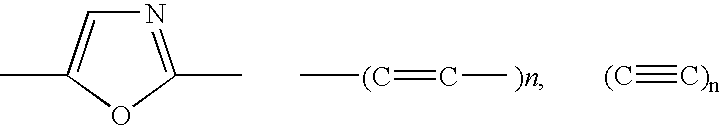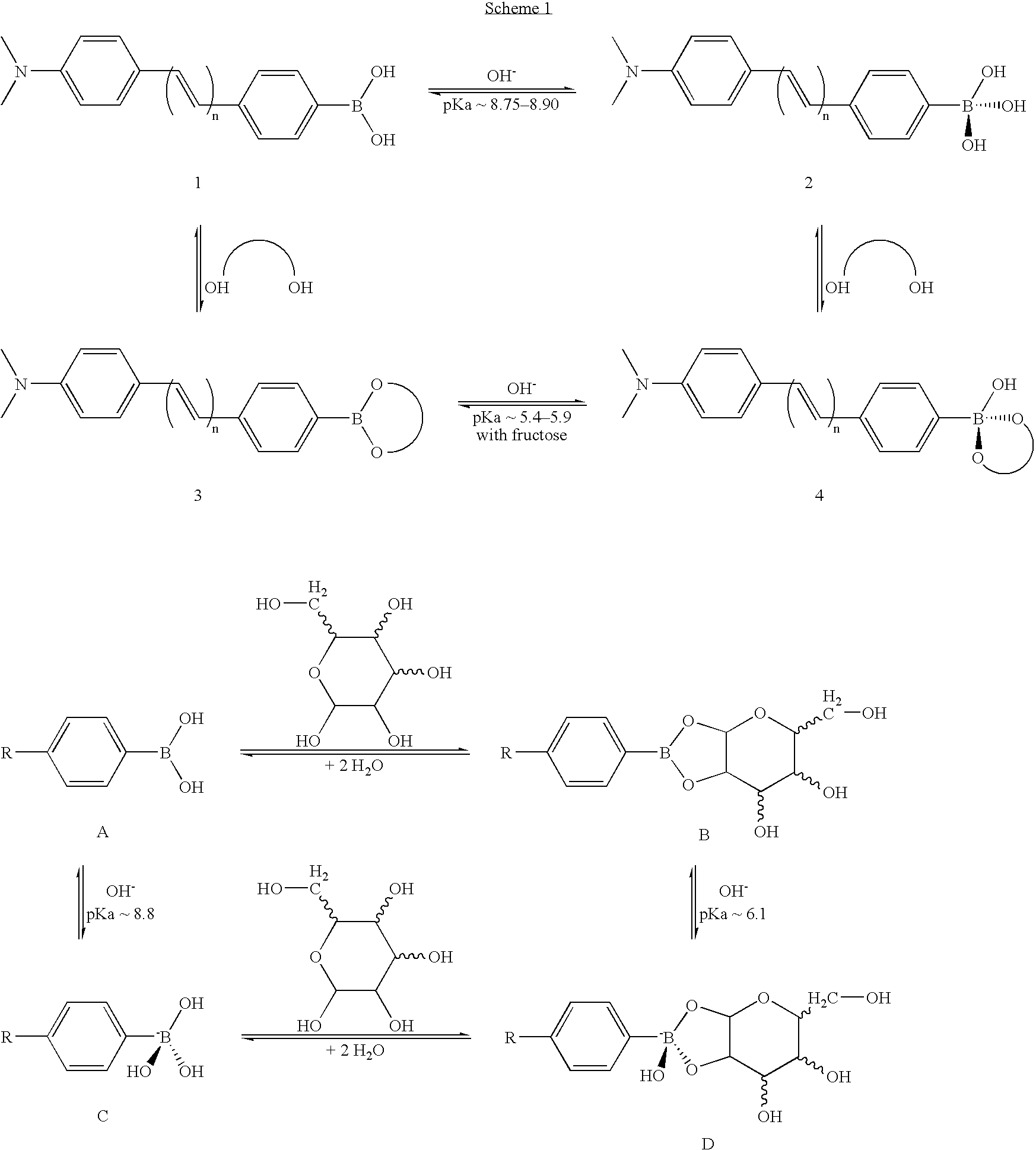Fluorescent probes for saccharrides
a technology of fluorescent probes and saccharides, applied in the field of fluorescent probes for saccharides, can solve the problems of inability to meet the needs of continuous implantable sensors, limited use of enzymes, and low stability, and achieve the effects of reducing sensitivity, increasing intensity, and reducing fluorescence emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
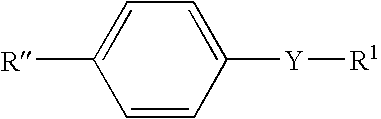
[0109] The probes of this Example are based on donor / acceptor diphenylbutadiene and diphenylhexatriene derivatives involving the boronic acid group that display useful shifts and intensity changes in their emission spectra. These changes are induced by the changes of the electron-withdrawing property between the boronic acid group and its anionic form. Compared to the analogous stilbene probes, which also displays the charge transfer mechanism, the charge transfer mechanism can be applied for longer wavelength probes. This mechanism could be extended to the development of red and / or near infrared probes using appropriate fluorophores. In addition, the charge transfer mechanism induces a change in the fluorophore lifetime of the probes, thus opening the door to the development of new probes for fluorescence lifetime based sensing for sugars.
[0110] This example demonstrates the effect of the wavelength of absorption and emission of the fluorophore on the efficiency on the CT mechanism...
example 3
[0123] In attempt to develop additional fluorescent probes for glucose detection, the following compound 6 was synthesized as shown in the following reaction scheme: 6
[0124] Compound 6 is readily synthesized from the reaction between the 2-amino-4'-dimethylaminoacetophenone hyrochloride (Synthesized from 4'-Dimethylaminoacetophenone (TCI america) according to the standard procedure described in the literature (57-58) and the 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoic acyl chloride, obtained from the commercially available 4-(4,4,5,5-tetramethyl-1,3,2-dio-xaborolan-2-yl)benzoic acid refluxed in SOCl.sub.2, following by the dehydratation of the product in POCl.sub.3 (59). Donor / acceptor derivatives of diphenyloxazole are well known to show high fluorescence quantum yields, long wavelength emission and to be very sensitive to small variations affecting the ICT property of the excited state. In this case, the ICT state is between the boronic acid, the electron-withdrawing gr...
example 4
[0129] The use of decay times (as opposed to intensities) would be a preferred method because the decay times are mostly independent of the probe concentrations or the signal of the fluorescence signal. The frequency-domain or phase-modulation method for sensing is well recognized for the instrumental simplicity, rapid data acquisition, and ability to detect small changes in phase angle or lifetime. A mean lifetime can be measured at a single modulation frequency using simple light emitted diodes or laser diodes (60-61). Importantly, lifetime can be measured in highly scattering media (62-63), and have been successfully measured through several layers of chicken skin (64). We present, in this study, the evaluation for fluorescence lifetime based sensing of two anthrancenes compounds having the phenyl boronic acid group and showing removal of PET quenching upon binding glucose. The results show a considerable change in the phase angle and in the modulation for both compounds. Evaluat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com