Stable cabazitaxel and albumin composition, and preparation method thereof
A technology of cabazitaxel and albumin, which is applied in the field of stable cabazitaxel albumin aseptic freeze-dried composition and its preparation, can solve problems such as safety, and achieve the goal of reducing production cost, lowering the concentration of use, and excellent stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
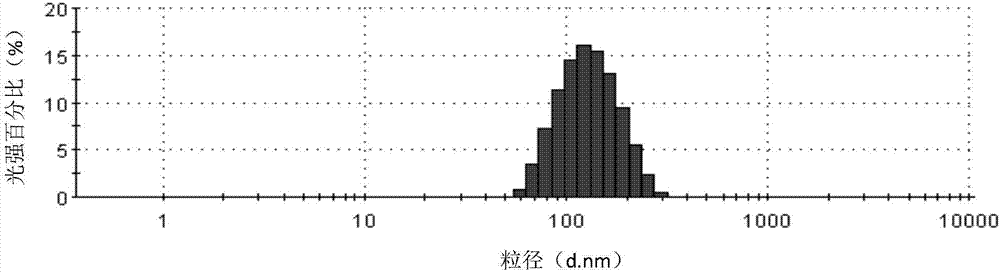
[0070] Dissolve 300mg of Cabazitaxel in 30ml of ethanol to form an organic phase, use 400ml of human serum albumin aqueous solution (15mg / ml) as the water phase, mix the water phase with the organic phase, place in a high-speed shearing machine at 10000rpm After shearing at a certain speed, transfer it to a high-pressure homogenizer, homogenize under a pressure of 35,000 psi, and circulate for 5 times to prepare a suspension with an average particle size of 119.8nm and a pH of 6.7. Pass the suspension through Filter and sterilize with a 0.22 μm sterile filter head, and directly freeze-dry for 60 hours to obtain a freeze-dried composition. After the freeze-drying is completed, the composition is reconstituted with physiological saline, and the measured zeta potential is -5.7mv. Particle size analysis was carried out using a Malvern particle size analyzer, and the measured average particle size was 120.3nm. After standing at room temperature, it was detected by high-performance l...
Embodiment 2
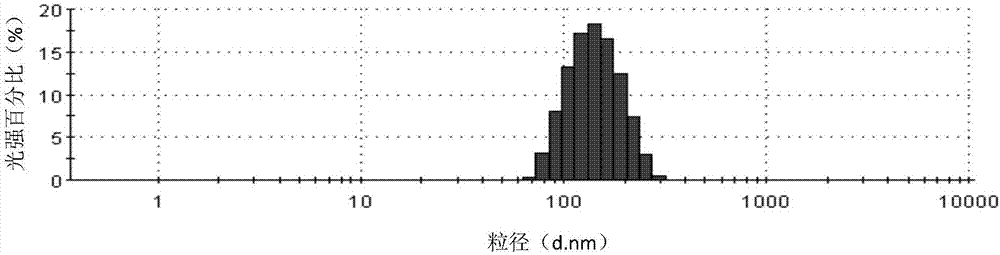
[0072] Dissolve 300mg of Cabazitaxel in 15ml of ethanol to form an organic phase, use 500ml of human serum albumin aqueous solution (6mg / ml) as the water phase, mix the water phase with the organic phase, place in a high-speed shearing machine at 7000rpm After shearing at a certain speed, transfer it to a high-pressure homogenizer, perform homogenization under a pressure of 30,000 psi, and circulate for 5 times to prepare a suspension with an average particle size of 134.4nm and a pH of 6.5. Pass the suspension through Filter and sterilize with a 0.22 μm sterile filter head, and directly freeze-dry for 60 hours to obtain a freeze-dried composition. After the freeze-drying is completed, the composition is reconstituted with physiological saline, and the measured zeta potential is -3.4mv. Particle size analysis was carried out using a Malvern particle size analyzer, and the measured average particle size was 135.4nm. After being placed at room temperature, it was detected by HPLC...
Embodiment 3
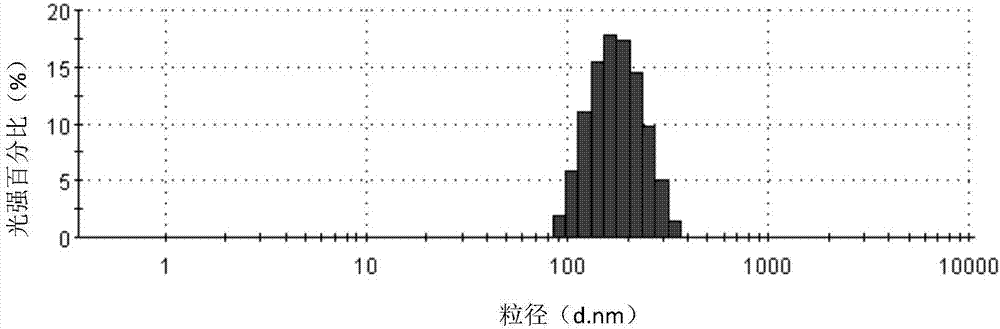
[0074] Dissolve 300 mg of Cabazitaxel in 10 ml of ethanol to form an organic phase, use 180 ml of human serum albumin aqueous solution (20 mg / ml) as the water phase, mix the water phase with the organic phase, and place in a high-speed shearing machine at 15000 rpm After shearing at a certain speed, transfer it to a high-pressure homogenizer, homogenize under a pressure of 40,000 psi, and circulate 6 times to prepare a suspension with an average particle size of 164.4nm and a pH of 7.0. Pass the suspension through Filter and sterilize with a 0.22 μm sterile filter head, and directly freeze-dry for 60 hours to obtain a freeze-dried composition. After the freeze-drying is completed, the composition is reconstituted with physiological saline, and the measured zeta potential is -7.5mv. Particle size analysis was carried out using a Malvern particle size analyzer, and the measured average particle size was 165.7nm. After being placed at room temperature, it was detected by HPLC that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com