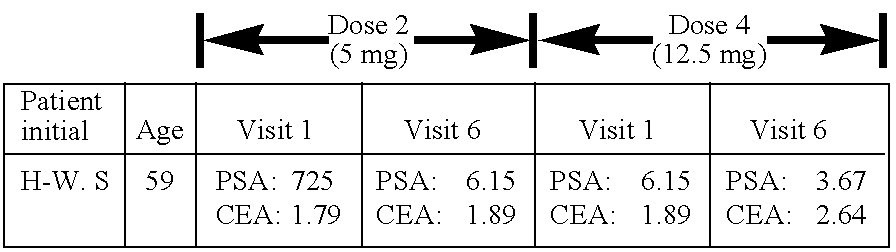
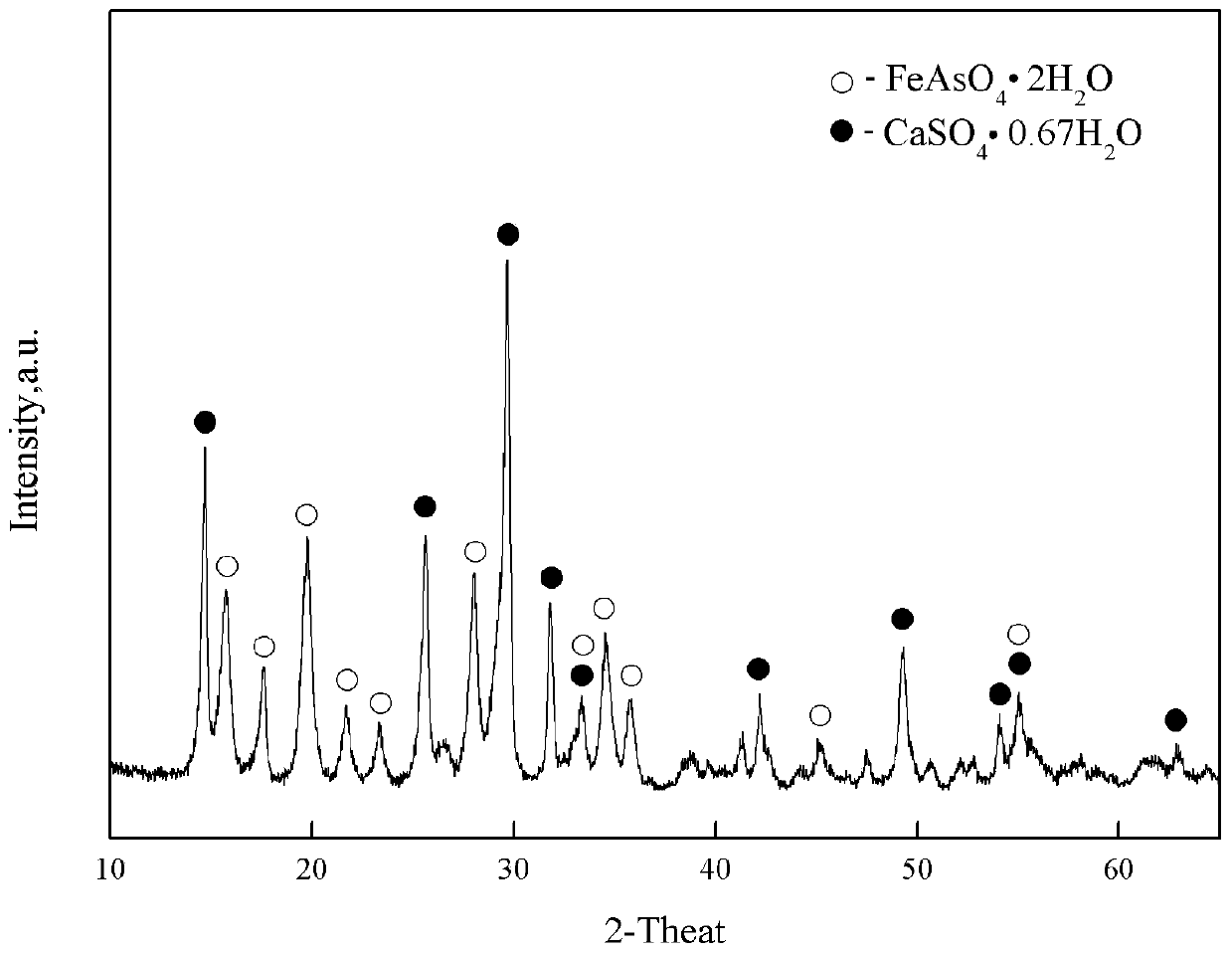
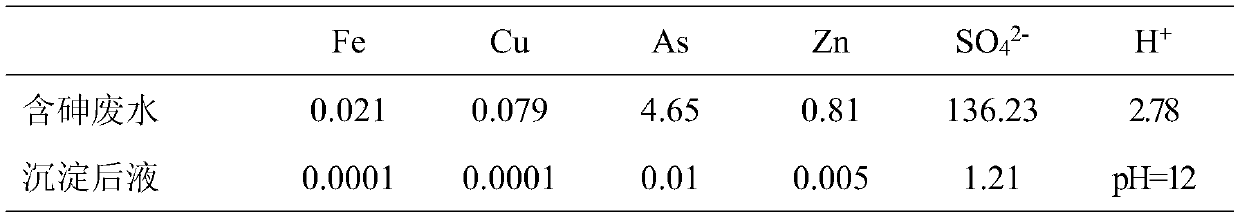
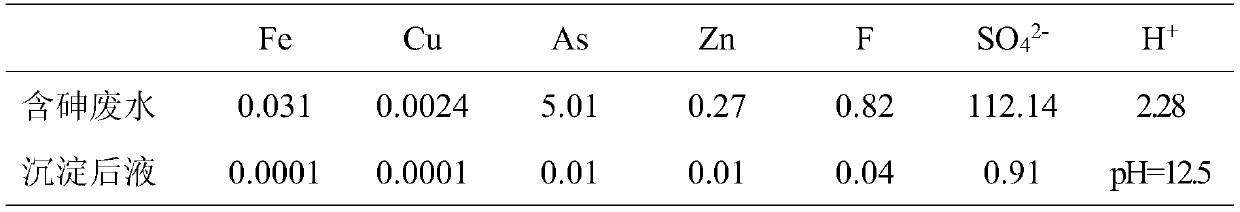
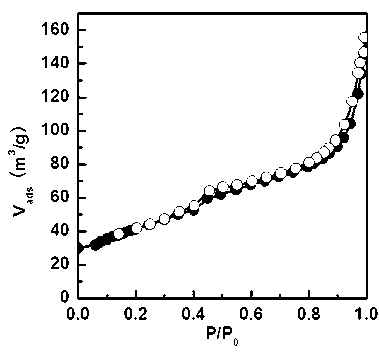
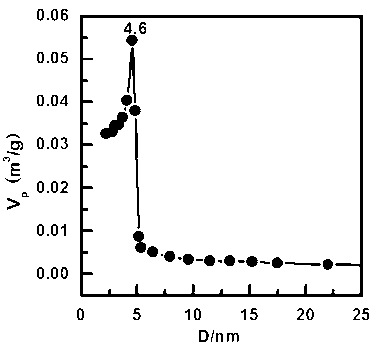
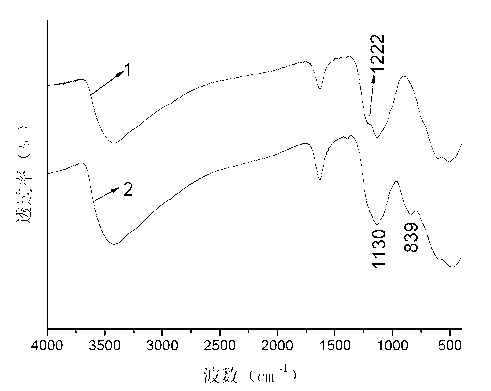
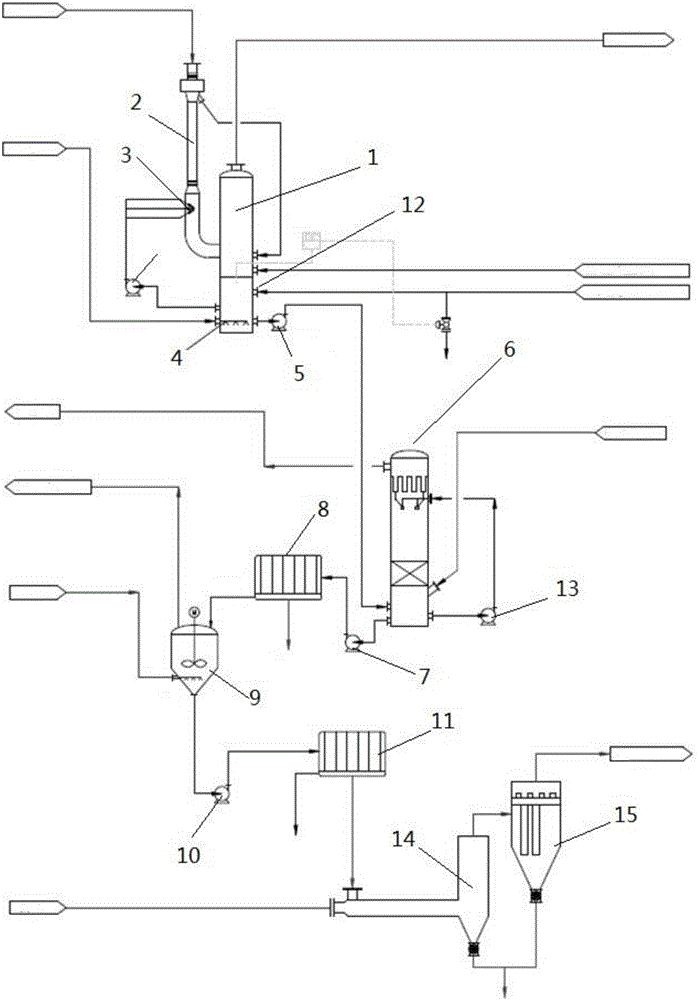
Patents
Literature
76 results about "Arsenous acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
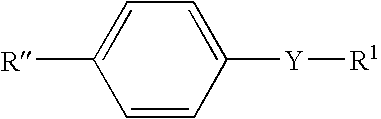
Arsenous acid (or arsenious acid) is the inorganic compound with the formula H₃AsO₃. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)₃.
Fluorescent probes for saccharrides
InactiveUS20040087842A1Decrease of fluorescence emissionReduce sensitivityAnalysis using chemical indicatorsChemiluminescene/bioluminescenceFluoProbesTelluric acid
The spectroscopic and photophysical properties of fluorescent probes comprising donor-acceptor derivatives comprising the boric acid group or a derivative of boric acid, B(OH)3 (or borate ion, BO(OH)2<-1>), arsenious acid, H3 AsO3 (or arsenite ion, H2AsO3<-1>), telluric acid, H6TeO6 (or tellurate ion, H5 TeO6<-1>) or germanic acid, Ge(OH)6 (or germanate ion, GeO(OH)3<-1>) are described. Method of using said probes are also provided.
Owner:LAKOWICZ JOSEPH R +1
Method for preparing arsenic trioxide from arsenic sulfide waste
InactiveCN102115166ALow costNothing producedSolid waste disposalArsenic compoundsSolubilityArsenic sulfide
The invention provides a method for preparing arsenic trioxide from arsenic sulfide waste. The arsenic sulfide waste is soaked in a sodium hydroxide solution to extract arsenic sulfide, arsenic sulfide mixes with an excessive amount of arsenic acid, and the mixture reacts at a temperature ranging from 40 DEG C to 95 DEG C for 0.5 to 3 hours, to produce a mixture containing arsenous acid and elemental sulfur; the mixture containing arsenous acid and elemental sulfur mixes with 2 to 5 times (by mass) of water and then oxygen gas is introduced so that arsenous acid is oxidized into arsenic acid, and the reaction product is filtered to remove elemental sulfur and thus to produce an arsenic acid solution; and the arsenic acid solution is reduced by sulfur dioxide gas to arsenous acid with a lower solubility, the reaction liquid is distilled under a reduced pressure to produce a saturated solution of arsenous acid, the saturated solution of arsenous acid is cooled so that arsenous acid is crystallized and then filtered to produce a filter cake of arsenous acid, and the filter cake of arsenous acid is dried and pulverized to obtain an arsenic trioxide product. The method has a short process flow, increases the recovery rate of arsenic, avoids the production of secondary pollutants, adopts simple equipment, greatly reduces the cost of arsenic recovery and is worthy to be widely applied.
Owner:JIANGSU ZHONGKE MACHINERY
Method for preparing arsenic trioxide by using arsenic-containing waste water
InactiveCN101125682ASimple processSave raw materialsWater/sewage treatmentArsenic compoundsFiltrationSlurry
The invention relates to a method to produce arsenic trioxide with waste water containing arsenium, which takes waste water containing arsenium as raw material to produce arsenic trioxide. A. alkali is added to remove impurities, cupric salt is added, the pH value of the solution is adjusted to 4-14 by alkali and green copper arsenite or copper arsenate precipitate is prepared; B. the prepared green copper arsenite or copper arsenate precipitate is made into slurry by water or sulphuric acid solution; sulfur dioxide is pumped into or sulphite is added to be reacted, arsenious acid solution and pink filtration residue are obtained after filtration; C. arsenious acid solution is crystallized, filtered and dried to get arsenic trioxide powder. The invention has the advantages of simple process, saving raw materials, reducing cost and high recovery yield.
Owner:CENT SOUTH UNIV
Pharmaceutical compositions comprising of arsenous acid, its sodium salt and its derivatives intended for the treatment or urogenital cancer and its metastasis
InactiveUS20090011047A1Telomeres shortenImprove the quality of lifeBiocideAntipyreticDiseaseAdjuvant
The present application relates to pharmaceutical compositions and methods for treatment of urogenital diseases and bone metastasis in a human, which pharmaceutical composition contains an effective amount of arsenous acid alkaline or earth alkaline metal salt and / or a pharmaceutically acceptable adjuvant. According to the present invention, the alkaline arsenous acid metal salt is sodium meta-arsenita (AsO2Na) or potassium meta-arsenite (AsO2K). The effective amount of arsenous acid alkaline or earth alkaline metal salt is 0.0001-1500 mg / kg, preferably 1-1000 mg / kg, more preferably 1-150 mg / kg, and most preferably 50-100 mg / kg of body weight / day. The administration form of the pharmaceutical compositions of the invention is preferably oral, such as a tablet, capsule, powder and / or solution with a pharmaceutically acceptable carrier, diluent or excipient.
Owner:KOMINOX
Method for treating arsenic-containing wastewater and solidifying arsenic
ActiveCN111170510AHigh removal rateReduce the temperatureWater contaminantsTreatment involving filtrationIron sulfateArsenous acid
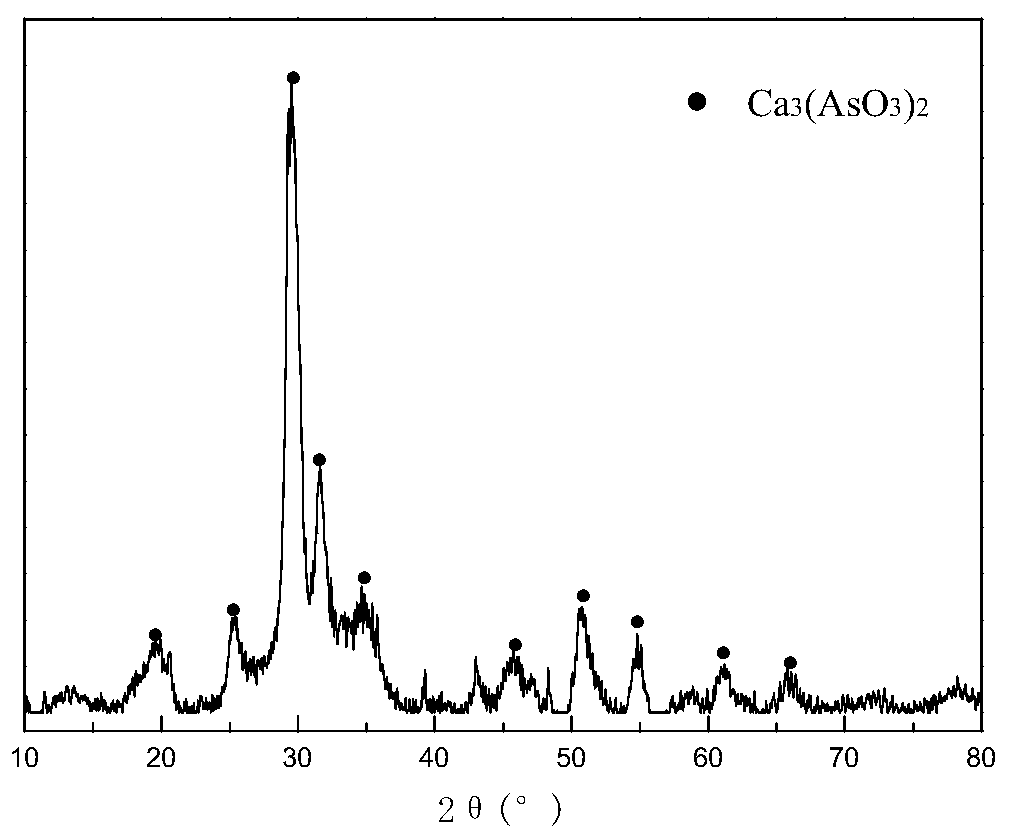
The invention discloses a method for treating arsenic-containing wastewater and solidifying arsenic. The method comprises the following steps: separating and enriching arsenic in the arsenic-containing wastewater in the form of calcium arsenate or / and calcium arsenite precipitate; and oxidizing the obtained arsenic enriched product in a ferric sulfate or ferrous sulfate solution, and carrying outnormal-pressure reaction or pressurized hydrothermal reaction or normal-pressure hydrothermal reaction, so that arsenic in the arsenic enriched product is solidified in the form of scorodite crystals.The obtained scorodite crystals are complete in grain development, uniform in grain distribution and stable in structure under acidic conditions. The method has the advantages of high operation efficiency, good arsenic fixation effect, convenience in operation, low arsenic solidification treatment cost and the like, and is suitable for industrial application of harmless treatment of arsenic-containing wastewater.
Owner:宁波弗镁瑞环保科技有限公司 +2
Reagent used for detecting urinary iodine by biochemistry analyzer and urinary iodine detecting method
ActiveCN102495203AReduce dosageAutomatic determination automatic fastBiological testingSodium iodidePotassium iodine
The invention discloses a reagent used for detecting urinary iodine by a biochemistry analyzer and a urinary iodine detecting method. The reagent mainly comprises the following components: digestion solution-ammonium persulfate solution, reagent R1-arsenious acid or arsenic trioxide solution, reagent R2-ceric ammonium sulfate or cerous sulfate solution, and iodine standard solution-potassium iodide (sodium iodide) or potassium iodate (sodium iodate) solution. The detecting method mainly includes the following steps: selecting the detecting reagent, digesting urine sample, setting parameters of the biochemistry analyzer, and determining through the biochemistry analyzer. The detecting method has high automation degree, small human error, little reagent dosage and short detecting time. By adopting the biochemistry analyzer to analyze the urinary iodine, the urinary iodine detecting method expands the detecting items of the instrument, is particularly suitable for the medical organizations equipped with the biochemistry analyzers, and meets the requirements of clinical detection on quick issuing of result and detecting automation.
Owner:WUHAN ZHONGSHENG BIOCHEM TECHN
Method for preparing arsenic trioxide by arsenic-containing waste water
InactiveCN102115165AHigh recovery rateLow costSolid waste disposalArsenic compoundsCopper sulfateArsenic sulfide
The invention provides a method for preparing arsenic trioxide by arsenic-containing waste water, which comprises the steps of: mixing arsenic sulfide waste residue with water, and extracting arsenic sulfide by sodium hydroxide solution; mixing the obtained arsenic sulfide with the water with 2-5 times of the weight of the arsenic sulfide to be sizing agent, and reacting with copper sulfate solution to obtain arsenous acid and copper sulphide-containing precipitate and sulfuric acid solution; mixing the arsenous acid and copper sulphide-containing precipitate with the water to feed the oxygen, oxygenizing arsenious acid to generate arsenic acid which is easy to dissolve in water, and filtering to obtain arsenic acid solution and copper sulphide precipitate; restoring the obtained arsenic acid to be arsenious acid by sulfur dioxide to obtain arsenious acid; and distilling the arsenious acid, concentrating, filtering and drying to obtain an arsenic trioxide product. The method is short in technological process, high in arsenic recovery rate, free of the generation of secondary pollutant, simple in equipment, and extremely lower in arsenic recovery cost, thereby being a method which is worth to popularize and apply.
Owner:马艳荣
Compound technology for removing As, Sb, Bi by utilizing copper electrolyte
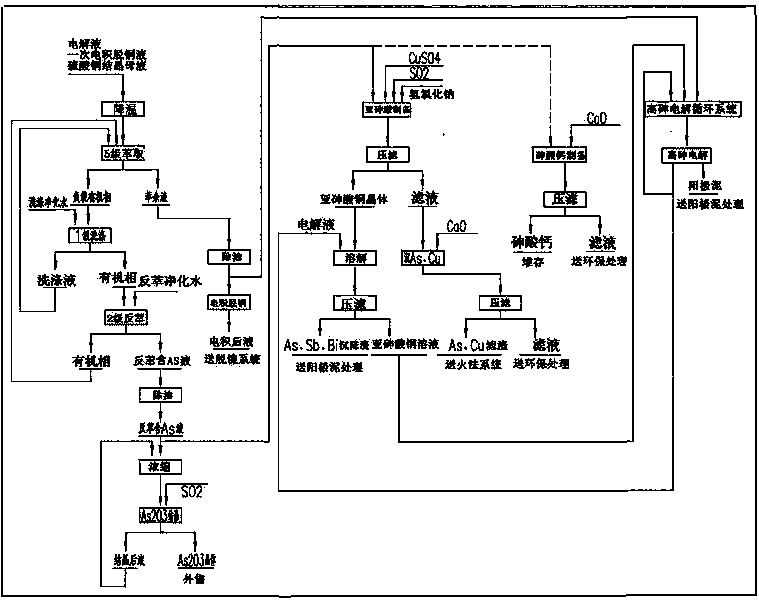
ActiveCN104018185AReduce power consumptionReduce energy consumptionElectrolysis componentsPhotography auxillary processesElectrolytic agentArsenic product
The invention discloses a compound technology for removing, Sb, Bi by utilizing copper electrolyte. A process for removing As by means of solvent extraction and a process of removing Sb and Bi by means of solution self-purification are combined; copper arsenite crystals are prepared by As in a back-extraction solution and is used as a high arsenic electrolytic additive; during high arsenic electrolysis, Sb and Bi in the solution can be effectively removed through As (III), and a majority of Sb and Bi in an anode plate enter anode mud. According to the compound technology, the aims of removing As, Sb and Bi can be fulfilled; meanwhile, an electro-deposition induced decoppering and dearsenification method is eliminated in the main process, and a small quantity of nickel removal liquid in the auxiliary process needs to be subjected to electro-deposition treatment. The compound technology is low in power consumption; the amount of H3As gas, black copper plates and black copper powder is obviously reduced; a dearsenification product is sold in a form of As2O3 or stacked in a form of calcium arsenate. According to the method, the operating environment is optimized, the defects that the As removal product contains a large amount of copper in the electro-deposition induced decoppering and dearsenification method and needs to be returned to a pyrogenic process system for separating copper and arsenic are overcome; the electro-deposition during the main impurity removal process is avoided, and the energy consumption is obviously reduced.
Owner:CHINA NERIN ENG
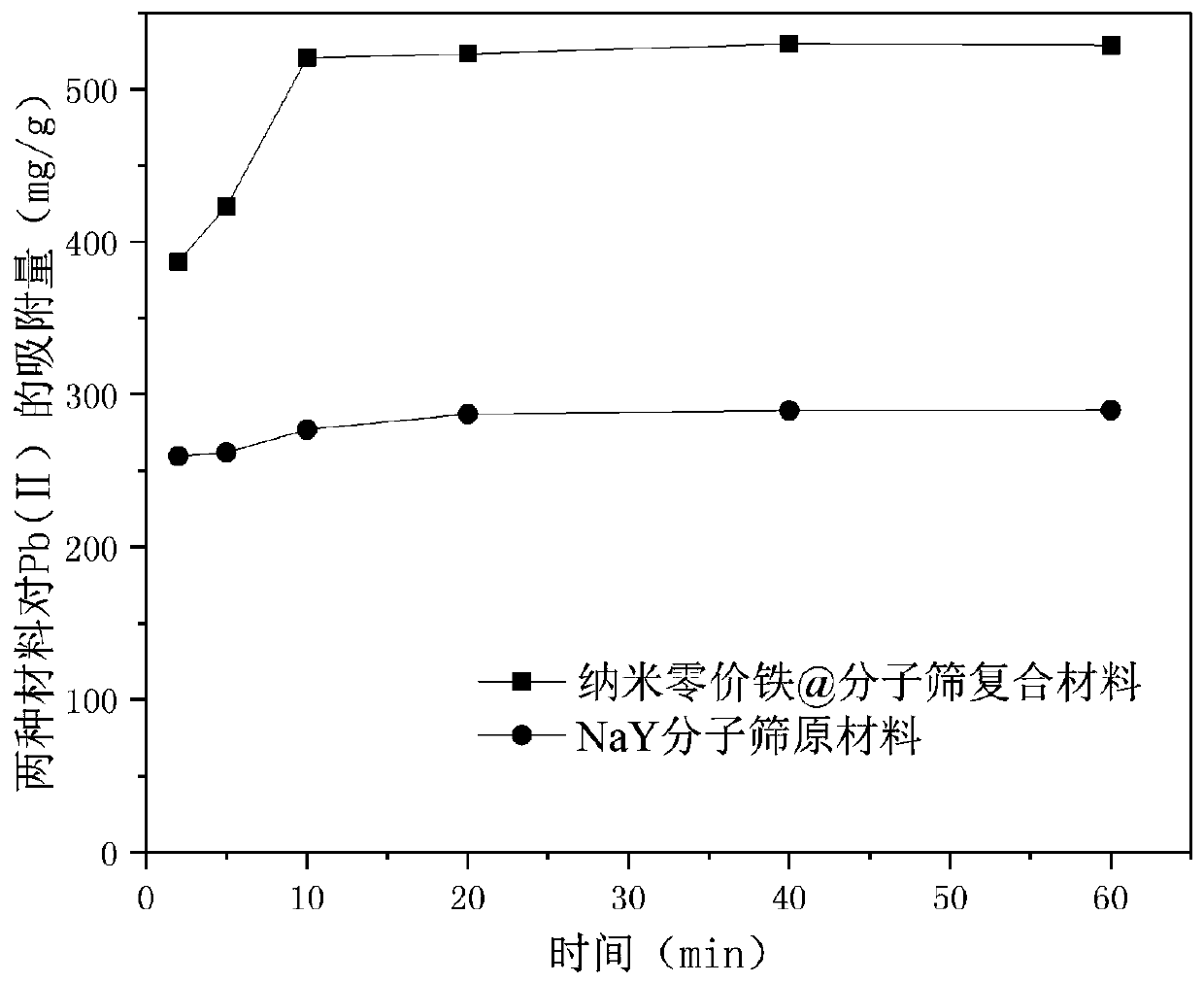
Nano zero valent iron @ molecular sieve composite material and preparation method and applications thereof
InactiveCN110451597AReduce reunionPrevent surface oxidationWater contaminantsWater/sewage treatment by sorptionIron saltsNitrogen gas
The invention relates to a nano zero valent iron @ molecular sieve composite material and a preparation method and applications thereof. The preparation method comprises following steps: preparing aniron salt solution; adding molecular sieves into the iron salt solution; mechanically stirring the iron salt solution under the protection of nitrogen so as to introduce iron ions into the molecular sieves to obtain a mixed solution (A) containing molecular sieves and iron salts; subjecting the mixed solution (A) to solid-liquid separation; after separation, mixing the solids with water to obtaina mixed reaction solution; under the protection of nitrogen and mechanical stirring, adding a solution of a reducing agent into the mixed reaction solution, keeping on stirring to obtain a mixed solution (B); subjecting the mixed solution (B) to solid-liquid separation; and finally washing and drying the obtained solids after separation to obtain the nano zero valent iron @ molecular sieve composite material. The synthesized nano zero valent iron @ molecular sieve composite material has a cation exchange function of molecular sieves and an adsorption-reduction function of nano zero valent ironand has a good effect on removing cations of heavy metals such as Pb<2+>, Cu<2+>, etc. and anions such as ions of arsenous acid.
Owner:WUHAN UNIV OF SCI & TECH
Arsenic sulfide waste residue recycling treatment method and device thereof
ActiveCN104438287AEffluent water quality is stable and up to standardAvoid secondary hazardous wasteSolid waste disposalReverse osmosisSulfidation
The invention discloses an arsenic sulfide waste residue recycling treatment method, which comprises the following steps: performing alkaline leaching to obtain sulfo-arsenite or sulfo-arsenate solution, filtering to remove waste residues through a filter, to obtain alkaline leachate, then performing oxidation desulfurization, so as to oxidize sulfo-arsenite or sulfo-arsenate into sulfo-arsenite or arsenate ions, then filtering and separating elemental sulfur through another filter to obtain arsenite or arsenate solution, recovering acid-base from the arsenite or arsenate solution by utilizing a bipolar membrane device, returning base solution for alkaline leaching and acid solution for crystalization and preparation of As2O3 or As2O5, performing electrodialysis or reverse osmosis concentration on residual liquor in a bipolar membrane salt chamber, returning concentrated liquor as inflowing water of the salt chamber of the bipolar membrane device, and draining or recovering fresh water. According to the arsenic sulfide waste residue recycling treatment method, the problems that for the traditional arsenic sulfide waste residue treatment method, consumption is large, the content of arsenic in effluent water does not reach the standard, the recycling rate is low, the technological process is complicated, secondary pollution exists and the like can be solved, the content of arsenic in effluent water reaches the standard, the recycling rate of As and S is high, the advantages of low consumption, low running cost, simple technological process and the like can be achieved.
Owner:BEIJING CYCLE COLUMBUS ENVIRONMENTAL SCI & TECH
Method for preparing arsenic adsorbent and method for treating waste water
InactiveCN102698703AImprove adsorption capacityThe synthesis method is simpleOther chemical processesWater/sewage treatment by sorptionSorbentWastewater
The invention provides a method for preparing an arsenic adsorbent. The arsenic adsorbent has zirconium oxide of nano-pore structure. The material can effectively treat arsenate and arsenite in the waste water, and can be recycled after waste water treatment. Another objective of the invention is to provide a method for treating waste water containing arsenate or arsenite ions by the arsenic adsorbent prepared by the method. The method contains a 'regeneration' step, so that the arsenic adsorbent can be reused cyclically.
Owner:CHONGQING THREE GORGES UNIV
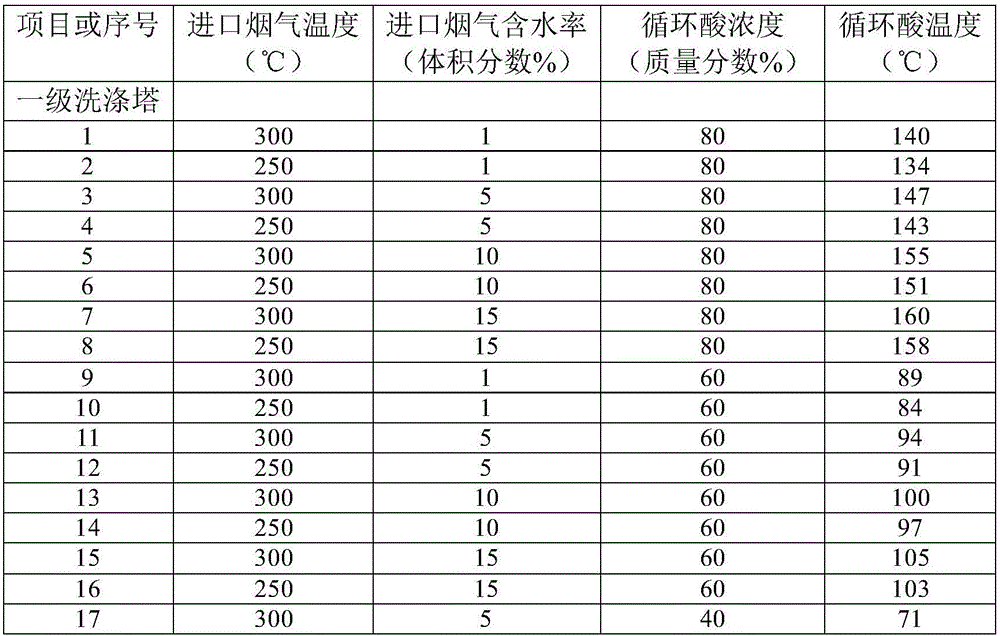
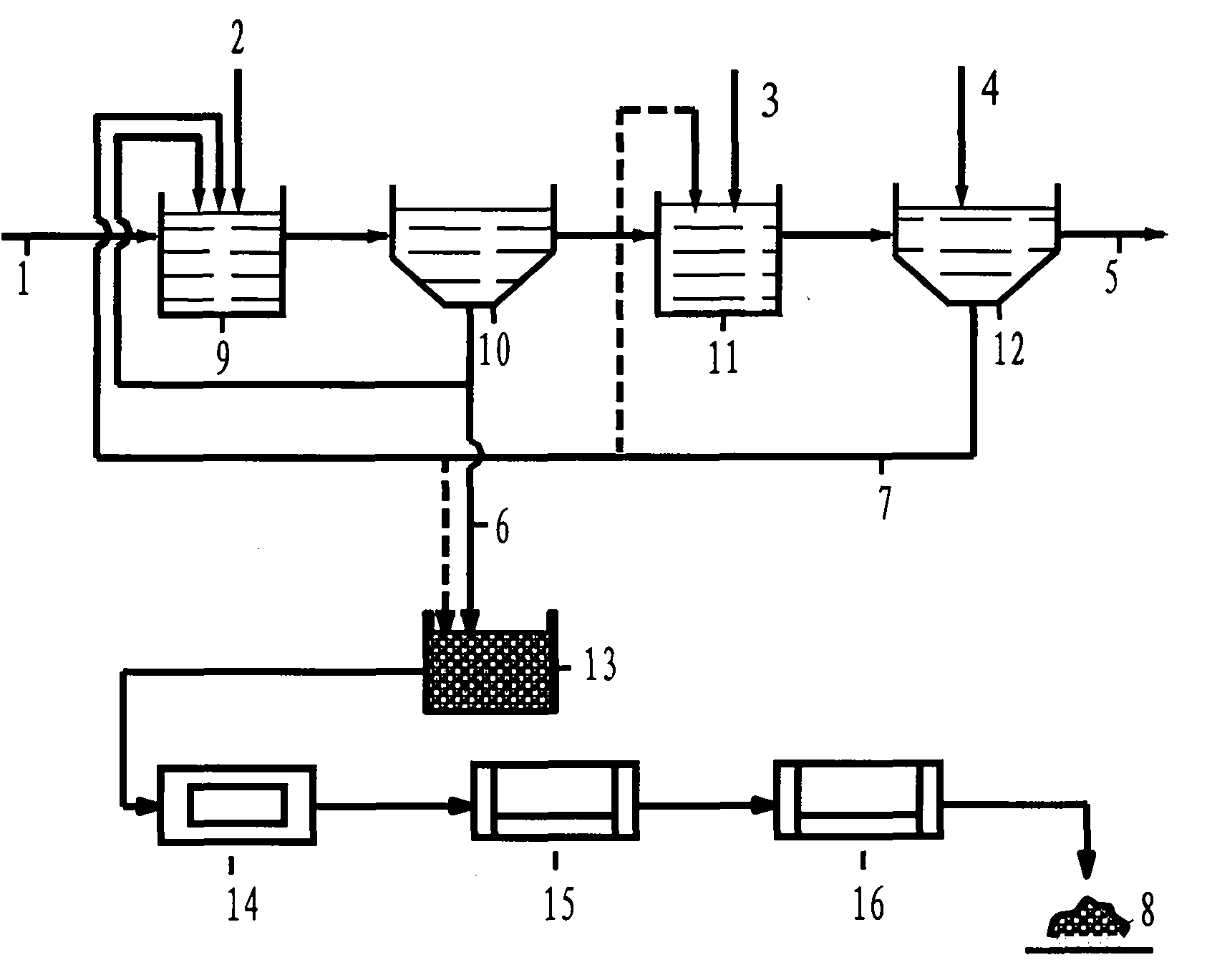
Energy saving process and system for comprehensively recycling waste acid of sulfuric acid purification
ActiveCN106268179ALow running costReduce solubilityGas treatmentArsenic oxides/hydroxides/oxyacidsHigh concentrationSlag
The invention discloses an energy saving process for comprehensively recycling waste acid of sulfuric acid purification. The energy saving process comprises the following steps: high-temperature flue gas is fed into a first-stage washing tower; acidic liquor at the bottom of a tower is pumped to an acid discharging spraying head in a gas inlet pipeline of the first-stage washing tower, and the acidic liquor is in contact with the high-temperature flue gas reversely to absorb SO3 in the flue gas; the flue gas washed by the acidic liquor is cooled and then enters a subsequent conventional purification process for continuous cooling, and condensed diluted acid generated in the subsequent cooling process is returned into the first-stage washing tower to keep the SO3 and water in the tower balanced; heavy metal dust and arsenic elements in the flue gas form sulfate and arsenious acid after being washed by the acidic liquor; a compressed air aeration device oxidizes the arsenious acid in the acidic liquor into arsenic acid; the acidic liquor containing the arsenic acid and the heavy metal acid salt is conveyed into a cooling and thickening tower; concentrated acid from the cooling and thickening tower is filtered by a slag filter to filter out heavy metal sulfate slag; filtrate enters an SO2 reducing tank, and high-concentration SO2 gas is fed into the tank, so that the arsenic acid is reduced into arsenious acid precipitates which can be slightly dissolved in the sulfuric acid; the acidic liquor containing the arsenious acid precipitates is subjected to solid-liquid separation to obtain an arsenious acid filter cake.
Owner:中铝环保节能集团有限公司
High-efficiency method for treating arsenic-containing wastewater
InactiveCN102107981ANo secondary impactReduce solubilitySludge treatment by oxidationWater contaminantsAfter treatmentSludge
The invention discloses a high-efficiency method for treating arsenic-containing wastewater, solves the problems in the prior art, and provides a complete method for treating the arsenic-containing wastewater. Arsenic (As3 plus) can be catalytically oxidized in advance through self-oxidation iron oxide flow bacilli; arsenious acid ions are oxidized to arsenic acid ions; then calcium compounds are added to gather arsenic-containing waste in the water; solid-liquid separation is conducted twice; and sludge can enter the wastewater again to circularly remove arsenic. After treatment, the arsenic content in the wastewater is very low, the discharged arsenic-containing sludge becomes non-toxic matters to be land-filled after being dried and roasted, and the water needing to be discharged can be discharged directly.
Owner:湖南布鲁斯凯碳资产管理有限公司
Iron-loaded cotton fiber material and preparation method thereof
ActiveCN102125819AImprove performanceStable quality and performanceOther chemical processesAlkali metal oxides/hydroxidesFibrillar morphologyCotton fibre
The invention discloses an iron-loaded cotton fiber material with absorbent cotton fiber as basic skeleton and a preparation method thereof, belonging to the technical field of macromolecular material. The fiber material structurally contains the hydroxide of amorphous iron. The preparation method thereof comprises the steps of: taking the absorbent cotton fiber as the basic skeleton, loading ferric iron salt solution through dispersion and hydrolysis so that the structure of the fiber contains the hydroxide of amorphous iron. The reaction process is simple, the conditions are moderate, and the reaction agents adopted are nontoxic and environmentally-friendly. The fiber material keeps fibrous shape of the absorbent cotton fiber, has stable performance in air, and causes no pH change of water when being used as adsorbent in the water, and the iron-loaded shape of the adsorbent is stable so that arsenite ions in the water can be adsorbed effectually.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Method for separating and recovering arsenic from metallurgical material acid leaching liquid
InactiveCN104831067ARealize resource utilizationAvoid secondary pollutionProcess efficiency improvementSorbentDesorption
A method for separating and recovering arsenic from a metallurgical material acid leaching liquid comprises the following steps: selectively adsorbing arsenic by adopting antimony or / and bismuth oxide and hydrate thereof as an adsorbent in order to remove arsenic, and filtering to obtain an arsenic removed liquid and a supported adsorbent; recovering valuable metals from the arsenic removed liquid, adding a sodium carbonate or / and sodium hydroxide solution to the supported adsorbent, carrying out stirring desorbing regeneration, and filtering to obtain a regenerated adsorbent and a desorption liquid; and returning the regenerated adsorbent to the arsenic adsorbing removal process for cycle use, carrying out cooling crystallization on the desorption liquid to precipitate disodium hydrogen arsenate crystals, and returning a disodium hydrogen arsenate crystallization mother liquor to the above supported adsorbent desorption process for cycle use. Obtained disodium hydrogen arsenate can be dried to obtain a product, and the product can be directly sold in the market, or can be used as an arsenous acid production raw material in order to realize recycling of As in the acid leaching liquid. The method has the advantages of simple process, simple operation, low production cost, good arsenic removal effect, no side effects on recovery of the valuable metal from the liquid, and thorough elimination of pollution of the arsenic removal process of the metallurgical material acid leaching liquid to environment.
Owner:CENT SOUTH UNIV
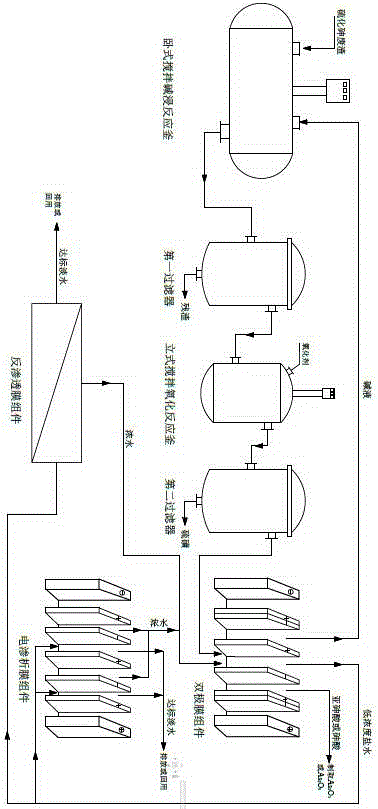
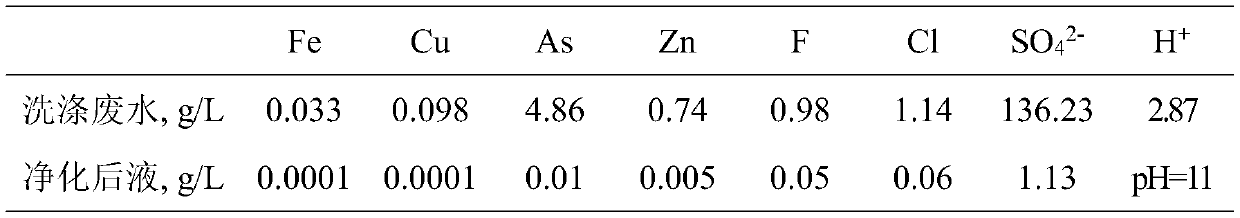
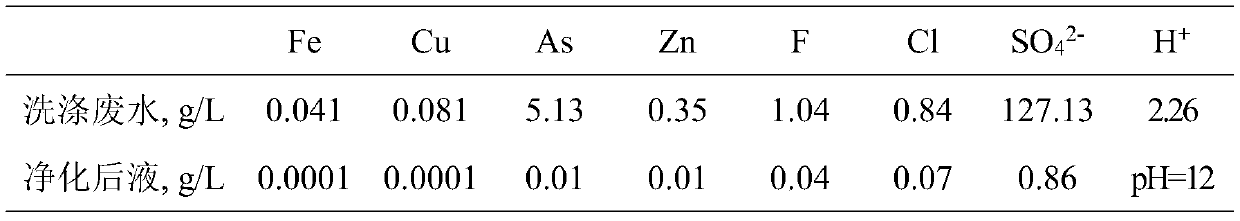
Resource treatment method for smelting flue gas washing wastewater
ActiveCN111302525ASimple recycling processProduce side effectsWater contaminantsTreatment involving filtrationSlagFlue gas
The invention discloses a resource treatment method for smelting flue gas washing wastewater. According to the method, smelting flue gas washing wastewater is subjected to resourceful treatment through the steps of reduction neutralization deacidification, step-by-step adsorption defluorination and dechlorination, neutralization purification and impurity removal and the like; the sulfuric acid inthe wastewater is neutralized with lime to obtain calcium sulfate containing less than 0.1% of As; the fluorine and the chlorine in the wastewater are separated and recovered in the form of sodium fluoride and sodium chloride solids respectively after adsorbing and enriching, the arsenic in the purification slag is recovered in the form of metallic arsenic or arsenious acid, and the valuable metals in the wastewater are enriched in the neutralization purification slag to facilitate comprehensive recovery; and the neutralized and purified liquid returns to smelting flue gas washing so as to berecycled. The method provided by the invention has the advantages of good treatment effect, high comprehensive utilization rate, zero wastewater discharge, no secondary pollution and the like, and issuitable for industrial application of smelting flue gas washing wastewater treatment.
Owner:CENT SOUTH UNIV +1
Method for treating arsenic-containing waste water
InactiveCN104016455ANothing producedLow costWater/sewage treatment by flocculation/precipitationArsenateToxic gas
The invention relates to a method for treating arsenic-containing waste water. The method comprises the following steps: (1) firstly adding FeSO4, wherein the dosage of the FeSO4 is four times more than the arsenic content of raw water; (2) then adding sodium hydroxide to regulate the pH value of the waste water to 8.8, and coprecipitating by utilizing the characteristic that generated colloid Fe(OH)3 can absorb ferric arsenate and ferric arsenite; and (3) finally adding negative ions of 2.5 ppm, stirring for 16 minutes, precipitating for 16 minutes, filtering, and measuring the arsenic content of filtered water. The method disclosed by the invention has the advantages that the pH of the waste water is regulated to 8.5-9.5 by firstly adding the FeSO4 and then adding the sodium hydroxide, the colloid Fe(OH)3 generated in reaction can adsorb the ferric arsenate and the ferric arsenite to realize coprecipitation, and finally the negative ions are added, sufficiently stirred, precipitated, filtered and detected. The method disclosed by the invention has the advantages of low cost, small dosage, easiness for operation, no secondary pollution, no toxic gas generation and high arsenic removal rate of 99.6%.
Owner:SHANGHAI FENGXIN ENVIRONMENTAL PROTECTION TECH
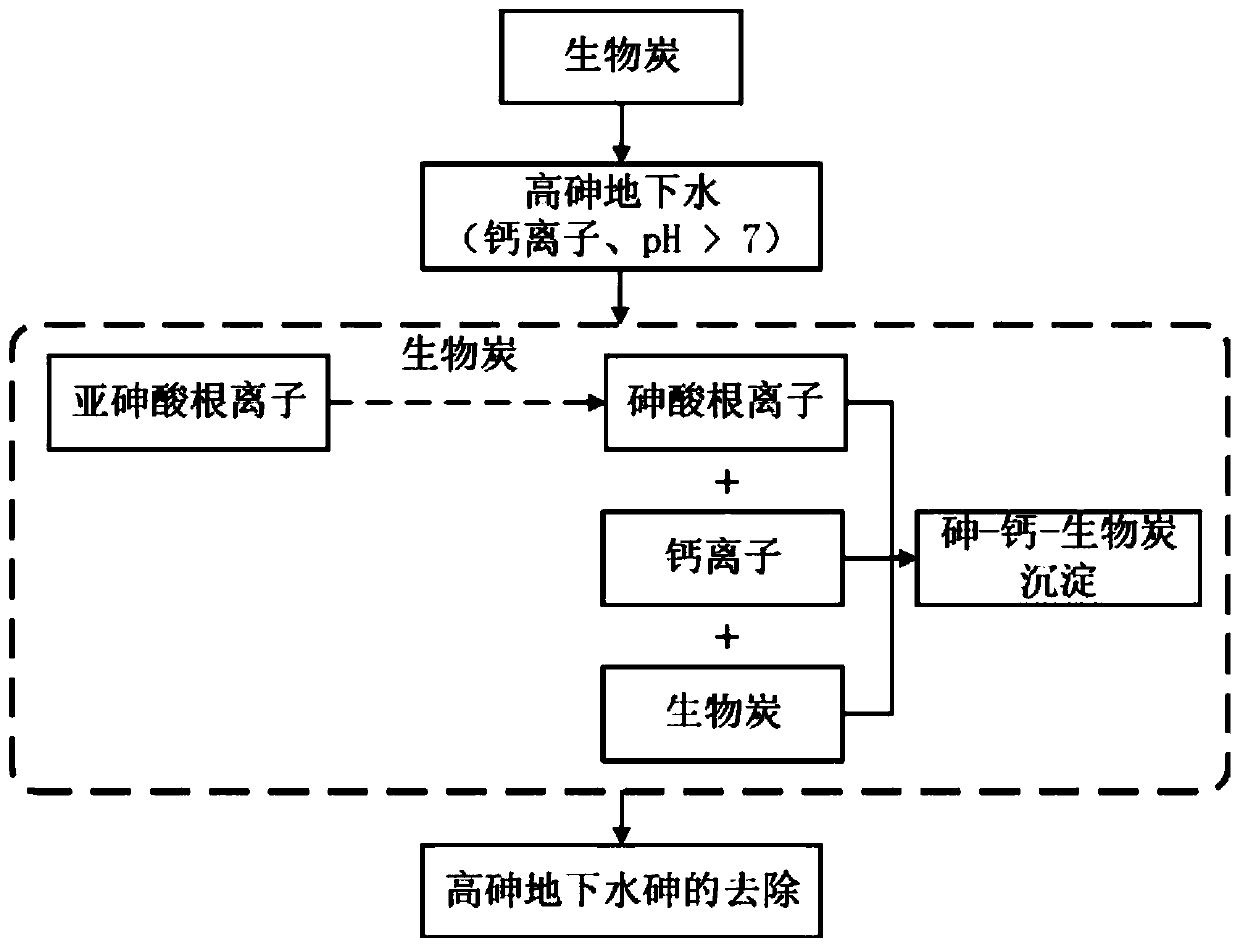
Method for removing arsenic in calcium-rich high-arsenic groundwater by using biochar
ActiveCN110143661AAchieve in situ removalAchieve removalWater contaminantsContaminated soil reclamationArsenic pollutionBiochar
The invention discloses a method for removing arsenic in calcium-rich high-arsenic groundwater by using biochar, belonging to the field of arsenic pollution treatment for groundwater. The method comprises the following steps: adding biochar into alkaline calcium-rich high-arsenic groundwater, and carrying out a stirring reaction for at least 4 hours to remove arsenic dissolved in the groundwater,wherein the concentration of arsenic contained in the groundwater is 0.1-10 mg / L, and the concentration of calcium ions is 20-2000 mg / L. According to the invention, the specific calcium-rich high-arsenic groundwater is used as the object in which arsenic is to be removed; oxidizing active groups on the surface of the biochar are employed to oxidize low-valent arsenite in the groundwater into high-valent arsenate, and the generated arsenate reacts with the calcium ions and biochar to formation an arsenic-calcium-biochar precipitate; so the characteristic that the groundwater is rich in calciumand is alkaline is fully utilized, oxidation and adsorption means are organically combined to achieve the purpose of integrated removal of arsenic from the groundwater, and the method can effectivelysolve the common problems of low efficiency, high operating cost, complicated operation and the like in conventional methods for removing arsenic in groundwater.
Owner:HUAZHONG UNIV OF SCI & TECH
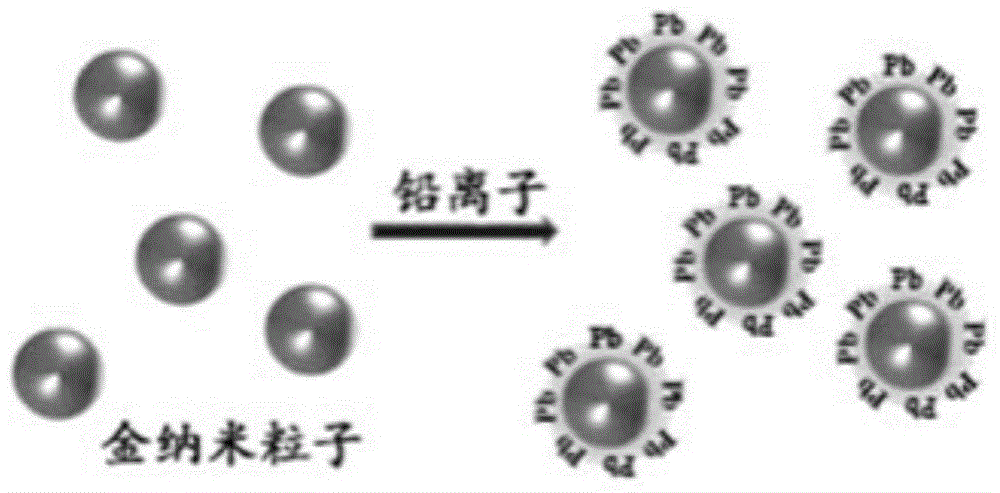
Gold nanometer probe, gold nanometer probe testing paper, preparation methods of gold nanometer probe and gold nanometer probe testing paper, and applications of gold nanometer probe and gold nanometer probe testing paper
InactiveCN105466867AHigh selectivityHigh sensitivityMaterial analysis by electric/magnetic meansColor/spectral properties measurementsNanoparticleAcid concentration
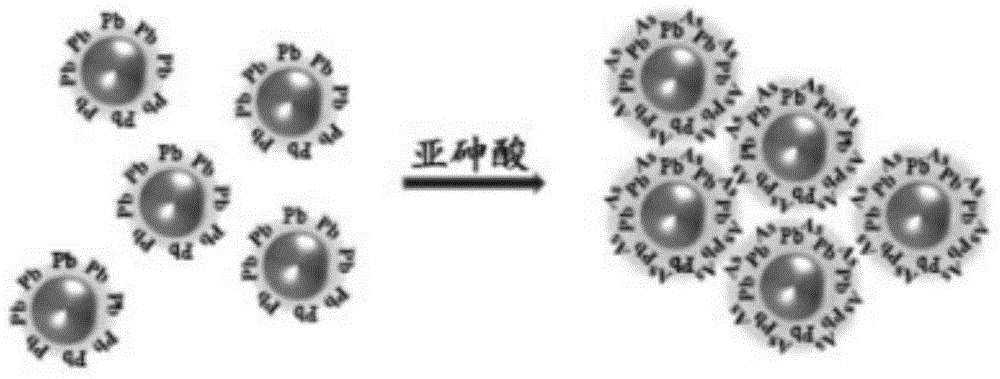
The present invention discloses a gold nanometer probe, which comprises gold nanoparticles and a lead compound coated on the surface of the gold nanoparticles. The preparation method comprises: preparing a gold nanoparticle solution; and adding the gold nanoparticle solution and a lead ion solution into a glycine-sodium hydroxide solution to carry out a reaction so as to make the lead compound be coated on the surface of the gold nanoparticles to obtain the gold nanometer probe. The present invention further discloses gold nanometer probe testing paper, which comprises a testing paper main body and the gold nanometer probe adsorbed on the testing paper main body. The present invention further discloses applications of the gold nanometer probe and the gold nanometer probe testing paper in detection of the arsenious acid concentration in water samples.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Material for purication anion polluted water
InactiveCN1830822AEfficient removalStrong exchange abilityWater/sewage treatment by ion-exchangeWhite powderIon
A laminar material for cleaning the anion-polluted water by removing the anions (F ions, arsenous radicals, or arsenic radicals) is prepared through proportionally dissolving Mg (or Zn) source and Al source in water, dissolving alkali and anions in water, quickly mixing them, stirring for 48-144 hr, pump filtering, water washing and baking to obtain white powder.
Owner:JILIN UNIV
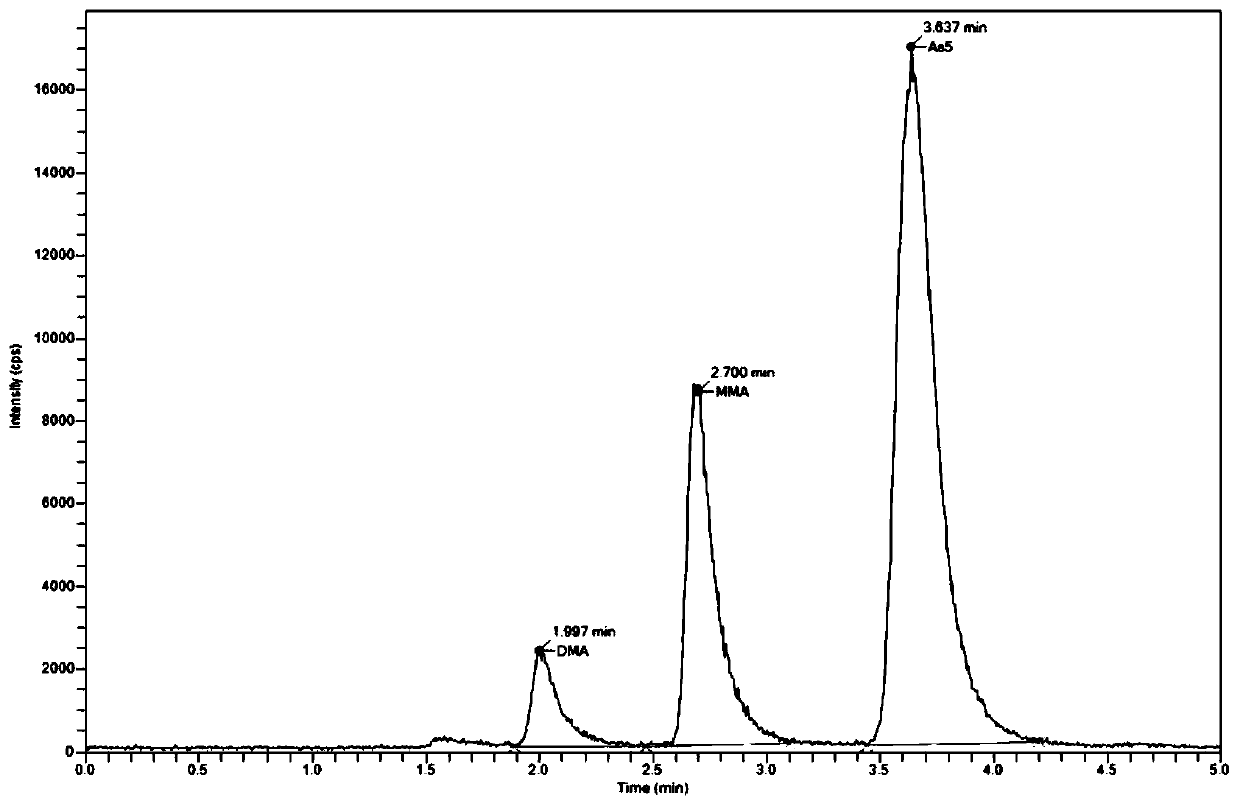
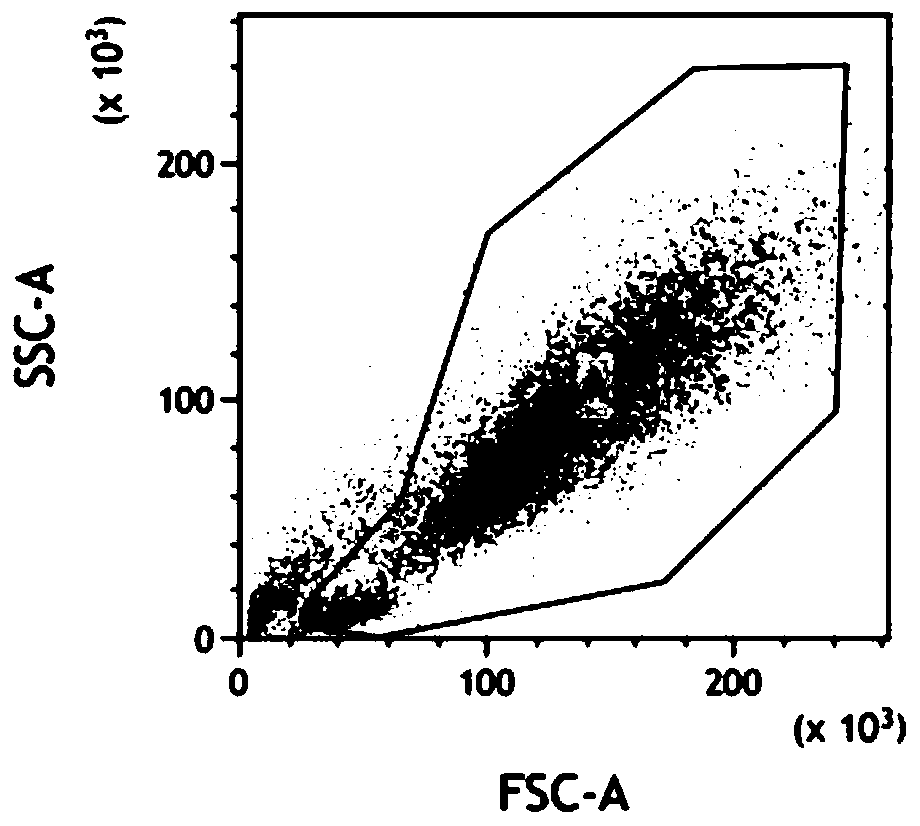
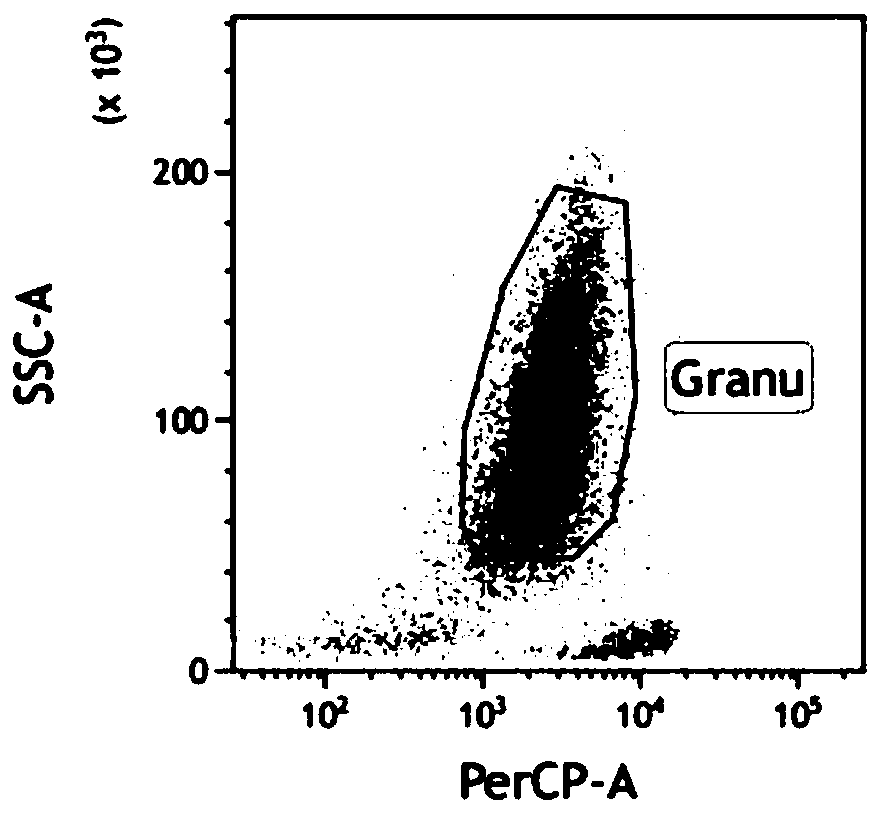
Method for measuring concentrations of four arsenic compounds in granulocytes by using HPLC (High Performance Liquid Chromatography)-ICP (Inductively Coupled Plasma)-MS method and application
ActiveCN110632217AMeet the sensitivity requirementsHigh precisionComponent separationInductively coupled plasmaToxic reaction
The invention relates to a method for measuring the concentrations of four arsenic compounds in granulocytes by using an HPLC (High Performance Liquid Chromatography)-ICP (Inductively Coupled Plasma)-MS method and an application, and belongs to the technical field of blood sample detection. In order to solve the problem that the existing method cannot accurately measure the concentrations of fourdifferent arsenic compounds AsIII, AsV, DAV and MMVA in granulocytes, the invention provides a method for measuring the concentrations of four arsenic compounds in granulocytes by using the HPLC-ICP-MS method. The method comprises the steps of determining a detection condition, preparing a standard solution, drawing a standard curve, collecting and processing a granulocyte sample, performing sample detection and data processing. According to the method for measuring the concentrations of four arsenic compounds in granulocytes by using the HPLC-ICP-MS method and the application, each arsenic compound in granulocytes can be effectively extracted and accurately quantified. When the method is applied to the detection of the concentrations of four arsenic compounds in peripheral blood granulocytes of a patient suffering from APL (Acute Promyelocytic Leukemia, the metabolism and distribution characteristics of each arsenic form can be clarified, a technical basis is provided for revealing ofthe action mechanism of arsenic acid and reduction of toxic reactions, and when the method is used for assisting clinics, personalized medicine and targeted therapy are implemented.
Owner:HARBIN MEDICAL UNIVERSITY
Process for conducting resource recycling on copper and arsenic from arsenic-containing waste acid
InactiveCN105695743ARealize resourcesWorkmanship is feasibleProcess efficiency improvementEnvironmental engineeringArsenous acid
The invention discloses a process for conducting resource recycling on copper and arsenic from arsenic-containing waste acid. Resource recycling of the arsenic-containing waste acid is realized through the steps of neutralization and impurity removal, arsenic precipitation, washing, leaching, evaporation crystallization and preparation of arsenic trioxide through dissolution. According to the process, arsenic in the arsenic-containing waste acid is precipitated, converted and prepared into arsenic trioxide, and the defects of preparing arsenic trioxide by reducing copper arsenite with sulfur dioxide are overcome; high-standard arsenic trioxide is obtained, and treatment of the waste acid and reutilization of copper and arsenic resources are realized.
Owner:方端
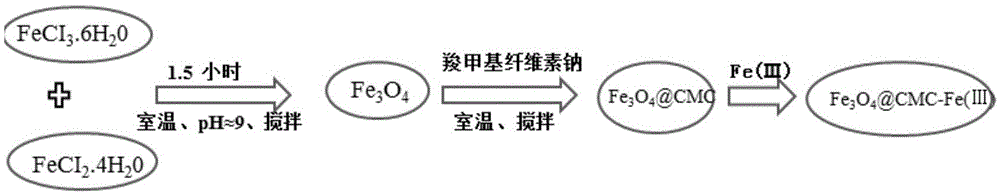
Preparation method of Fe (III)-containing magnetic nano material
ActiveCN105617977AReduce energy consumptionHigh yieldOther chemical processesWater contaminantsSorbentReaction temperature
The invention discloses a preparation method of a Fe (III)-containing magnetic nano material, relates to a preparation method of an iron-containing magnetic nano material, and aims at solving the problems that in an existing preparation method of an iron-containing magnetic nano material for adsorbing arsenic, the reaction temperature is high, energy consumption is high, and the reaction conditions are complex. The preparation method comprises the steps that 1, magnetic particles are prepared; 2, blending is performed to obtain the Fe (III)-containing magnetic nano material. The invention further discloses application of the Fe (III)-containing magnetic nano material, and the Fe (III)-containing magnetic nano material is used for adsorbing arsenous acid ions by serving as an adsorbing agent. The preparation method has the advantages that energy consumption is low, and the reaction can be performed under stirring at room temperature; environmental friendliness is achieved, cheap metal salts FeCl3.6H2O and FeCl2.4H2O and sodium carboxymethylcellulose are used as reagents, deionized water is taken as solvent, and no template or other toxic reagent needs to be added; the yield is high, and large-scale preparation can be achieved. The preparation method is mainly used for preparing the Fe (III)-containing magnetic nano material.
Owner:NORTHEAST INST OF GEOGRAPHY & AGRIECOLOGY C A S
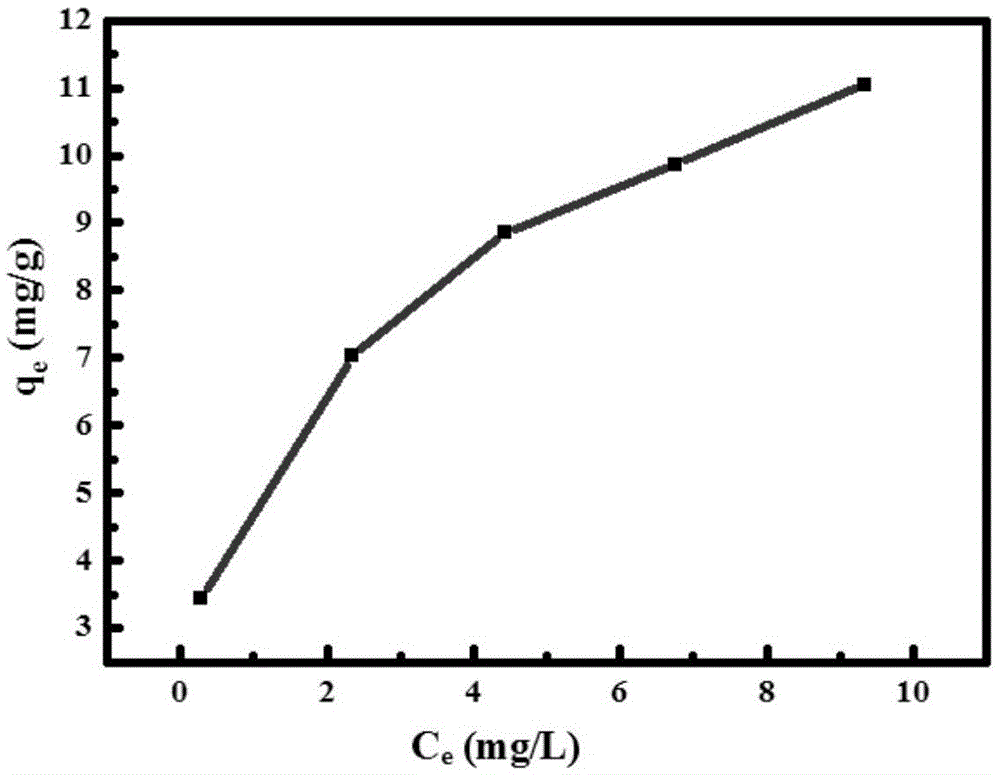
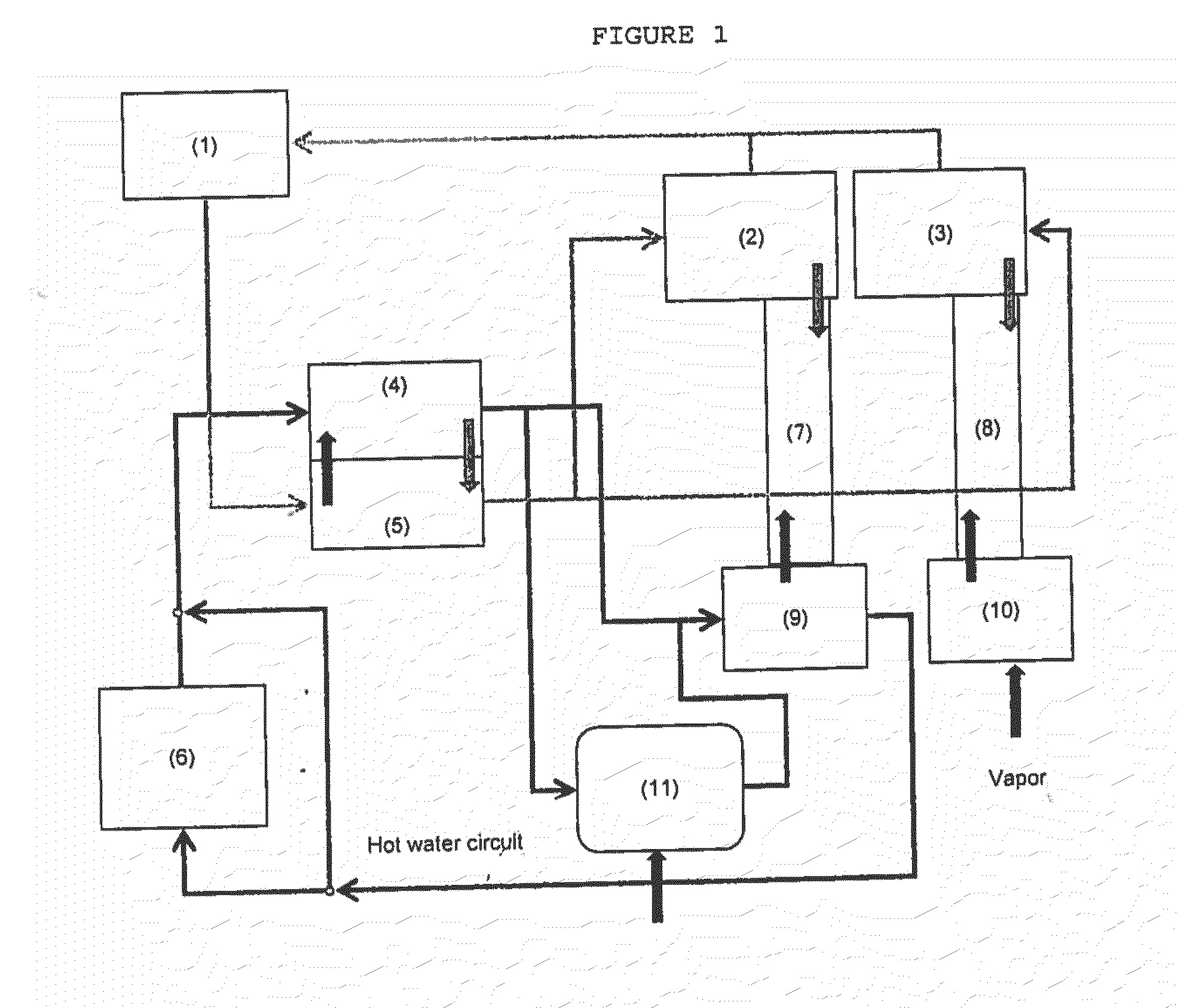
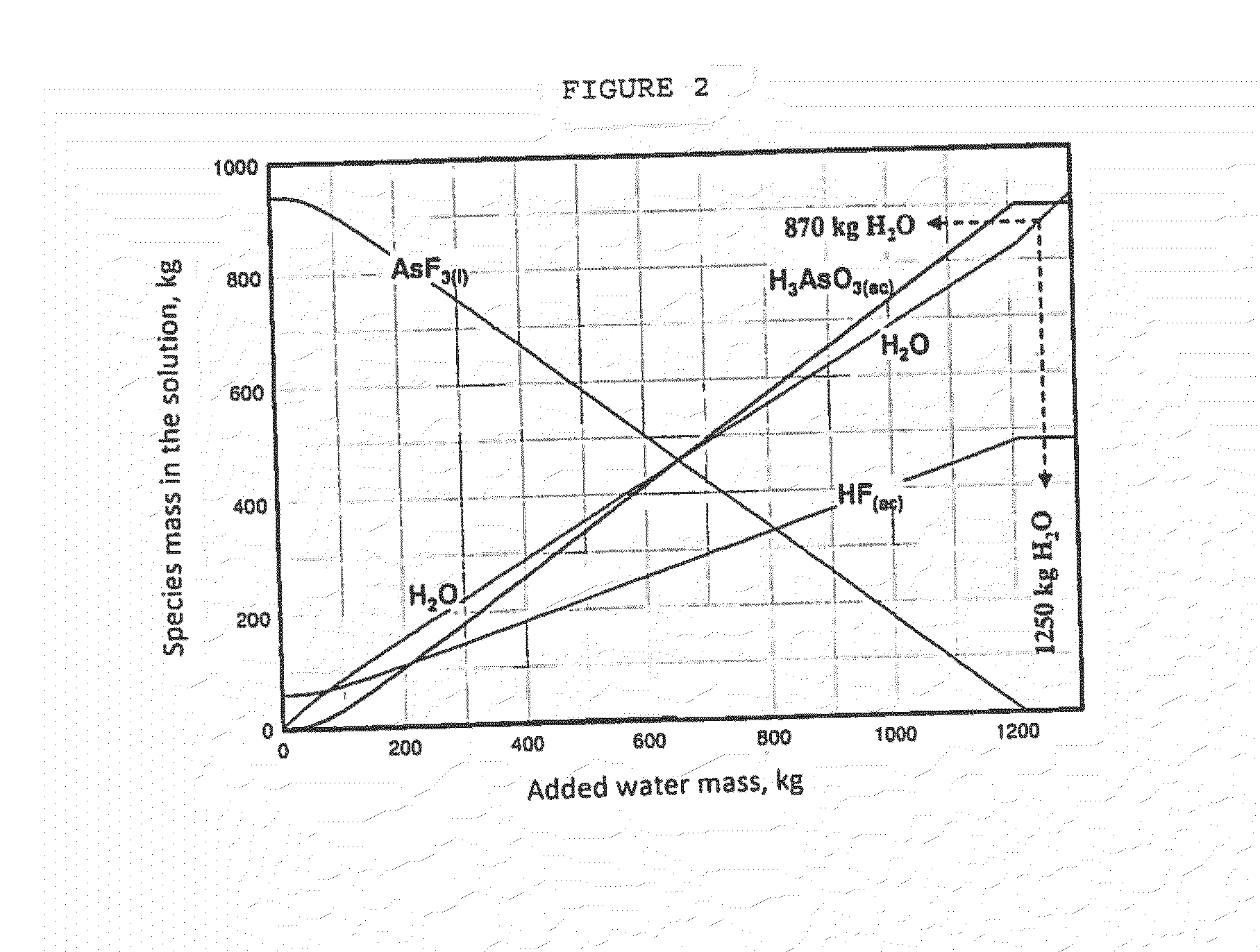
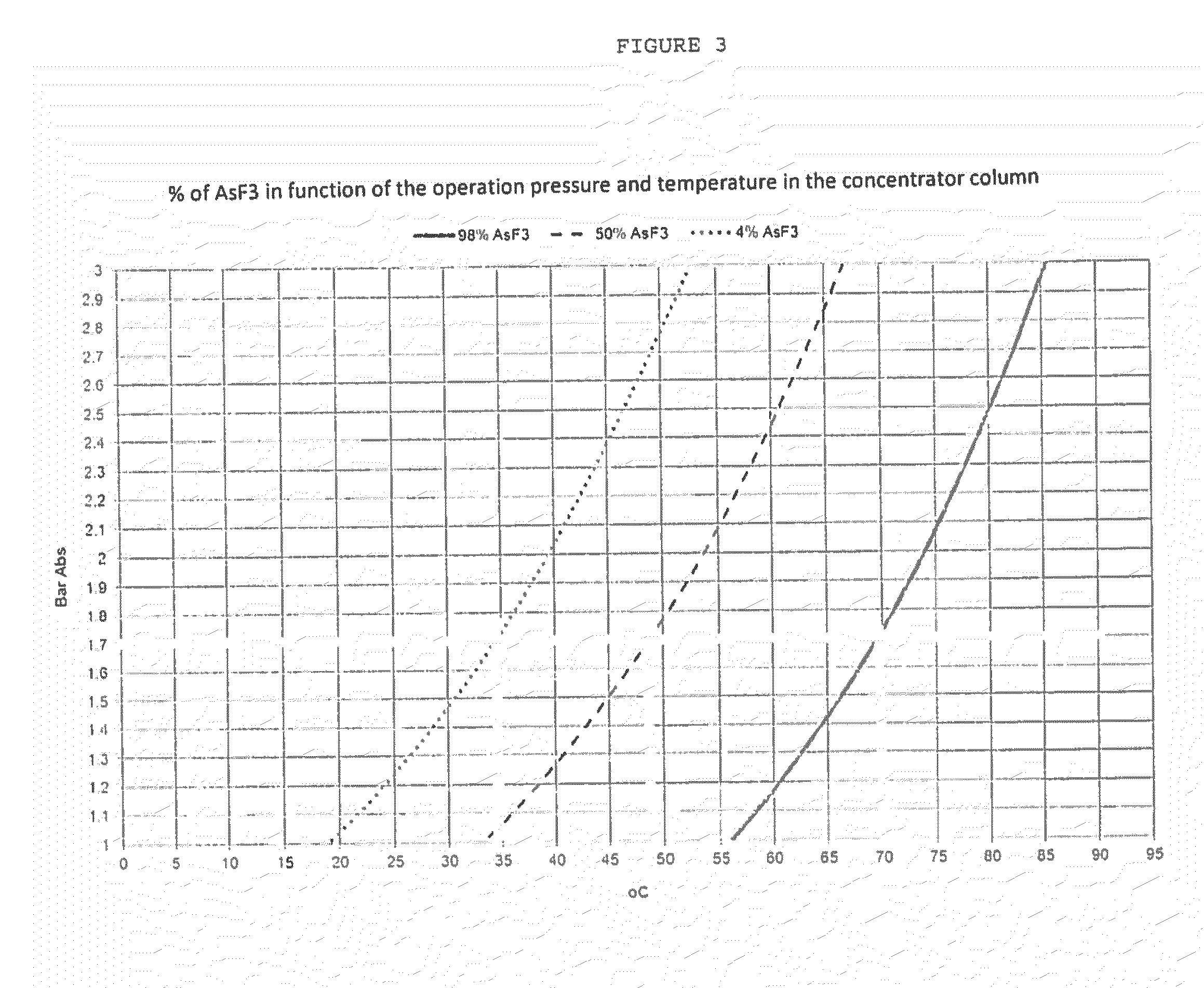
Process for purification of hydrofluoric acid including obtaining arsenious acid by-product
ActiveUS20160176711A1Arsenic oxides/hydroxides/oxyacidsDistillation regulation/controlProcess engineeringEnvironmental engineering
The process for purification of hydrofluoric acid as herein disclosed is able to reduce the content of heavy metals, including arsenic, to values lower than 5 ppm without using any chemicals and with an integrated design of hot and cold streams that provide low energy consumption. Likewise, it achieves the extraction of heavy metals, especially arsenic, with minimal waste generation and maintaining its original oxidation state, which for the case of arsenic is +3, so that the residue can be converted into a product with commercial value, that in this process is obtained as arsenious acid.The process consists of four systems: HF purification system, arsenic concentration system, hot water system, and cold water system. This document discloses the synchronized operation of these 4 systems that together achieve what is mentioned in the previous paragraph.
Owner:MEXICHEM FLUOR DE CAPITAL VARIABLE
Method for preparing arsenic adsorbent and method for treating waste water
InactiveCN102698703BImprove adsorption capacityThe synthesis method is simpleOther chemical processesWater/sewage treatment by sorptionSorbentWastewater
The invention provides a method for preparing an arsenic adsorbent. The arsenic adsorbent has zirconium oxide of nano-pore structure. The material can effectively treat arsenate and arsenite in the waste water, and can be recycled after waste water treatment. Another objective of the invention is to provide a method for treating waste water containing arsenate or arsenite ions by the arsenic adsorbent prepared by the method. The method contains a 'regeneration' step, so that the arsenic adsorbent can be reused cyclically.
Owner:CHONGQING THREE GORGES UNIV
Rapid iodized salt detection liquid
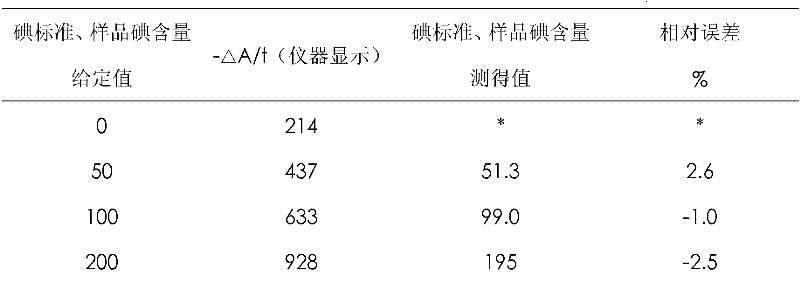
ActiveCN103472057AQuick responseThe detection process is fastMaterial analysis by observing effect on chemical indicatorIodised saltPreservative
The invention provides rapid iodized salt detection liquid. The rapid iodized salt detection liquid is prepared from the following raw materials: a reducing agent, namely arsenious acid with the concentration of 0.005-0.010g / mL, an indicator, namely amylose with the concentration of 0.002-0.006g / mL, a preservative, namely zinc chloride with the concentration of 0.0005-0.0015g / mL and sodium hydroxide with the concentration of 0.0100-0.0150g / mL; the pH of the detection liquid is 1-1.5. The rapid iodized salt detection liquid provided by the invention is suitable for the detection of iodized salt, the iodine preparation of which is potassium iodate, and iodized salt, the iodine preparation of which is potassium iodide. The rapid iodized salt detection liquid has the advantages that the reducing agent is the arsenious acid, so that the detection speed is relatively rapid; the appropriate pH and the purified amylose are used for preparation, so that the sensitivity and stability of reaction colors are ensured; the zinc chloride is used as the preservative, so that after the rapid iodized salt detection liquid is stored for a long term, the color gradation of the rapid iodized salt detection liquid is still obvious during detection.
Owner:HARBIN MEDICAL UNIVERSITY
Method for harmlessly treating arsenic in arsenic-containing waste liquid
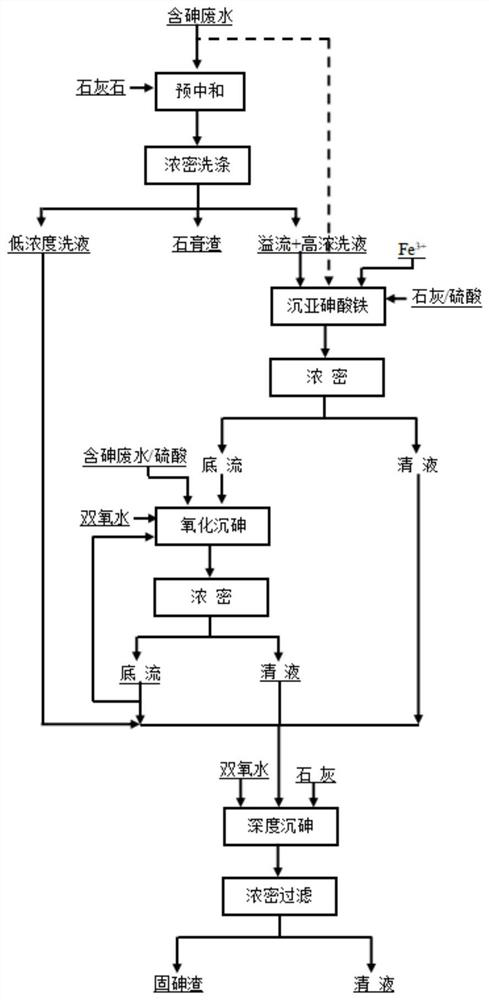
InactiveCN112897739AReduce consumptionProcessing cost economyArsenites/arsenatesWater contaminantsEnvironmental engineeringArsenous acid
The invention discloses a method for harmlessly treating arsenic in arsenic-containing waste liquid, which is characterized in that the arsenic-containing waste liquid is harmlessly treated by adopting a process of settling iron arsenite, oxidizing and settling arsenic and deeply settling arsenic, the arsenic is solidified in a most stable scorodite form and enters a landfill for landfill, and the treated waste liquid can be directly recycled or discharged. The method can be suitable for harmless treatment of arsenic in arsenic-containing solutions with various concentrations.
Owner:ZIJIN MINING GROUP +1
Detection reagent and method for urinary and salivary iodine via microplate reader after normal-temperature pretreatment
InactiveCN109540877AReduce the error valueReduce sample loading timeMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationSodium hypochlorite solutionArsenous acid
The invention discloses a detection agent for urinary and salivary iodine via a microplate reader after normal-temperature pretreatment. The agent is characterized by comprising an agent A, a 0.5-2.2mol / L of sodium hypochlorite solution NaClO in which Mr=74.44; an agent B, a 0.5-2.2mol / L of sulphuric acid solution H2SO4 in which Mr=98.078; an agent C, a0.01-0.2mol / L of arsenous acid solution H3AsO3; an agent D, a 0.01-0.2mol / L of ammonium cerous sulphate solution Ce(NH4)(SO4)4.H2O in which Mr=632.6, and a calibration liquid, a iodine standard series solution (a Potassium Iodate solution KIO3,Mr=214.0).
Owner:王九宏
Pharmaceutical compositions comprising sodium meta arsenite for treatment of multiple myeloma
The present application relates to pharmaceutical compositions and methods for treatment of urogenital diseases and bone metastasis in a human, which pharmaceutical composition contains an effective amount of arsenous acid alkaline or earth alkaline metal salt and / or a pharmaceutically acceptable adjuvant. According to the present invention, the alkaline arsenous acid metal salt is sodium meta-arsenita (AsO2Na) or potassium meta-arsenite (AsO2K). The effective amount of arsenous acid alkaline or earth alkaline metal salt is 0.0001-1500 mg / kg, preferably 1-1000 mg / kg, more preferably 1-150 mg / kg, and most preferably 50-100 mg / kg of body weight / day. The administration form of the pharmaceutical compositions of the invention is preferably oral, such as a tablet, capsule, powder and / or solution with a pharmaceutically acceptable carrier, diluent or excipient.
Owner:KOMINOX
Method for preparing arsenic trioxide from arsenic sulfide waste
InactiveCN102115166BHigh recovery rateLow costSolid waste disposalArsenic compoundsSolubilityArsenic sulfide
The invention provides a method for preparing arsenic trioxide from arsenic sulfide waste. The arsenic sulfide waste is soaked in a sodium hydroxide solution to extract arsenic sulfide, arsenic sulfide mixes with an excessive amount of arsenic acid, and the mixture reacts at a temperature ranging from 40 DEG C to 95 DEG C for 0.5 to 3 hours, to produce a mixture containing arsenous acid and elemental sulfur; the mixture containing arsenous acid and elemental sulfur mixes with 2 to 5 times (by mass) of water and then oxygen gas is introduced so that arsenous acid is oxidized into arsenic acid,and the reaction product is filtered to remove elemental sulfur and thus to produce an arsenic acid solution; and the arsenic acid solution is reduced by sulfur dioxide gas to arsenous acid with a lower solubility, the reaction liquid is distilled under a reduced pressure to produce a saturated solution of arsenous acid, the saturated solution of arsenous acid is cooled so that arsenous acid is crystallized and then filtered to produce a filter cake of arsenous acid, and the filter cake of arsenous acid is dried and pulverized to obtain an arsenic trioxide product. The method has a short process flow, increases the recovery rate of arsenic, avoids the production of secondary pollutants, adopts simple equipment, greatly reduces the cost of arsenic recovery and is worthy to be widely applied.
Owner:JIANGSU ZHONGKE MACHINERY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com