Albumin-purification method comprising a nanofiltration step, solution, and composition for therapeutic use containing the same
a technology of nanofiltration and purification method, which is applied in the direction of instruments, extracellular fluid disorder, peptide/protein ingredients, etc., can solve the problems the production cost of recombinant albumin may be too high in comparison to that of plasma albumin, and the chance of virus passing through, etc., to achieve efficient optimisation of albumin recovery yield, high degree of safety, and optimise the effect of operation and filtering duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
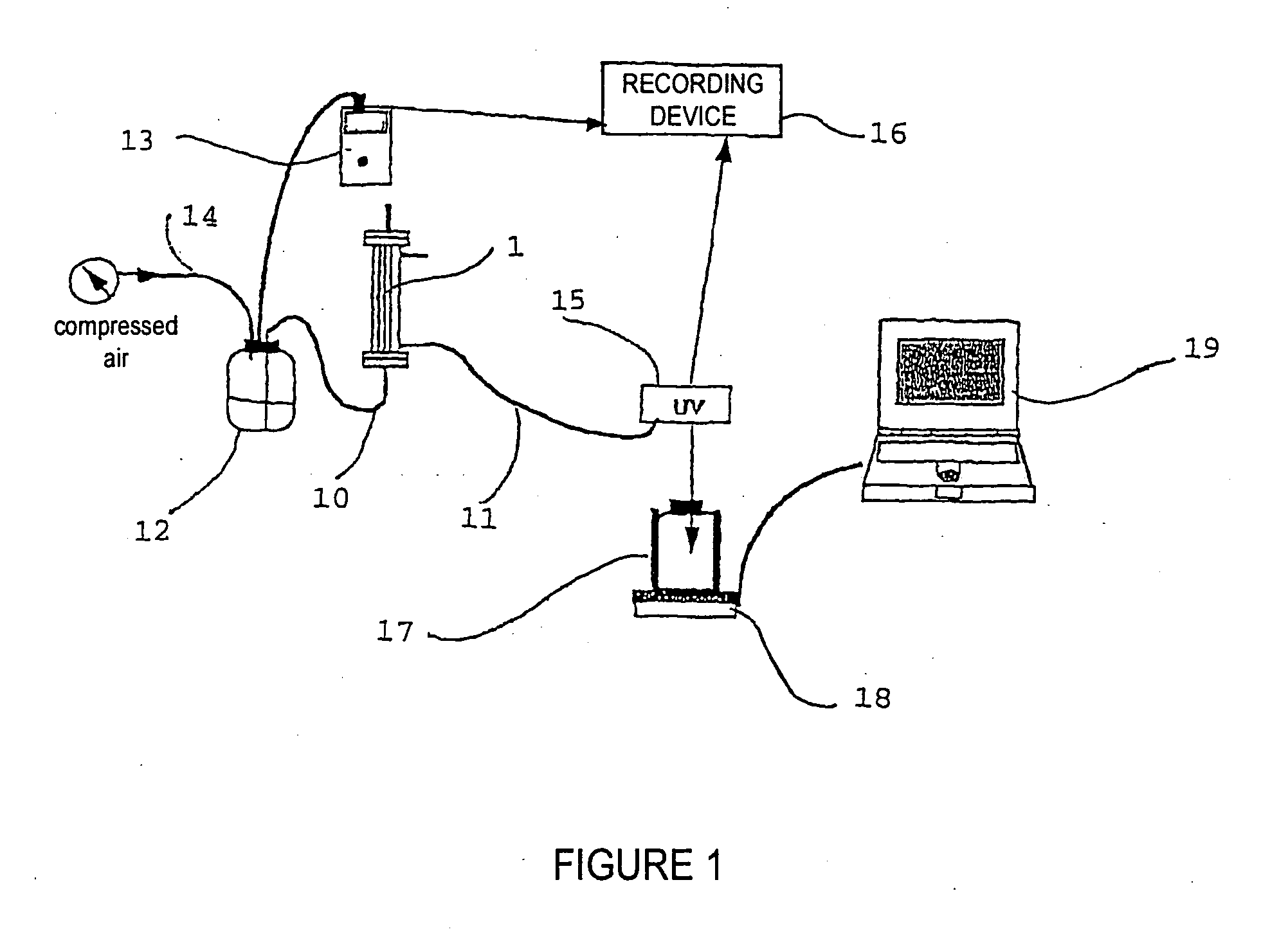
1.1 Methodology for Implementing the Nanofiltration Method (FIG. 1)
[0040] A filtration device 1 containing a PLANOVA® 15-nm filter with a surface area of 0.01 m2 (from Asahi, Japan) is equipped with tubes 10, 11 at the retentate (pre-filtration solution) outlet and at the filtrate (post-filtration solution) inlet and outlet, made of pharmaceutically compatible materials, with diameters of about 5 mm, closed by clamps. The device is arranged on a stand (not shown) in vertical position with the help of graspers.
[0041] The inlet of the 15-nm filter is connected, through the tube 10, to a pressure vessel 12 the pressure of which is measured with a digital pressure gauge 13 connected to the upstream filter circuit.
[0042] Before use, an integrity test is carried out on the 15-nm filter in accordance with the manufacturer's procedure: “Air leakage test during the integrity pre-and post-filtration test of PLANOVA® 15, 35, 75-nm filters”.
[0043] Then, the system is rinsed. For that purpos...
example 2
[0048] Two preliminary tests are carried out to assess the feasibility of the nanofiltration in terms of. protein load, duration and yield, when the nanofiltration is implemented with albumin solutions A (Example 1) filtered at 20° C. and under 0.5-bar pressure. Volumes of solutions A are prepared with protein loads of 0.5 kg / m2 and 1 kg / m2. The results are shown in Table 1.
TABLE 1FiltrationProteinProtein loaddurationrecoverySolutions(kg / m2)(min.)(%)A0.519098A128299
[0049] The above results show that, depending on the selected conditions, the nanofiltration of albumin solutions A provides a protein load of 1 kg / m2 without filter clogging and with an excellent yield. It also appears that doubling the protein load does not result in a nanofiltration duration twice as long.
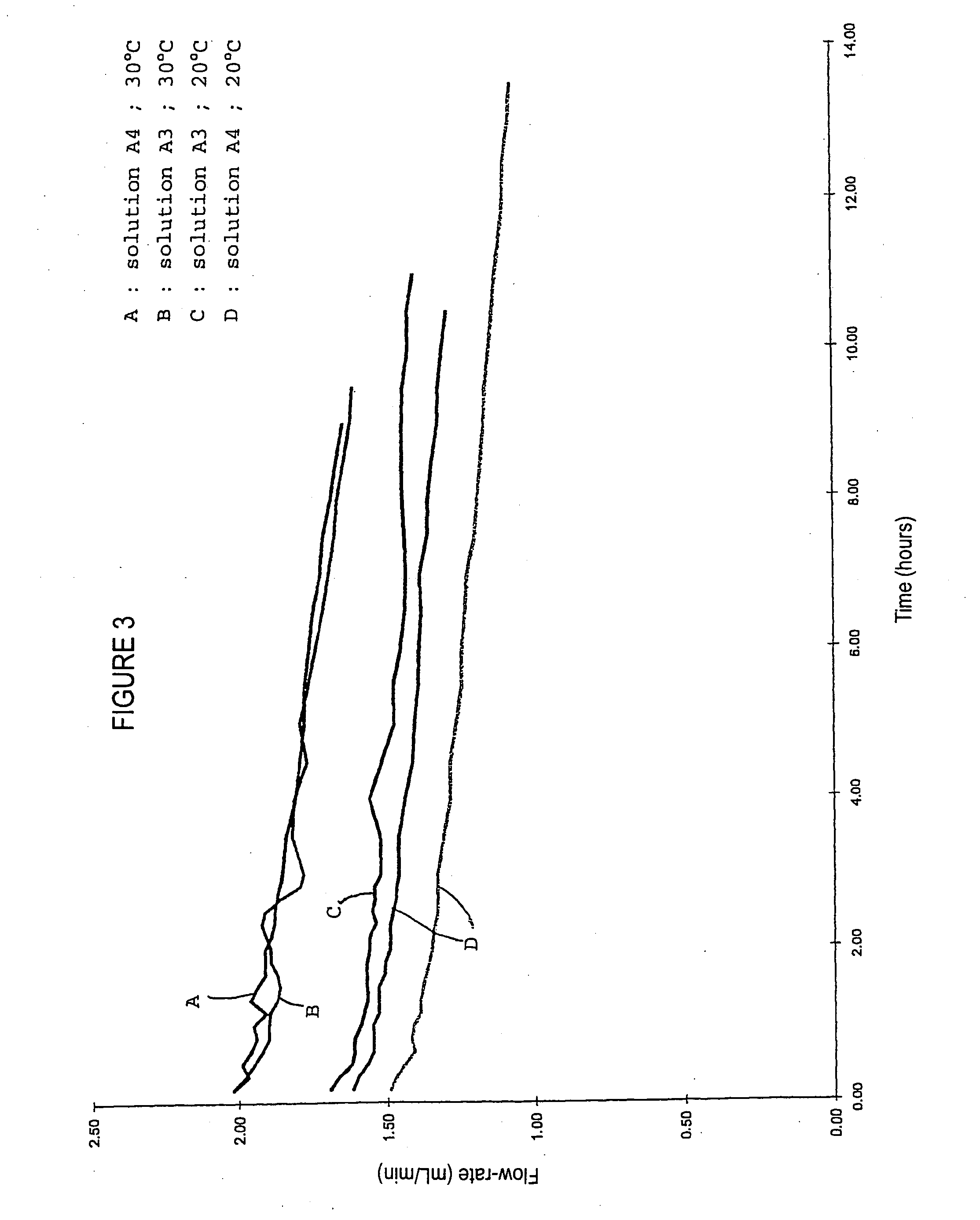
[0050] The results of the tests have therefore prompted the Applicant to consider the nanofiltration of albumin solutions with a view to provide higher protein loads, while optimising some influencing parameters if...
example 3
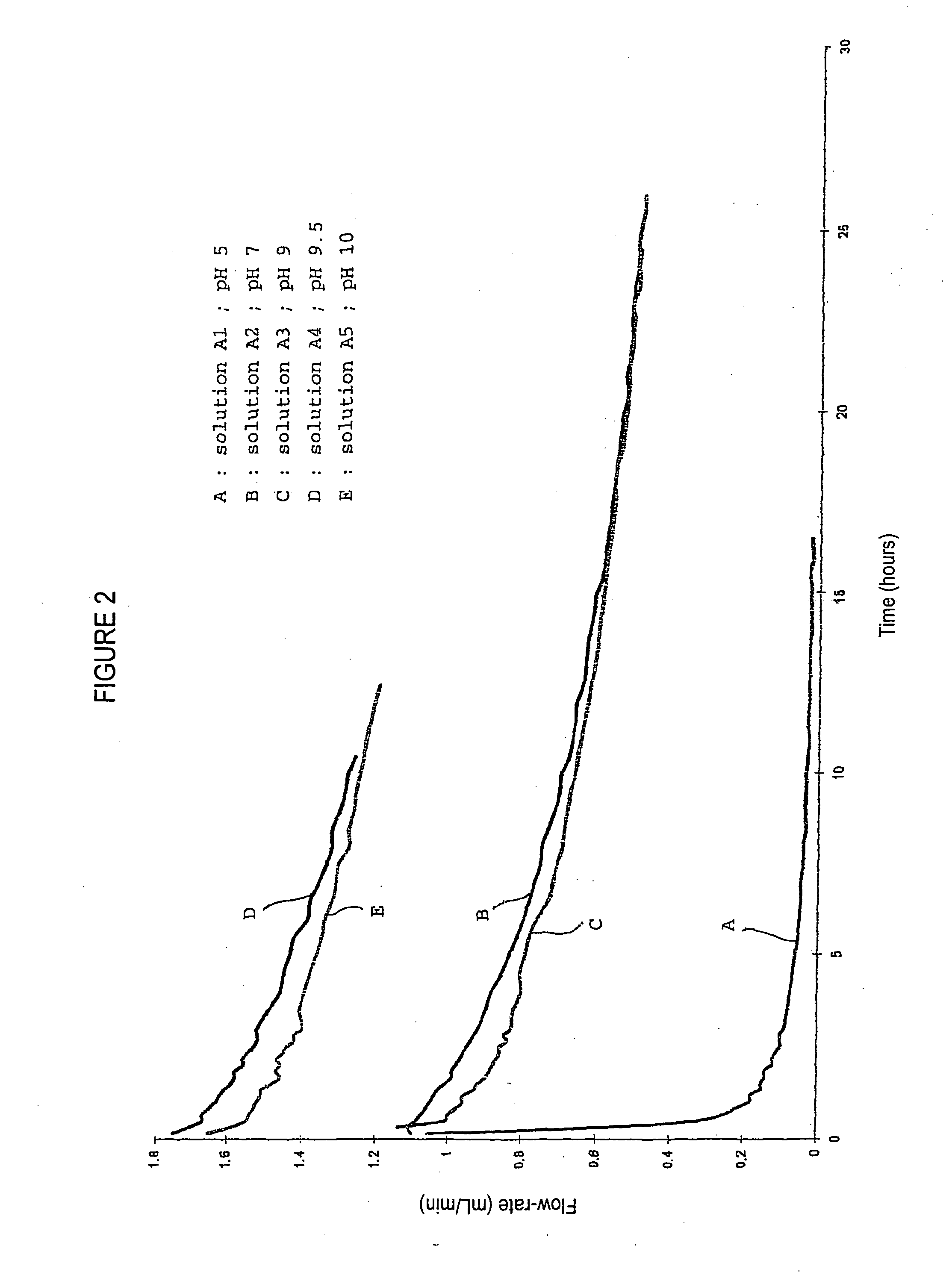
[0051] The dependence of the filtration durations and yields to the pH of the albumin solutions in a such a way as a protein load of 4 kg / m2 is provided is determined using a batch of albumin A as starting material. Five solutions of albumin at 40 g / L, A1 to A5, are prepared in a solution of NaCl at 9 g / L, and adjusted to pH 5, 7, 9, 9.5 and 10 respectively, with 0.5 M solutions of HCl or NaOH, and submitted to a nanofiltration. The nanofiltration tests are carried out at 20° C. at a pressure of 0.5 bar. FIG. 2 shows the plots of filtration flow rate (mL / min) versus nanofiltration duration for each of the solutions considered.
[0052] An analysis of these plots shows, with solution A1 (pH 5), a rapid reduction in flow rate and, as a consequence, filter clogging. With solutions A2 and A3, there is a reduction in the initial flow rate which is faster with solution A3, but with quite acceptable variations, no filter clogging being observed after more than 25 hours of filtration. The bes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com