Kit and method for separating mononuclear cells from umbilical cord blood
A kit and the technology of umbilical cord blood, which are applied in the field of separation of mononuclear cells, can solve the problems of unsatisfactory separation effect and achieve good effect, high efficiency and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
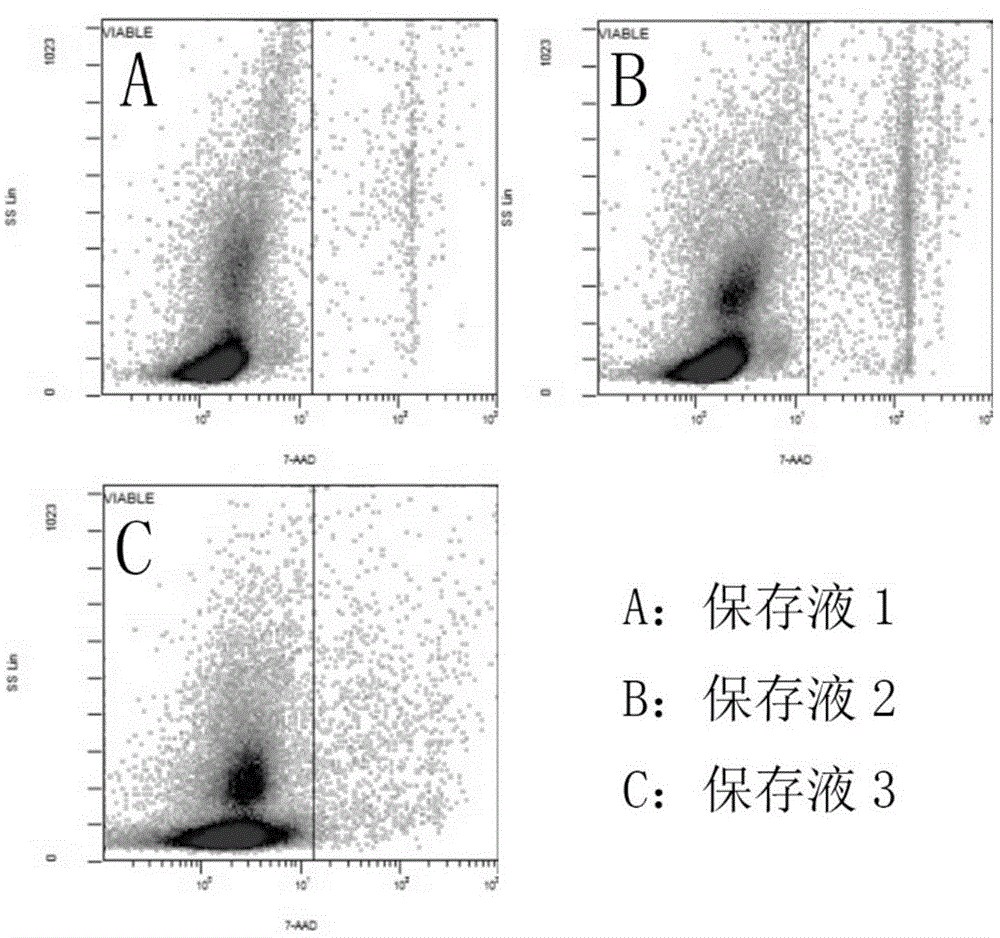
[0044] Embodiment 1 Kit of the present invention and separation method
[0045] 1. Kit composition
[0046] Diluent: 0.9% sodium chloride solution by mass percentage.
[0047] Precipitating agent: 6% hydroxyethyl starch solution by mass percentage.
[0048] Separation liquid: a suspension of silica gel particles treated with polyvinylpyrrolidone with a density of 1.078 g / ml.
[0049] Resuspension: 20% human AB plasma.
[0050] 2. Separation method
[0051] 1. Collect human cord blood in a sterile environment and store it in anticoagulant blood bags. In the ultra-clean bench, divide the collected human cord blood into 50ml centrifuge tubes, and put 20ml cord blood into each tube;
[0052] 2. Pour an equal volume of diluent into the centrifuge tube containing 20ml of umbilical cord blood for dilution, then add 10ml of precipitating agent, gently invert and mix, and let stand for 30-50 minutes;
[0053] 3. Place the supernatant obtained from the above sedimentation into a ne...
Embodiment 2
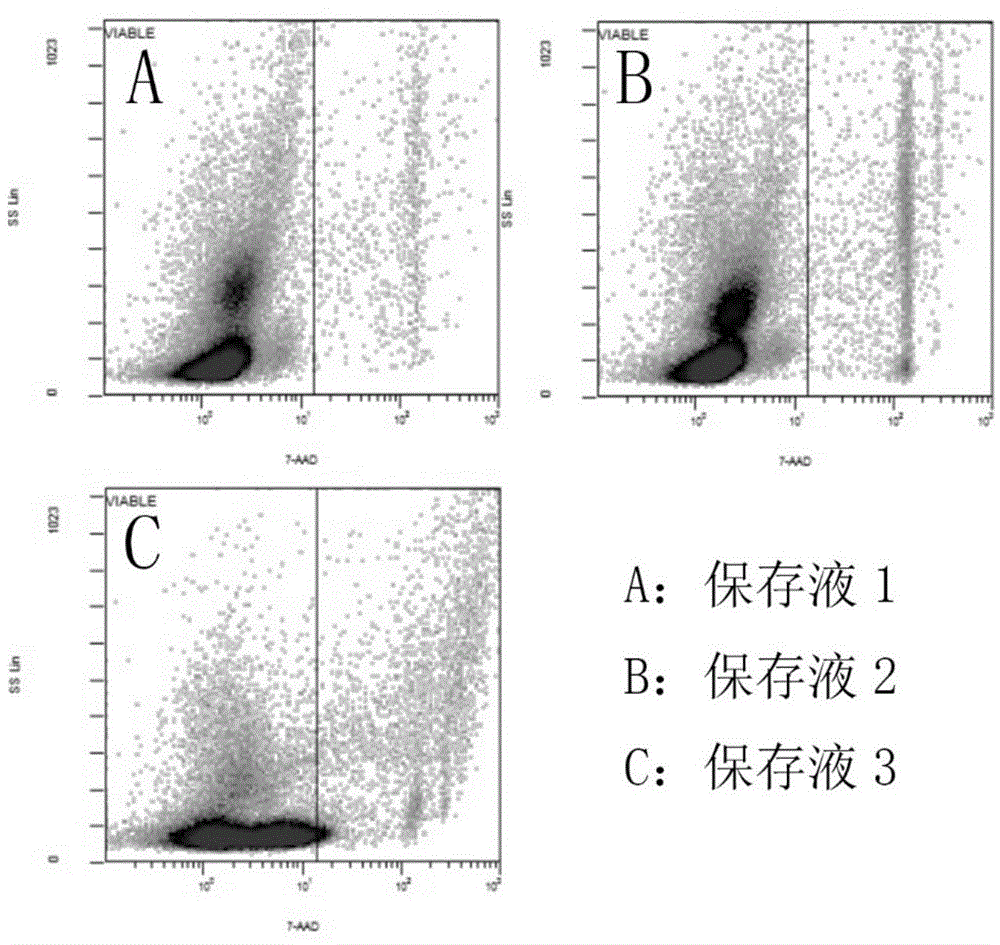
[0061] Embodiment 2 Kit of the present invention and separation method
[0062] 1. Kit composition
[0063] Diluent: diluted with 0.1% sodium chloride solution by mass percentage.
[0064] Precipitating agent: 2.4% hydroxyethyl starch solution in mass percentage.
[0065] Separation liquid: a suspension of silica gel particles treated with polyvinylpyrrolidone with a density of 1.072 g / ml.
[0066] Resuspension: 10% human AB plasma.
[0067] 2. Separation method
[0068] 1. Collect human cord blood in a sterile environment and store it in anticoagulant blood bags. In the ultra-clean bench, divide the collected human cord blood into 50ml centrifuge tubes, and put 20ml cord blood into each tube;
[0069] 2. Inject an equal volume of diluent into the centrifuge tube containing 20ml of umbilical cord blood to dilute, then add 10ml of precipitant, gently invert and mix, and centrifuge for 5-10min under 50g centrifugal force;
[0070] 3. Place the supernatant obtained from the ...
Embodiment 3
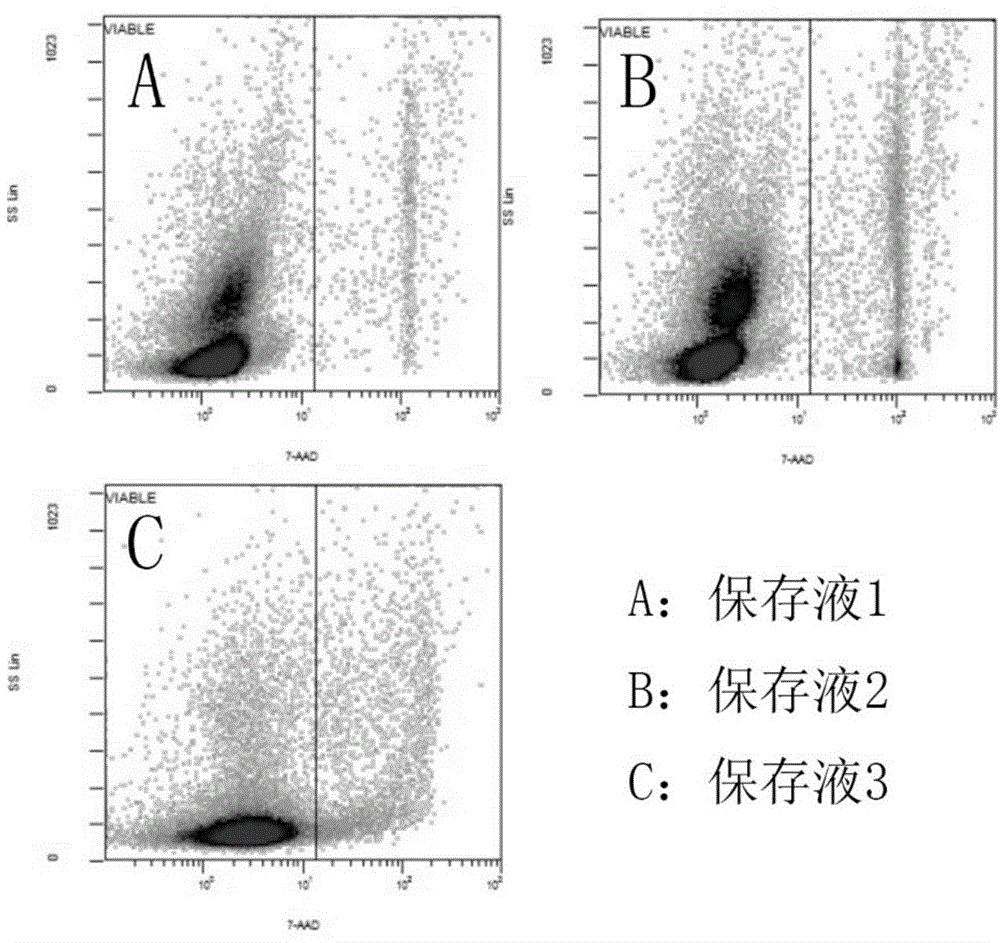
[0078] Embodiment 3 Kit of the present invention and separation method
[0079] 1. Kit composition
[0080] Diluent: the mass percentage is diluted with 5% sodium chloride solution.
[0081] Precipitating agent: 12% hydroxyethyl starch solution by mass percentage.
[0082] Separation liquid: a suspension of silica gel particles treated with polyvinylpyrrolidone with a density of 1.086 g / ml.
[0083] Resuspension: 30% human AB plasma.
[0084] 2. Separation method
[0085] 1. Collect human cord blood in a sterile environment and store it in anticoagulant blood bags. In the ultra-clean bench, divide the collected human cord blood into 50ml centrifuge tubes, and put 20ml cord blood into each tube;
[0086] 2. Pour an equal volume of diluent into the centrifuge tube containing 20ml of umbilical cord blood for dilution, then add 10ml of precipitating agent, gently invert and mix, and let stand for 30-50 minutes;
[0087] 3. Place the supernatant obtained from the above sedimen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com