Preparation method of As2O3 albumin nano medicine
A technology of arsenic trioxide albumin and arsenic trioxide, applied in the field of biomedicine, can solve the problems of complex preparation process, lack of targeting, short half-life, etc., and achieve the effect of good dispersion, good solubility and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
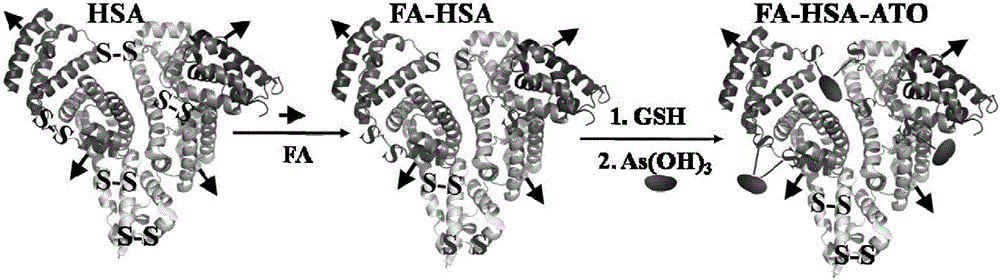
[0026] The preparation of embodiment 1 arsenic trioxide albumin nano drug delivery system (FA-HSA-ATO), such as figure 1 Shown:
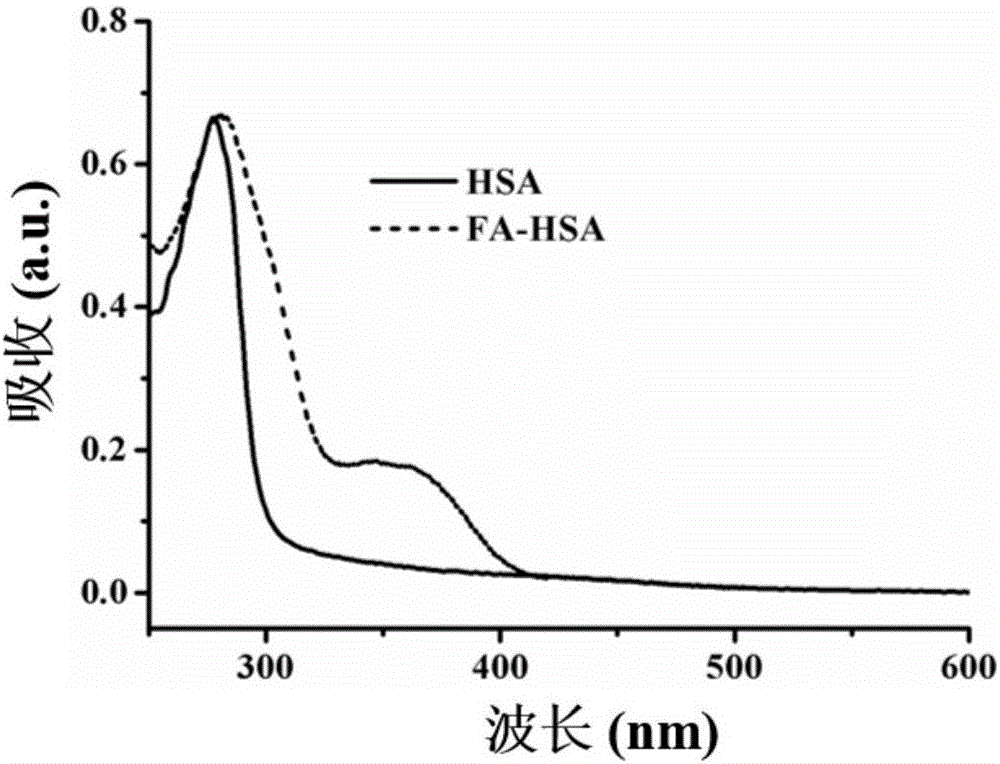
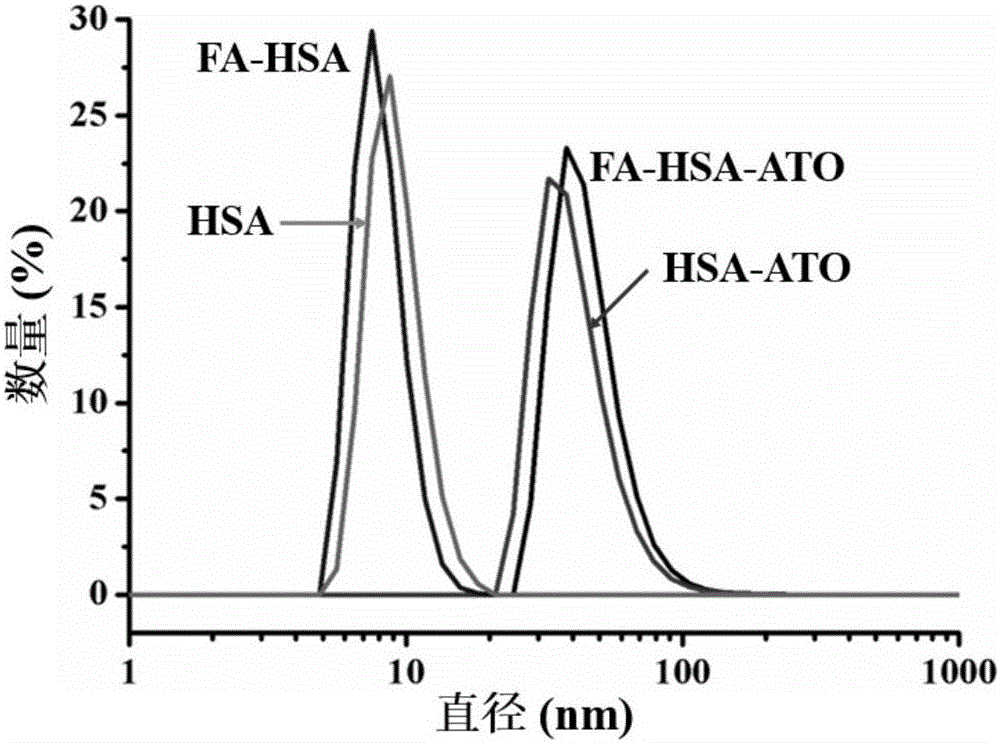
[0027] (1) Diarsenic trioxide (As 2 o 3 , ATO) powder 10 mg dissolved in a small volume of sodium hydroxide solution, then adjust the pH to about 5.0-6.5 with 1M dilute hydrochloric acid, and prepare an arsenic solution with an ATO concentration of 5 mg / mL for drug loading on albumin; label folic acid Molecular human serum albumin (Human serum albumin, HSA) 100mg (see figure 2 ) was dissolved in 2ml of Dulbecco's Phosphate Buffered Saline (D-PBS) without calcium and magnesium ions (50mg / mL), and glutathione (GSH) was added to react gently in a 37°C water bath for 1h , the final concentration of glutathione (GSH) added in the human serum albumin solution is 50mM;
[0028] (2) Add an aqueous solution containing 5 mg of ATO into the GSH-treated albumin solution, and stir at room temperature for 2 hours;
[0029] (3) Put the ATO-albumin mixed solu...
Embodiment 2 3
[0031] The preparation of embodiment 2 arsenic trioxide albumin nano drug delivery system (FA-HSA-ATO), such as figure 1 Shown:
[0032] (1) Diarsenic trioxide (As 2 o 3 , ATO) powder 20mg dissolved in a small volume of sodium hydroxide solution, then adjust the pH to about 5.0-6.5 with 1M dilute hydrochloric acid, and prepare an arsenic solution with an ATO concentration of 10mg / mL, which will be used for albumin drug loading; the labeled folic acid Molecular human serum albumin (Human serum albumin, HSA) 160mg (see figure 2 ) was dissolved in 2ml of Dulbecco's Phosphate Buffered Saline (D-PBS) (80mg / mL) without calcium and magnesium ions, and glutathione (GSH) was added and stirred gently in a water bath at 37°C for reaction 1 Hours, the final concentration of glutathione (GSH) added in the human serum albumin solution is 80mM;
[0033] (2) Add an aqueous solution containing 10 mg of ATO into the GSH-treated albumin solution, and stir at room temperature for 2 hours;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com