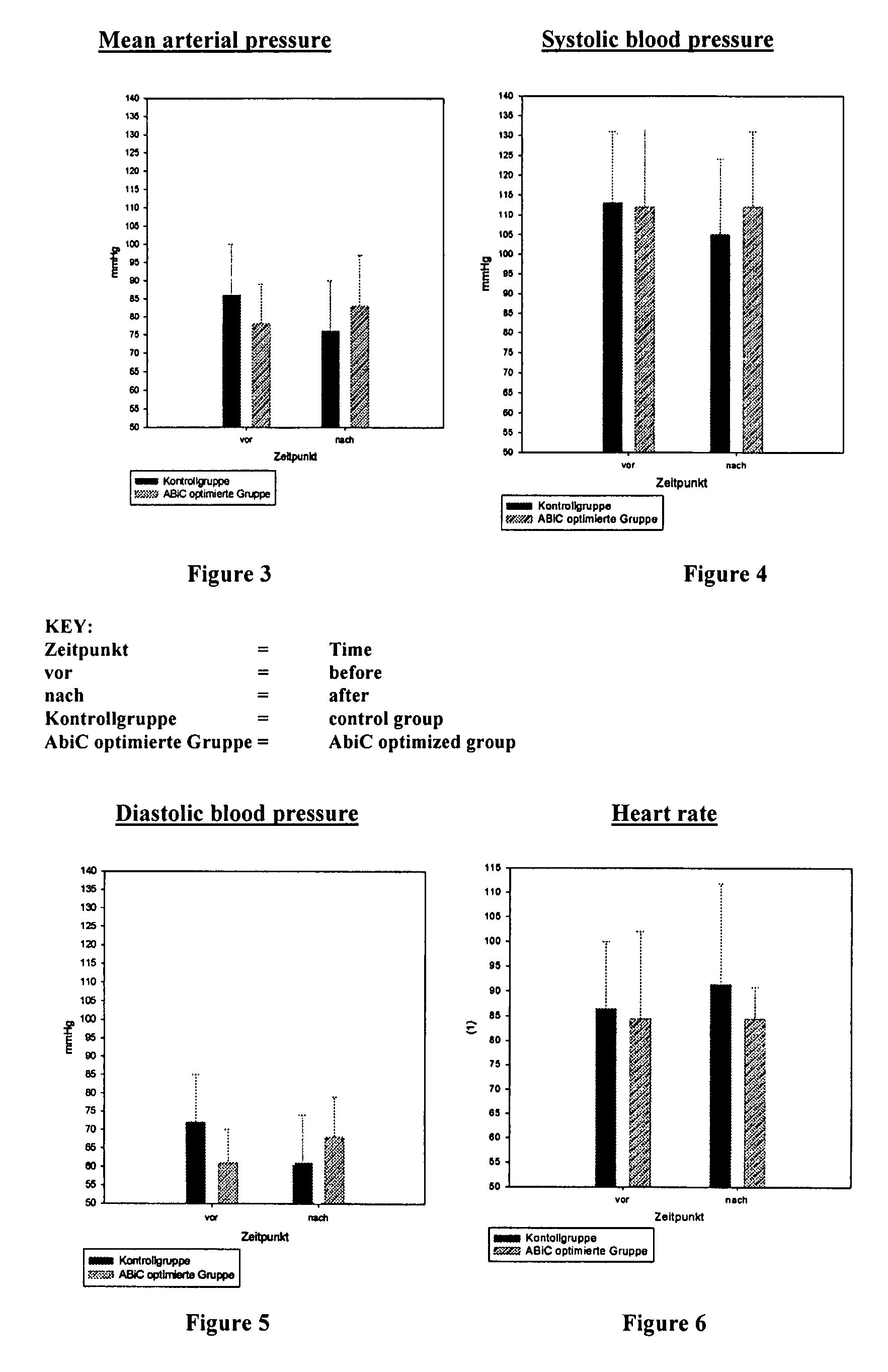
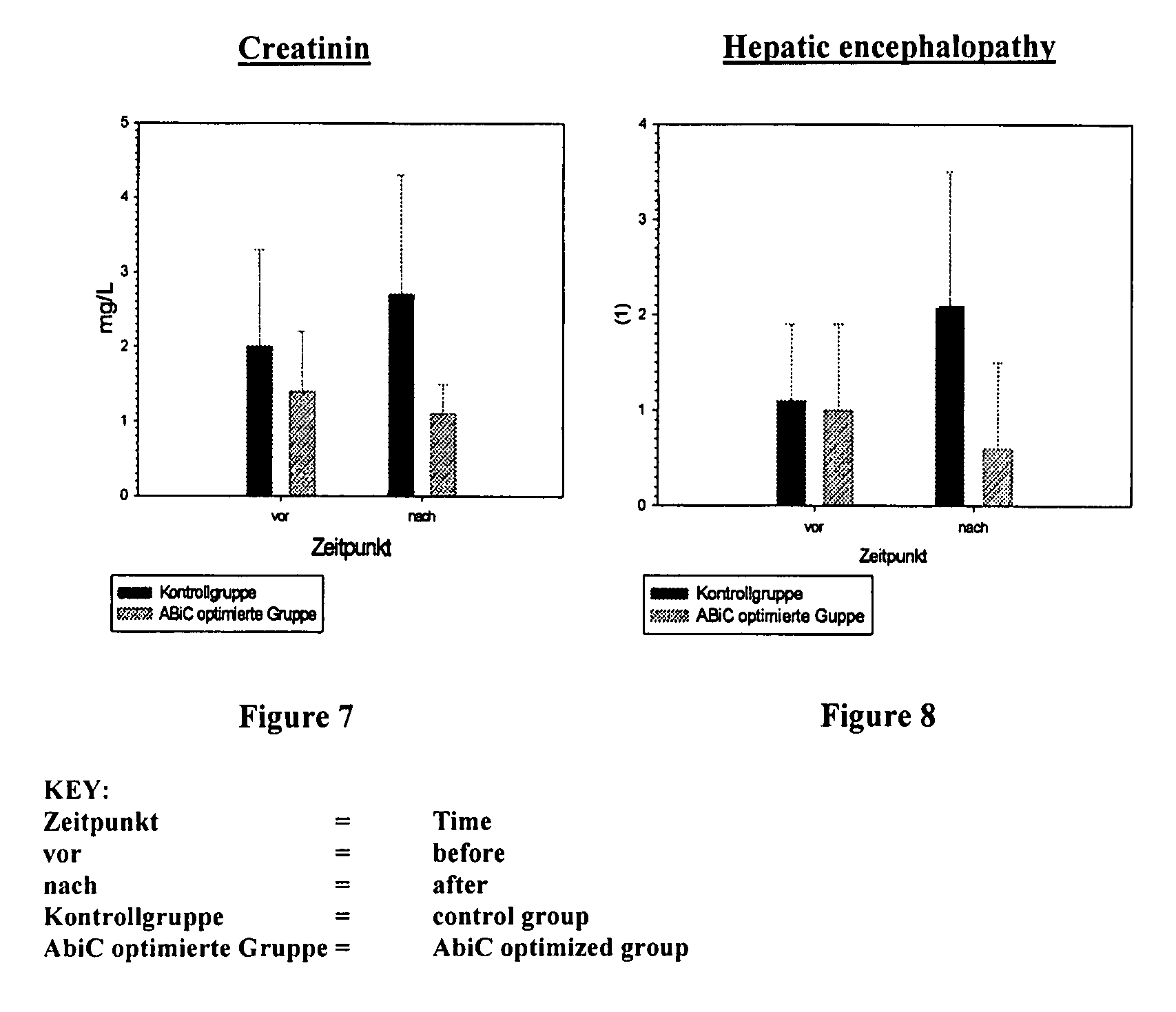
The invention concerns a method for producing an aqueous albumin solution from a starting albumin solution which contains stabilizer molecules which are capable of occupying binding sites of the albumin, wherein in a method for increasing the albumin binding capacity (ABiC) for other molecules, at least a portion of the stabilizer molecules is removed from the albumin of the starting albumin solution and separated from the starting albumin solution. To carry out such a method, by means of which a stabilized commercial starting albumin solution can be prepared in a manner which is simpler, faster, cheaper and in a manner which is gently on the albumin by removing the majority of the stabilizers and increasing the albumin binding capacity, the method comprises steps in which the starting albumin solution is brought into contact with a solid adsorption material the affinity of which for at least a portion, preferably all of the stabilizer molecules is higher than the affinity of the albumin for the corresponding stabilizer molecules, and the albumin is separated from the adsorption material; wherein the method is carried out at a pH of >3.
Owner:ALBUTEK GMBKH
Portable multiple organ support integration machine
ActiveCN103251996AReduce in quantitySmall device sizeDialysis systemsVenous accessVolume replacement
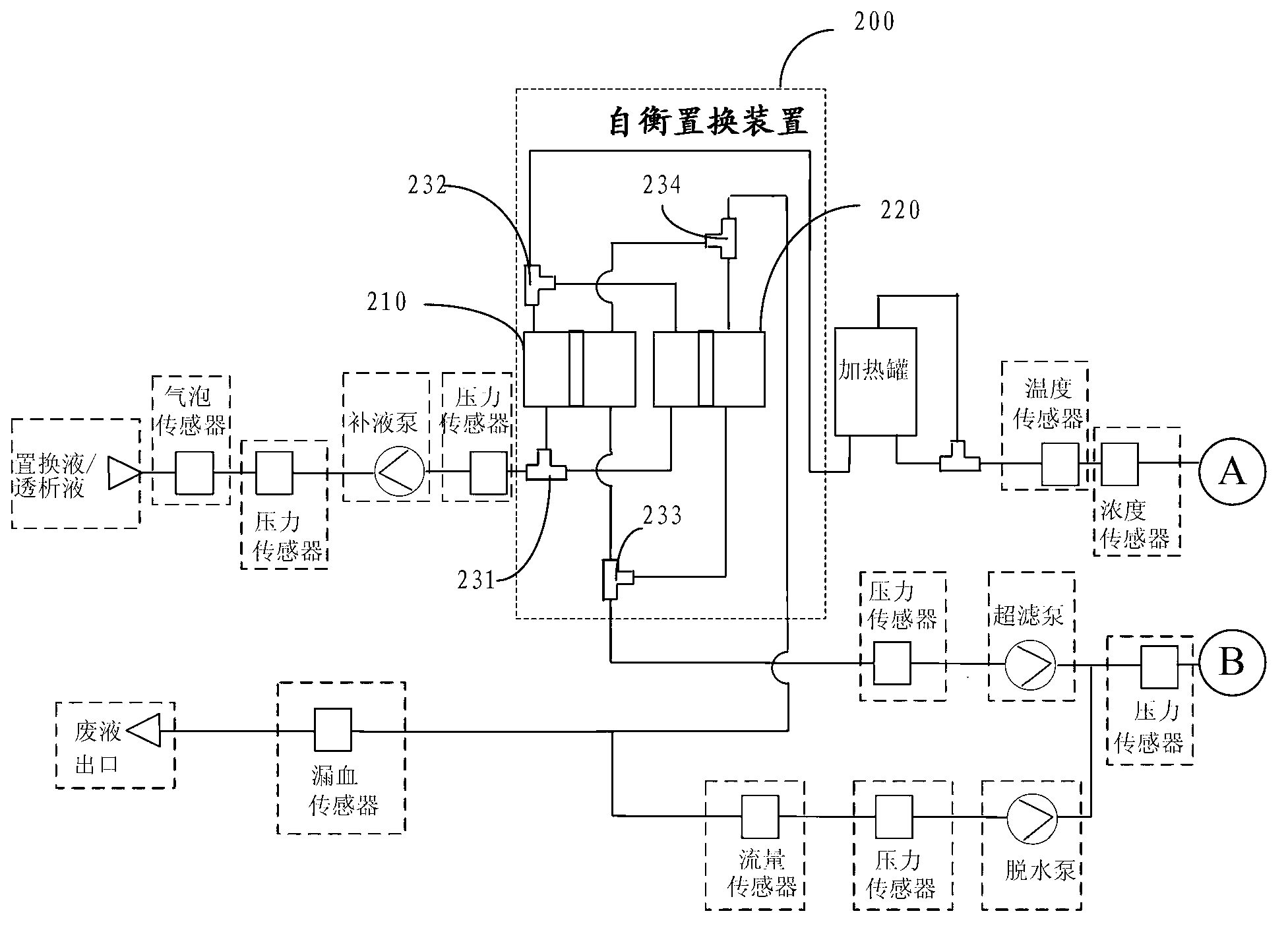
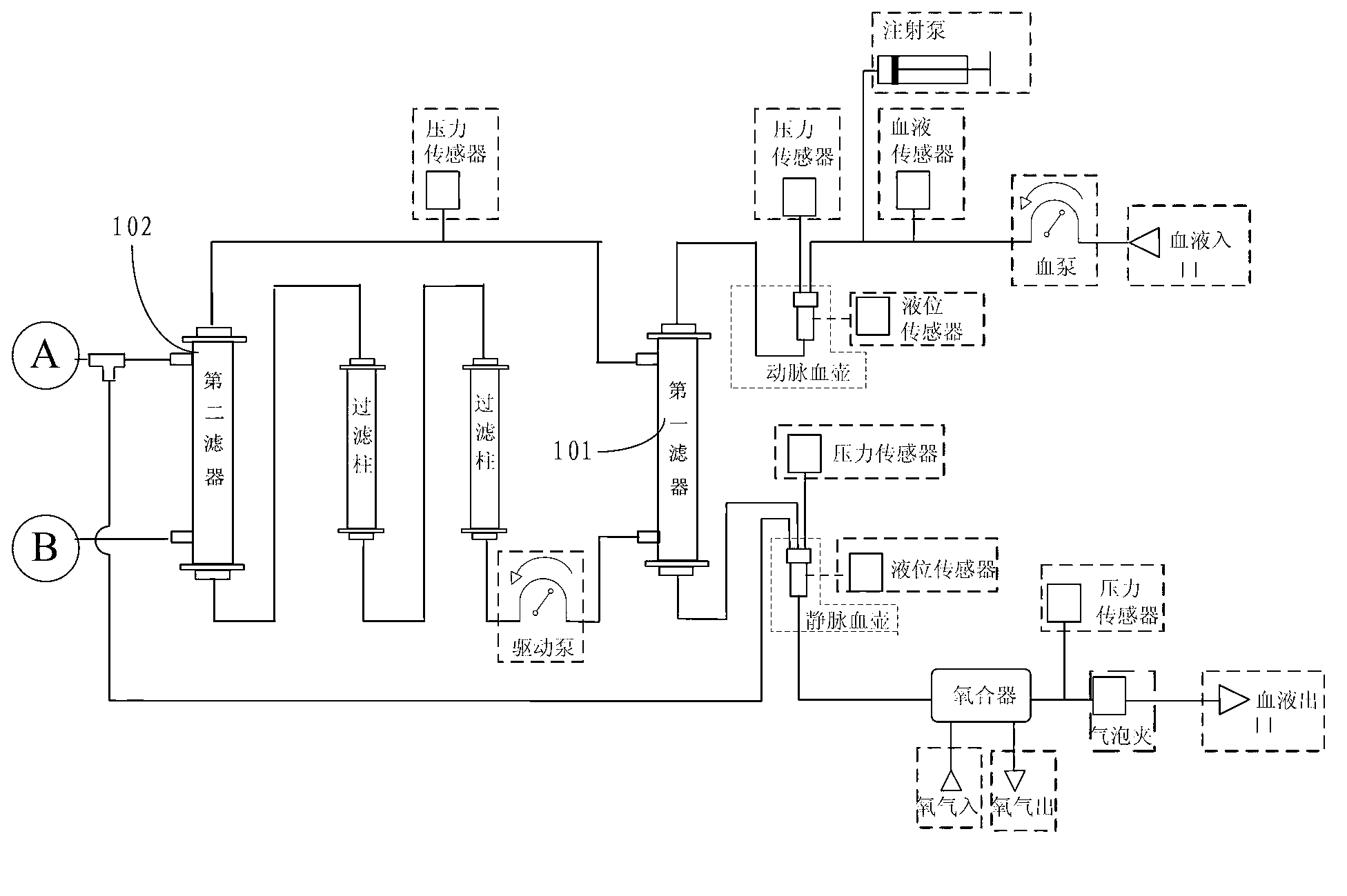
The invention discloses a portable multiple organ support integration machine which comprises an arterial closed circuit, a venous closed circuit, an albumin solution return circuit, a fluid infusion closed circuit and an ultrafiltrate closed circuit. A self-balance replacement device adopting a hard-type cavity and a hard-type piston is arranged on the fluid infusion closed circuit and the ultrafiltrate closed circuit, and flow balance of the fluid infusion closed circuit and the ultrafiltrate closed circuit is maintained by a method of volume replacement. The problems that existing multiple organ support integration machines are large in size, difficult to carry, poor in portability and hard for staff not in the profession to use, and cannot be used for treating and curing in special environments like in sports.
Owner:周春华 +1
Preparation method of As2O3 albumin nano medicine
ActiveCN105832676AGood dispersionUniform sizeInorganic active ingredientsGranular deliveryWater bathsPhosphate
The invention discloses a preparation method of an As2O3 albumin nano medicine. The preparation method includes the following steps: 1) dissolving As2O3 powder in a NaOH solution and regulating the pH with diluted hydrochloric acid to prepare an As2O3 solution for drug supporting of albumin in later use, dissolving human serum albumin marked by folic acid molecules in a Dulbecco's phosphate buffer liquid without calcium and magnesium ions to obtain a human serum albumin solution, and adding a disulfide bond reducing agent to the human serum albumin solution and performing a reaction in a water bath pot with gentle stirring to obtain the albumin solution treated by the disulfide bond reducing agent; 2) adding the As2O3 solution to the albumin solution treated by the disulfide bond reducing agent, and stirring the mixture at room temperature to obtain an As2O3 albumin mixed solution; and 3) dialyzing the As2O3 albumin mixed solution in a dialysis bag at low temperature with a D-PBS solution to remove residual As2O3, disulfide bond reducing agent and side products thereof to obtain the As2O3 albumin nano medicine.
Owner:HUNAN UNIV
Preparation process for glycyrrhizic acid-mediated hydroxycamptothecine albumin liver cancer targeting nanoparticle lyophilized powder
InactiveCN103432083AActive liver cancer targetingHigh drug loadingOrganic active ingredientsPowder deliveryEvaporationGlycyrrhizin
The invention relates to a preparation process for glycyrrhizic acid-mediated hydroxycamptothecine albumin liver cancer targeting nanoparticle lyophilized powder. The glycyrrhizic acid-mediated hydroxycamptothecine albumin liver cancer targeting nanoparticle lyophilized powder is obtained by the steps of reacting glycyrrhizic acid and albumin under a certain conditions to successfully couple glycyrrhizic acid and albumin; removing glycyrrhizic acid uncoupled with albumin by a dialysis method; then lyophilizing albumin coupled with glycyrrhizic acid to obtain lyophilized powder of albumin coupled with glycyrrhizic acid; dissolving albumin coupled with glycyrrhizic acid in water at a certain concentration and hydroxycamptothecine in a mixed solution of chloroform and ethanol; dropwise adding the hydroxycamptothecine solution to the albumin solution slowly; homogenizing at a certain rotation speed and time; homogenizing the homogenized mixed suspension for a certain times under a certain pressure to obtain an oil-in-water emulsion; removing organic solvents-ethanol and chloroform from the mixed suspension by a rotary evaporation method; and then removing water from the mixed suspension in a lyophilization manner.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
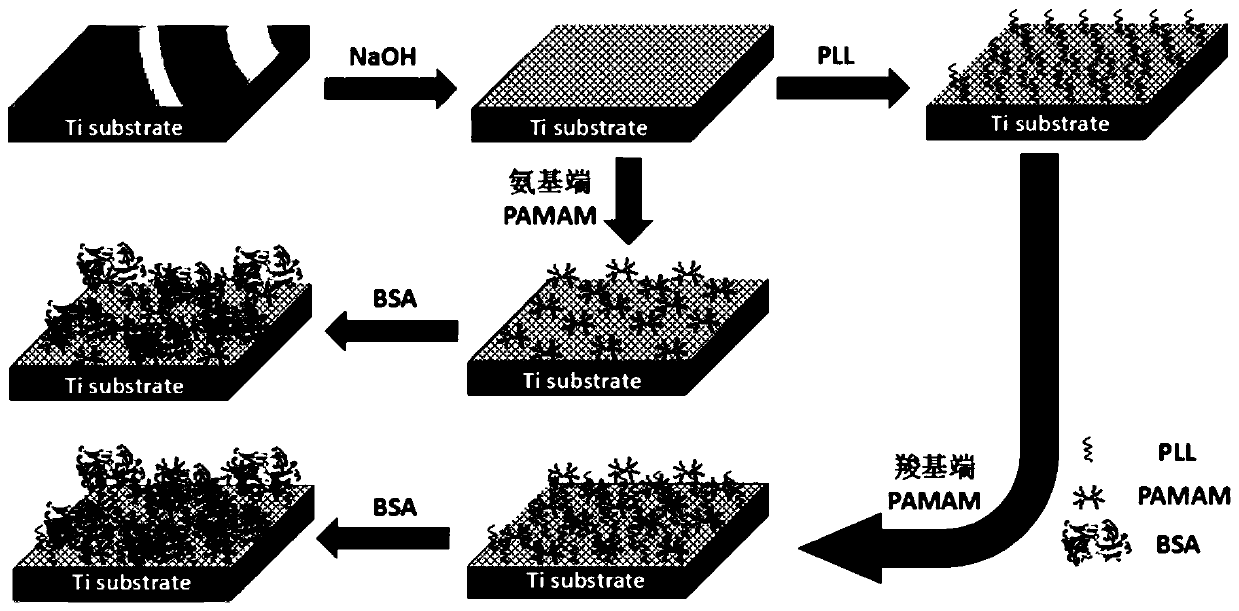
Method for forming albumen layer by mediating albumin on surface of material, biological material and application thereof
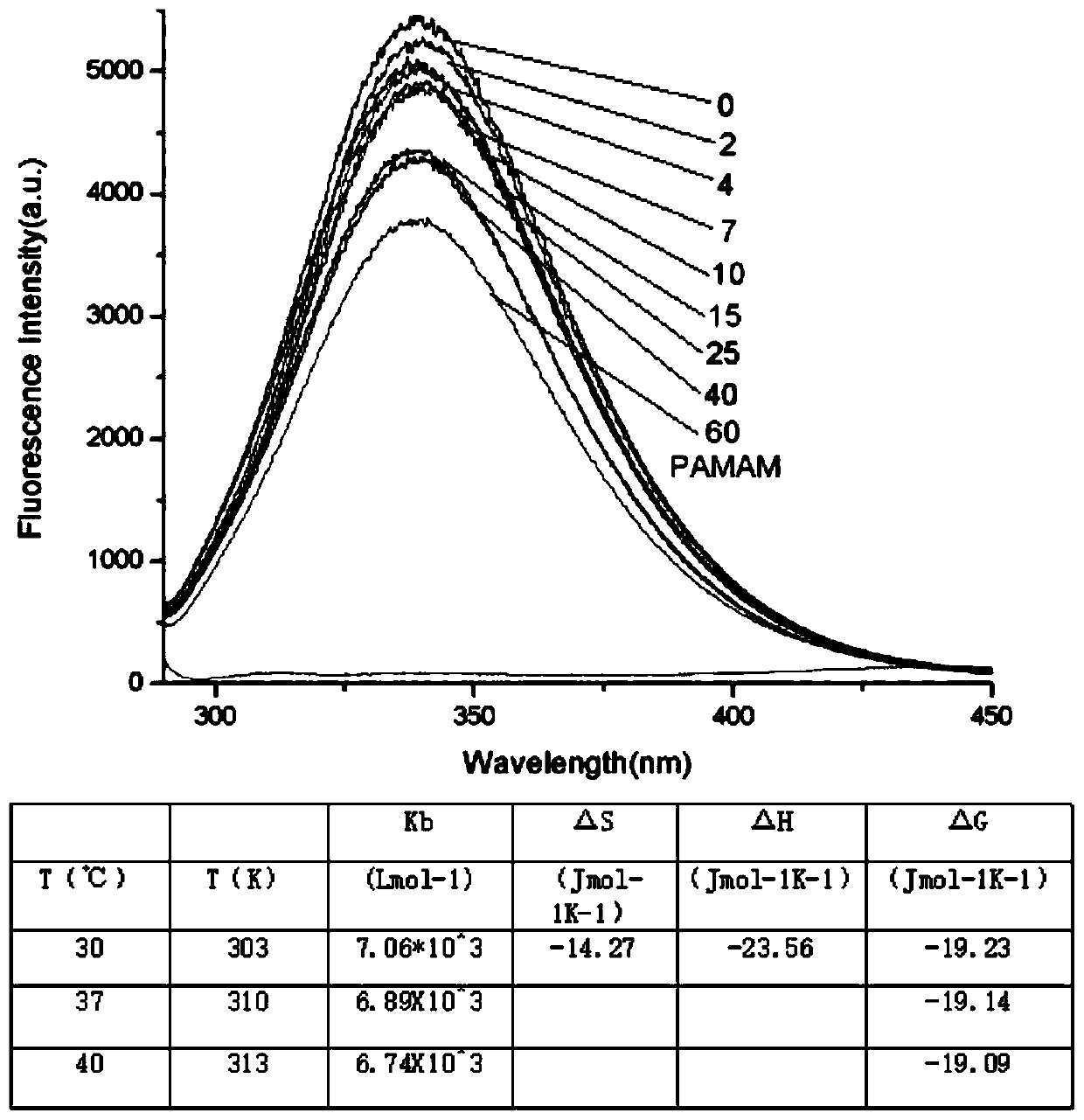
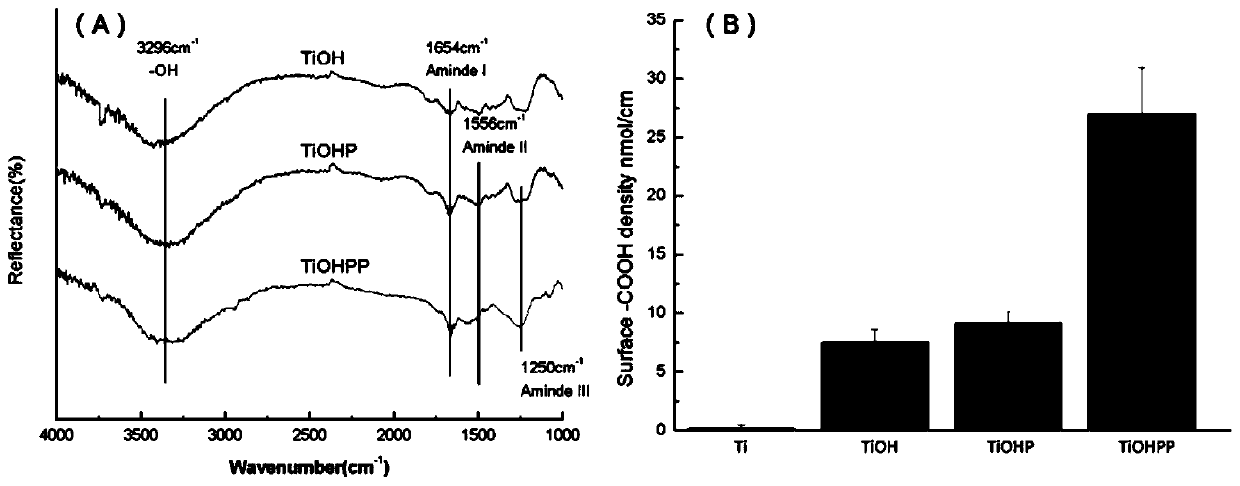
The invention discloses a method for forming an albumen layer by mediating albumin on the surface of a material, a biological material and application thereof and relates to the technical field of biomaterials. The method for forming an albumen layer by mediating albumin on the surface of a material includes standing a to-be-supported material in an alkaline solution and then obtaining an alkali-activated material; fixing dendrimers on the surface of the alkali-activated material, and then immersing the modified material in an albumin solution for standing reaction. Preferably, the dendrimer is a polyamide-amine dendrimer PAMAM; and more preferably, the molecular weight of PAMAM is 6000 to 10000. The biological material forms a PAMAM layer and the albumin layer on the surface of the to-be-supported material, and the albumin is mediated to form the albumen layer. The biological material is used for preparing a cardiovascular implant material and can reduce the adhesion of platelets to achieve anticoagulation, and the biocompatibility of the material is improved.
Owner:SOUTHWEST JIAOTONG UNIV
Stabilized albumin preparations
InactiveUS7351800B2Improve stabilityGood depressing effectPeptide/protein ingredientsDigestive systemSide effectInjectable Solution
This invention provides albumin preparations with safety and without any risk of side effects, which are free from viruses or contaminating proteins and can be stably stored over a long time while showing neither changes in appearance nor decrease in content.There are provided a stabilized albumin preparation produced by uniformly mixing a medium-chain fatty acid or a salt thereof and a sulfur-containing amino acid or a derivative thereof with an aqueous albumin solution (e.g., a buffer such as phosphate buffer which can be administered as pharmaceutical preparations, injection water, or a physiological saline) and dissolving them therein, and then processing the mixture solution into a formulation suitable for parenteral administration such as an intravenous fluid preparation or an injectable solution, and a stabilization method for an albumin preparation.
Owner:NIPRO CORP
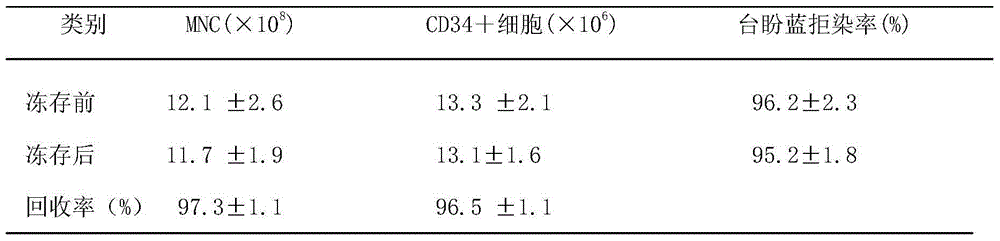
Kit and method for separating mononuclear cells from umbilical cord blood
InactiveCN105754938AImprove efficiencyPreservation solution has an excellent effect on preserving mononuclear cellsBlood/immune system cellsHydroxyethyl starchVolumetric Mass Density
The present invention discloses a kit and a method for separating mononuclear cells from umbilical cord blood. The kit for separating the mononuclear cells from the umbilical cord blood comprises the following components: a dilution solution which is a sodium chloride solution with the mass percentage of 0.1% to 5% or a phosphate buffer with pH of 7 to 7.5; a precipitating agent which is a hydroxyethyl starch solution with the mass percentage of 2.4%-12%, a hydroxymethyl starch solution with the mass percentage of 2.4%-12% or a methyl cellulose solution with the mass percentage of 2.4%-12%; a resuspension solution which is an albumin solution with the concentration of 5 to 10% or human AB blood plasma with the concentration of 10-30%; and a separation liquid which is a silica gel particle suspension treated by polyvinylpyrrolidone, and the separation liquid has a density of 1.072-1.086g / ml. The kit and the method can effectively separate and save the mononuclear cells, and have good application prospect. The effect is better than that of commercially available kits.
Owner:SICHUAN NEO LIFE STEM CELL BIOTECH
Plastic container containing albumin solution
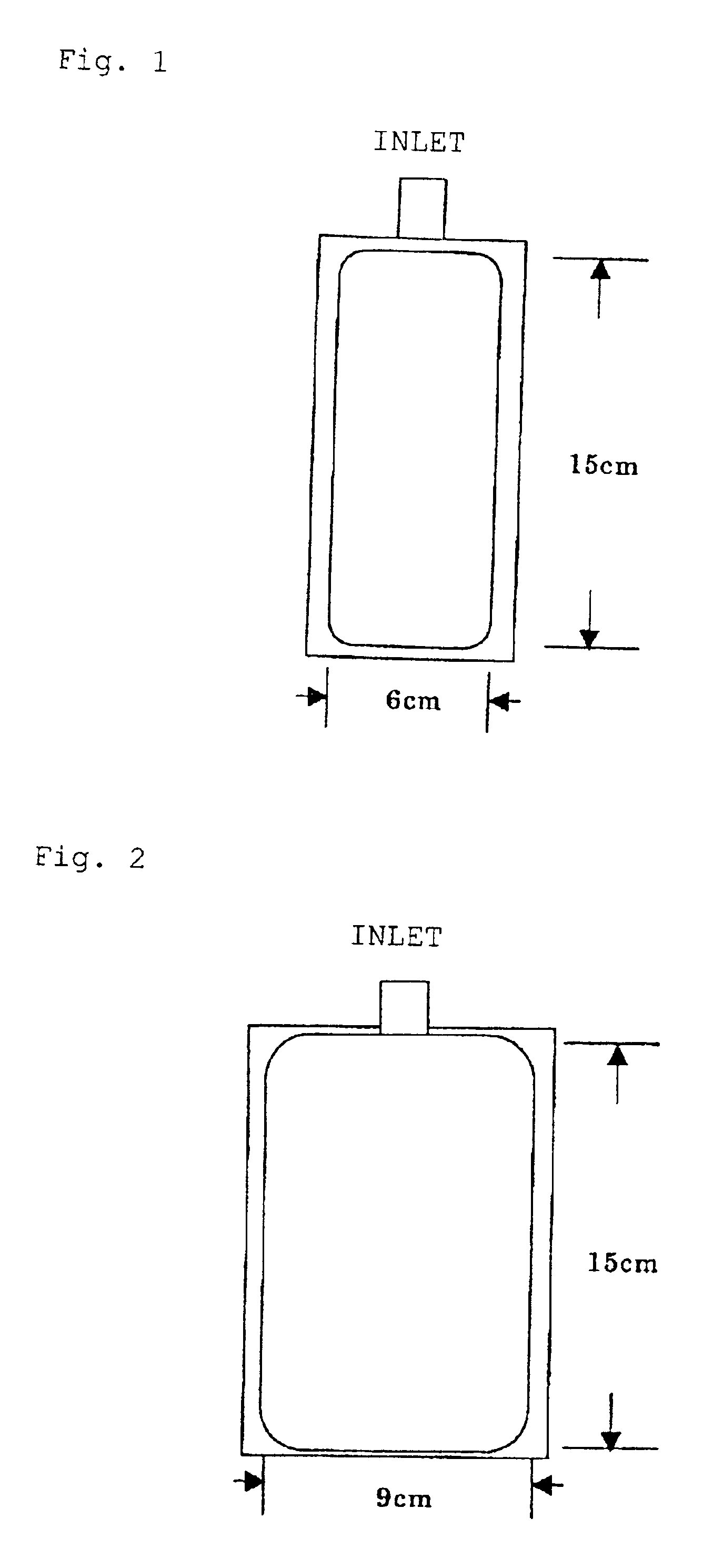
InactiveUS6777052B2Maintain performanceImprove discharge performancePeptide/protein ingredientsSurgical needlesWater vapor permeabilityAlbumin solution
The present invention provides a plastic container containing albumin solution and a packaged plastic container containing an albumin solution. The plastic container containing an albumin solution having an albumin concentration of 1 to 500 mg / ml, has at least one inlet / outlet for a liquid, and has a water vapor permeability of 1.5 g / m<2> / day.1013.25 hPa or less when the vapor permeability is measured at a pressure of 1013.25 hPa per surface area of 1 m<2 >for 24 hours at a temperature of 25° C. and at a relative humidity of 60%. The plastic container containing an albumin solution can be packaged with an outer packaging material to provide the packaged plastic container.
Owner:NIPRO CORP
Composition for regulating enteric microorganisms and preparation method thereof
InactiveCN107029214AIncrease lethalitySpeed up the removal processAntibacterial agentsPeptide/protein ingredientsIntestinal microorganismsAlbumin solution
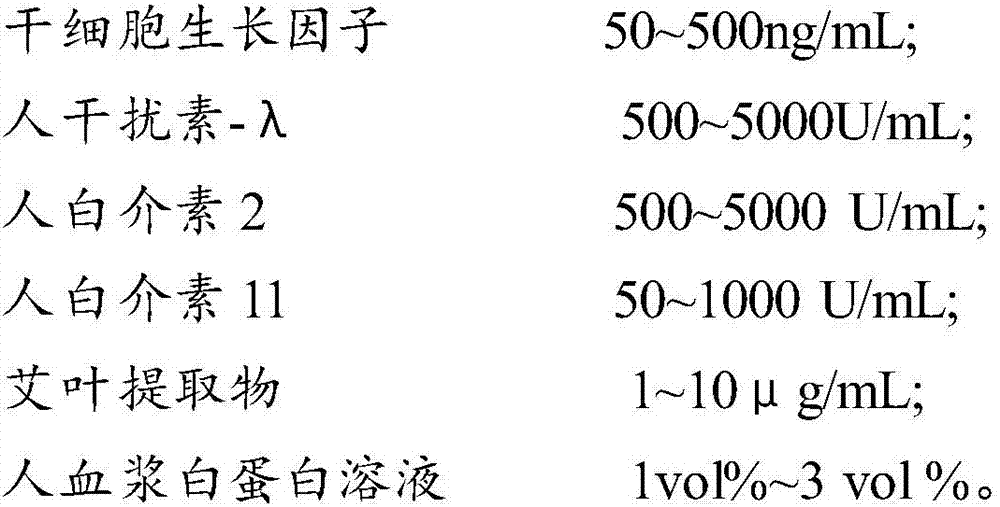
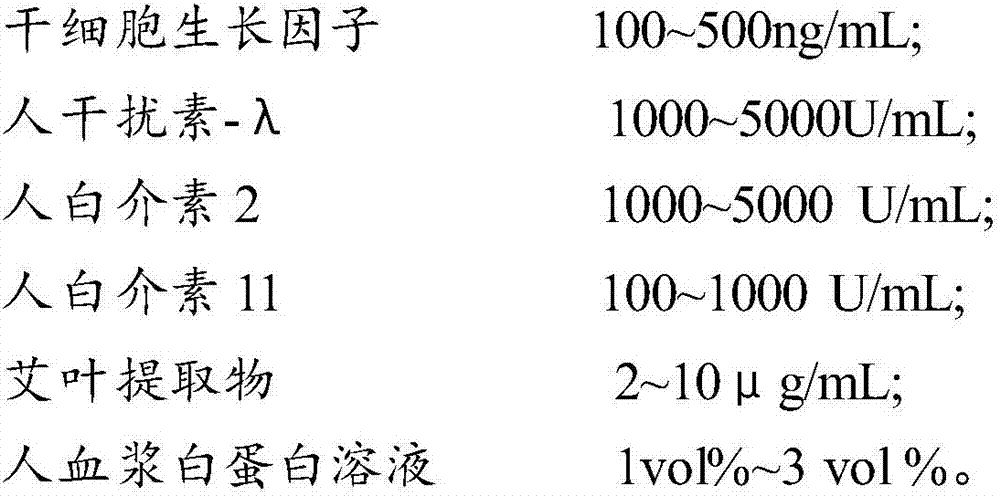
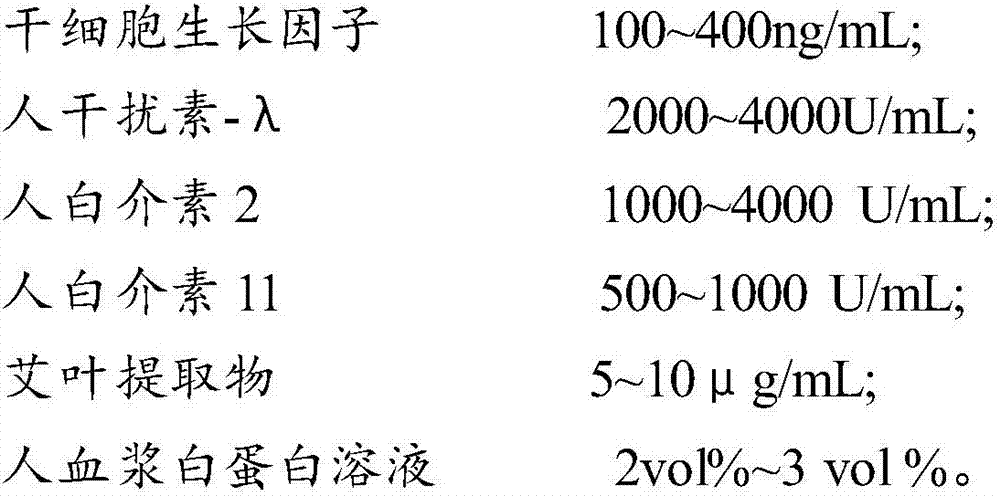
The invention provides a composition for regulating enteric microorganisms. The composition comprises solvent and components, such as, by weight, 50-500ng / mL of stem cell growth factor, 500-5000 U / mL of human interferon-Lambda, 500-5000 U / mL of human interleukin 2, 50-1000 U / mL of human interleukin 11, 1-10 mu g / mL of folium artemisiae argyi extractive, and 1vol%-3vol% of human plasma albumin solution. Through synergistic effect of the stem cell growth factor, the human interferon-Lambda, the human interleukin 2, the human interleukin 11, the human plasma albumin solution and the folium artemisiae argyi extractive, the composition can improve the whole immune system of a human body and strengthen the killing and removing ability of own immune cell to harmful flora; meanwhile, the composition can effectively regulate the intestinal microflora, increase probiotics, reduce harmful bacteria, and maintain a healthy environment of the whole intestinal canal.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Separation technology for human blood albumin through low-temperature ethanol two-step method
PendingCN110317262AReduce usageReduce processing timeSerum albuminPeptide preparation methodsWhole blood productSeparation technology
The invention relates to a separation technology for plasma protein through a low-temperature ethanol method. The two-step low-temperature ethanol separation method is adopted for separating a human blood albumin product. The technology is characterized in that the step of separating a component IV precipitate does not exist in the low-temperature ethanol separation technology for the human bloodalbumin, after components I, II and III are precipitated, the component V precipitate is directly separated, the ultrafiltration process for a human blood albumin solution is a two-step ultrafiltration technology, firstly, an ultrafiltration filtrate is reserved through ultrafiltration, macromolecule impure protein is removed through ultrafiltration, and then the human blood albumin is subjected to ultrafiltration to remove micromolecule protein, residual ethyl alcohol and possible aluminium ions. The technology has the advantages that the high yield of the human blood albumin product is fundamentally guaranteed, the possibility of repeated production or the reproduction process in the production technology of the human blood albumin is completely eliminated, and it is guaranteed that production of a blood product more effectively meets the demand of GMP for the medicine.
Owner:发贵科技(贵州)有限公司
Thymidylate synthase preparation loaded albumin nano-microsphere and preparation method thereof
InactiveCN104306339AUniform particle sizeGood dispersionOrganic active ingredientsGranular deliveryOrganic solventMicrosphere
The invention provides a thymidylate synthase preparation loaded albumin nano-microsphere. The nano-microsphere is prepared by the following steps: adding a solution of a thymidylate synthase preparation and an organic solvent into an albumin solution to develop and self-assemble the albumin to form a nano-microsphere; coating the thymidylate synthase preparation in the albumin nano-microsphere to form the thymidylate synthase preparation loaded albumin nano-microsphere, wherein the diameter of the albumin nano-microsphere is 40-500nm, and the thymidylate synthase preparation is 7-20 percent by mass of the albumin nano-microsphere. The nano-microsphere has the advantages of excellent biocompatibility, simple preparation process and stable physiochemical property, is capable of improving the drug stability, has a certain sustained release effect, and has potential application of clinical cancer treatment. The invention further discloses a preparation method of the thymidylate synthase preparation loaded albumin nano-microsphere.
Owner:NANJING UNIV +1
Preparation and redissolution method of composite curcumin-myofibrillar protein solid beverage
ActiveCN108771076AImprove solubilityRich in amino acidsFood ingredient functionsProtein composition from meatSolubilityUltra high pressure
The invention belongs to the field of solid beverages in a dry composition. The invention provides a preparation method of a composite curcumin-myofibrillar protein solid beverage. High pressure microfluidization treatment is adopted in the extraction of myofibrillar protein in chicken; during the reaction between myofibrillar protein and curcumin, pH value is firstly raised and then lowered to promote the combination of curcumin and myofibrillar protein; and finally through ultra-high pressure cold sterilization and freeze-drying, the composite curcumin-myofibrillar protein solid beverage isprepared. The invention also provides a redissolution method for redissolving the solid beverage by the use of water or an ovalbumin solution at the concentration of 4% according to a certain ratio. The solid beverage myofibrillar protein-curcumin compound prepared by the invention has high utilization rate and high nutritional value. In the solid beverage solution after redissolution, the myofibrillar protein-curcumin compound has good solubility, curcumin is greatly dispersed in the solution with no layering, and absorptivity is higher. The solid beverage is especially suitable for middle-aged and elderly people as well as those who have limited chewing function or poor digestive function.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com