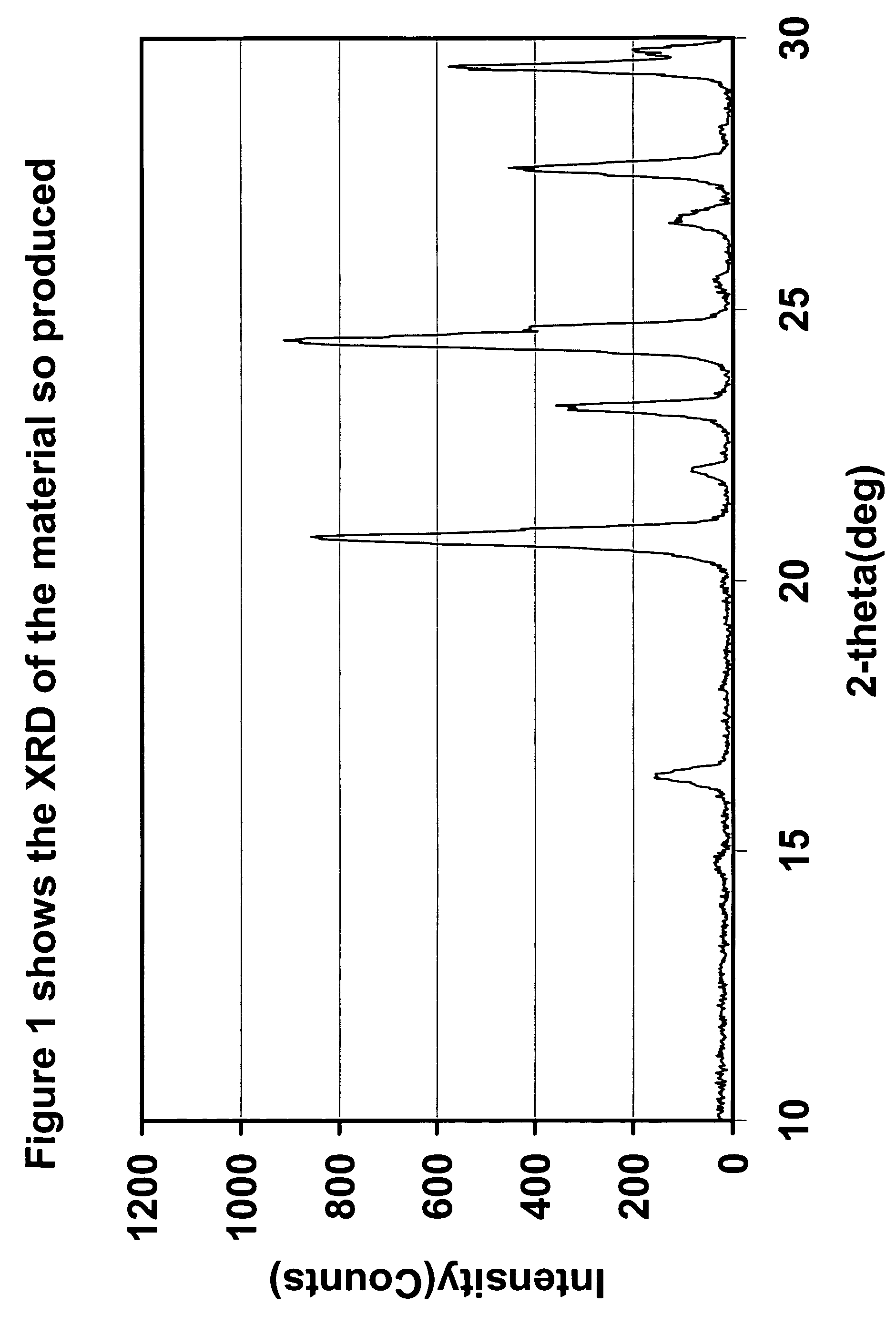
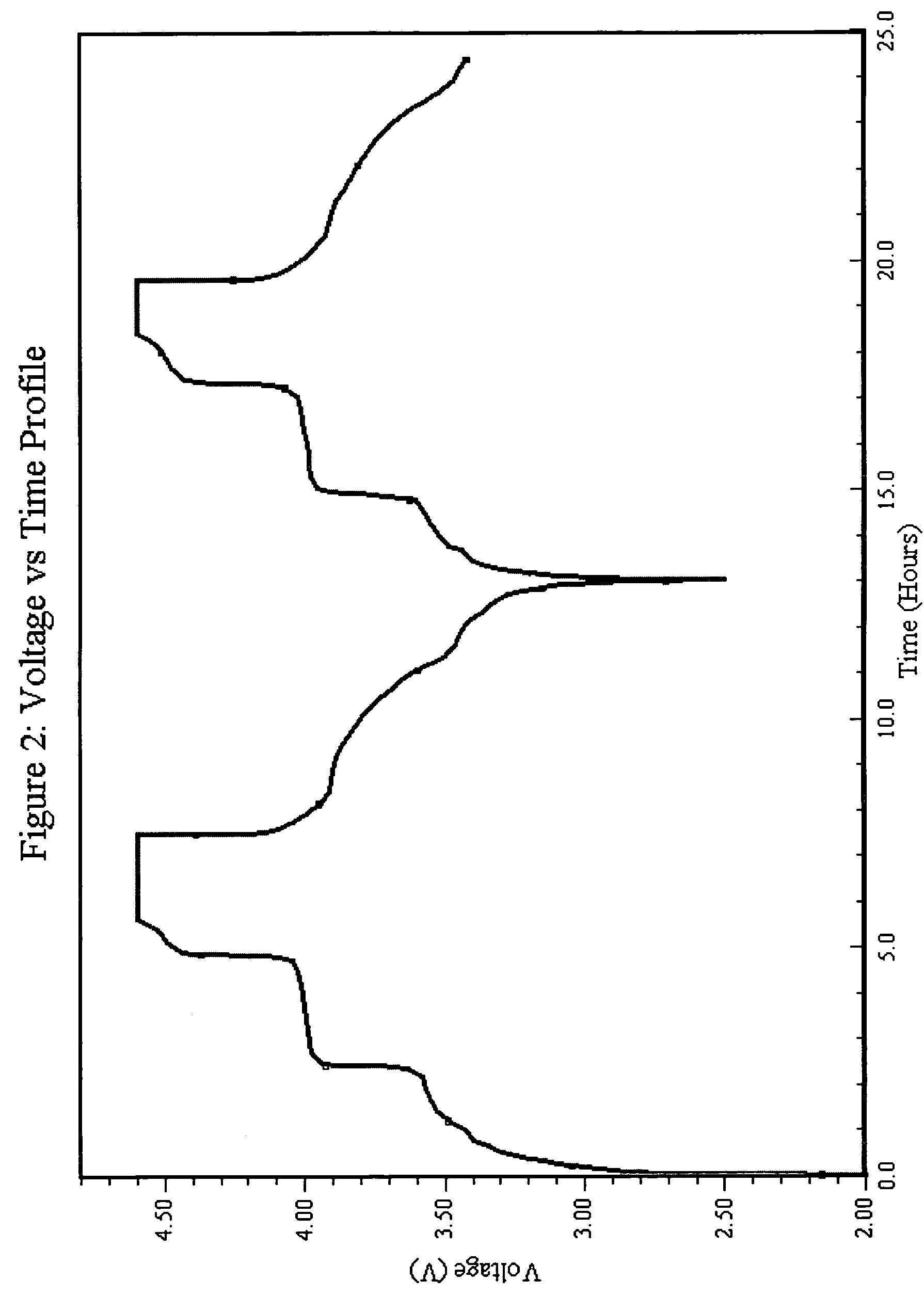
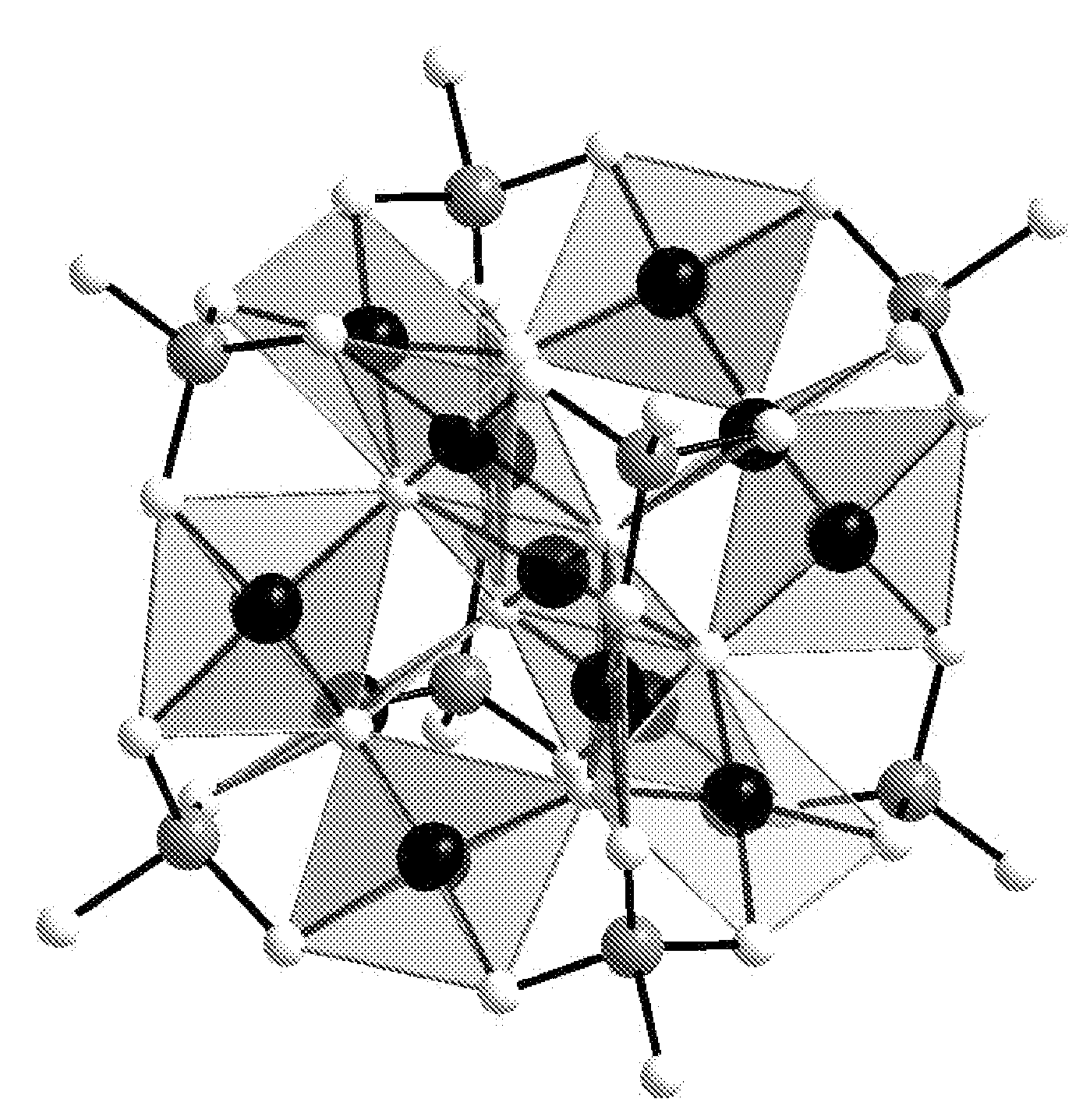
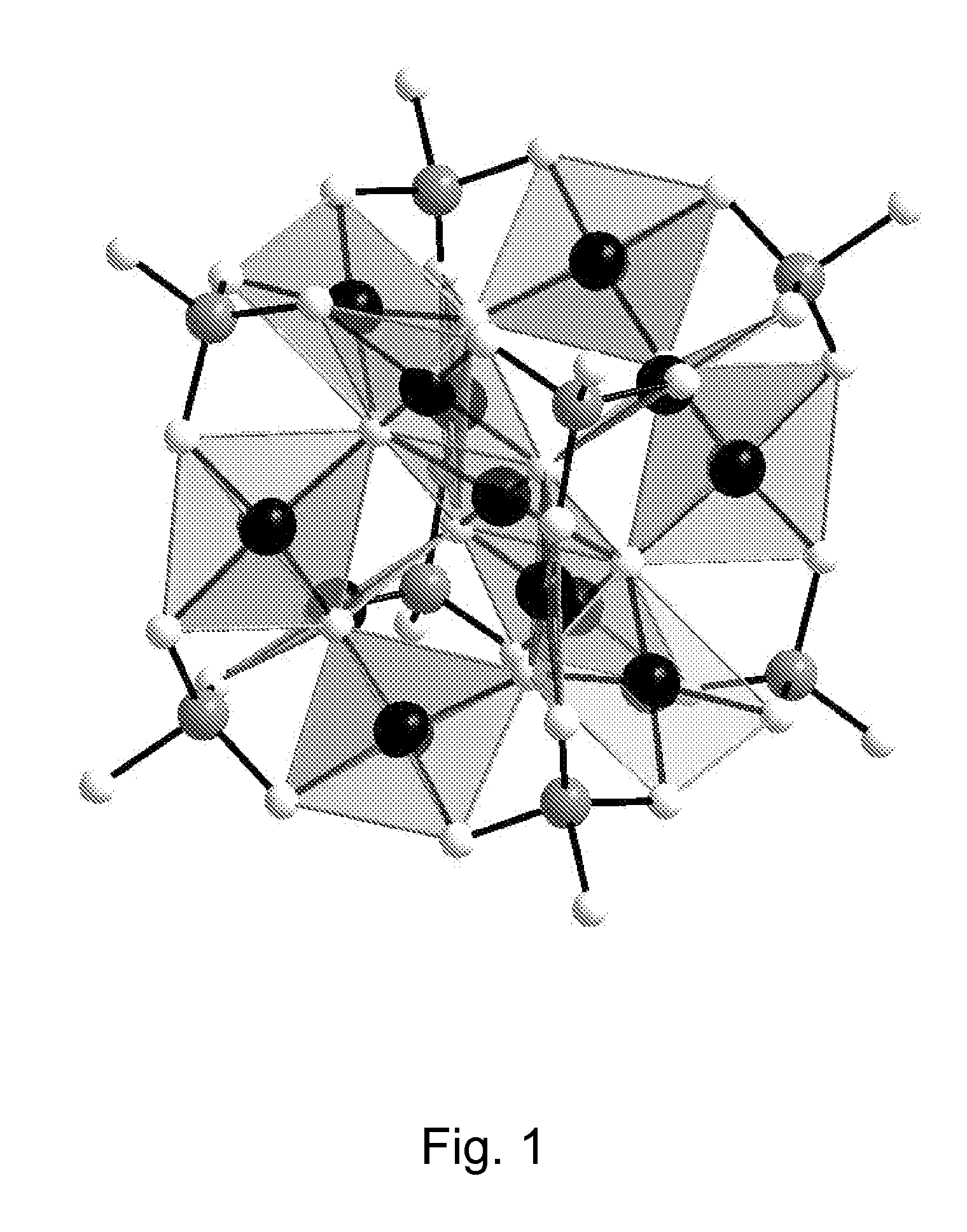
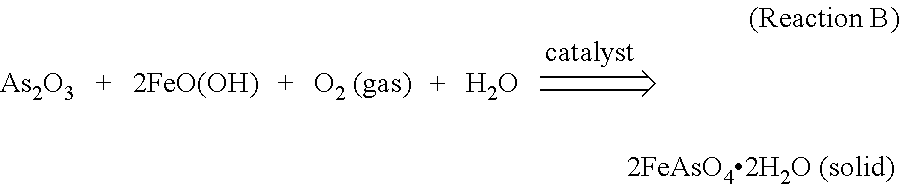Patents
Literature
102results about "Arsenites/arsenates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of forming inorganic pigments
The present invention provides a method of forming inorganic pigments using one or more metal alloys. Metal alloys used in the method of the invention are preferably milled to a mean particle size of less than about 10 microns, may be mixed with other metal oxides, and calcined in the presence of oxygen in a rotary kiln. Inorganic pigments formed in accordance with the method of the invention can be used in a wide variety of applications, including the coloration of glass matrixes, ceramic bodies, polymers, and paints.
Owner:FERRO CORP
Synthesis of cathode active materials
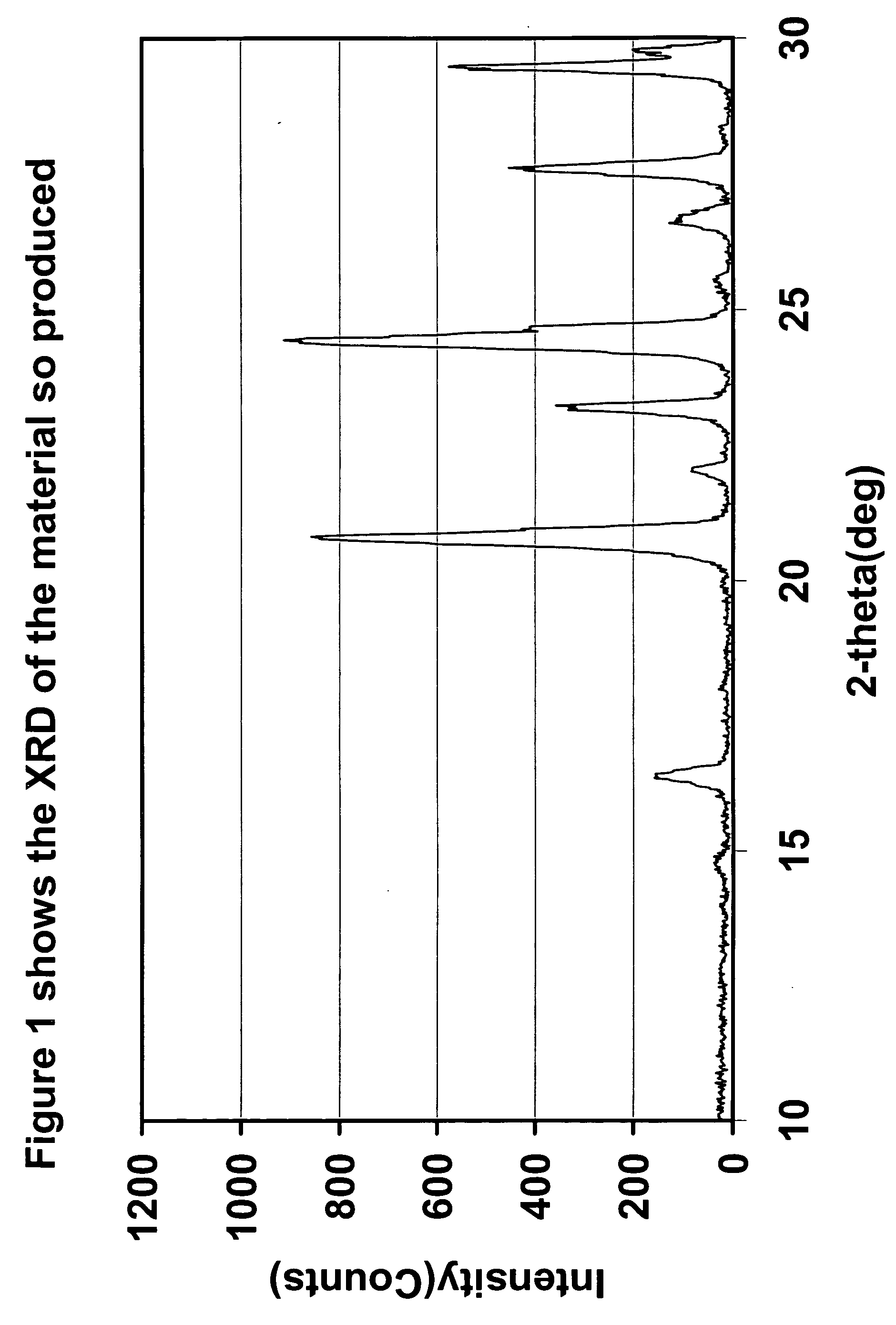
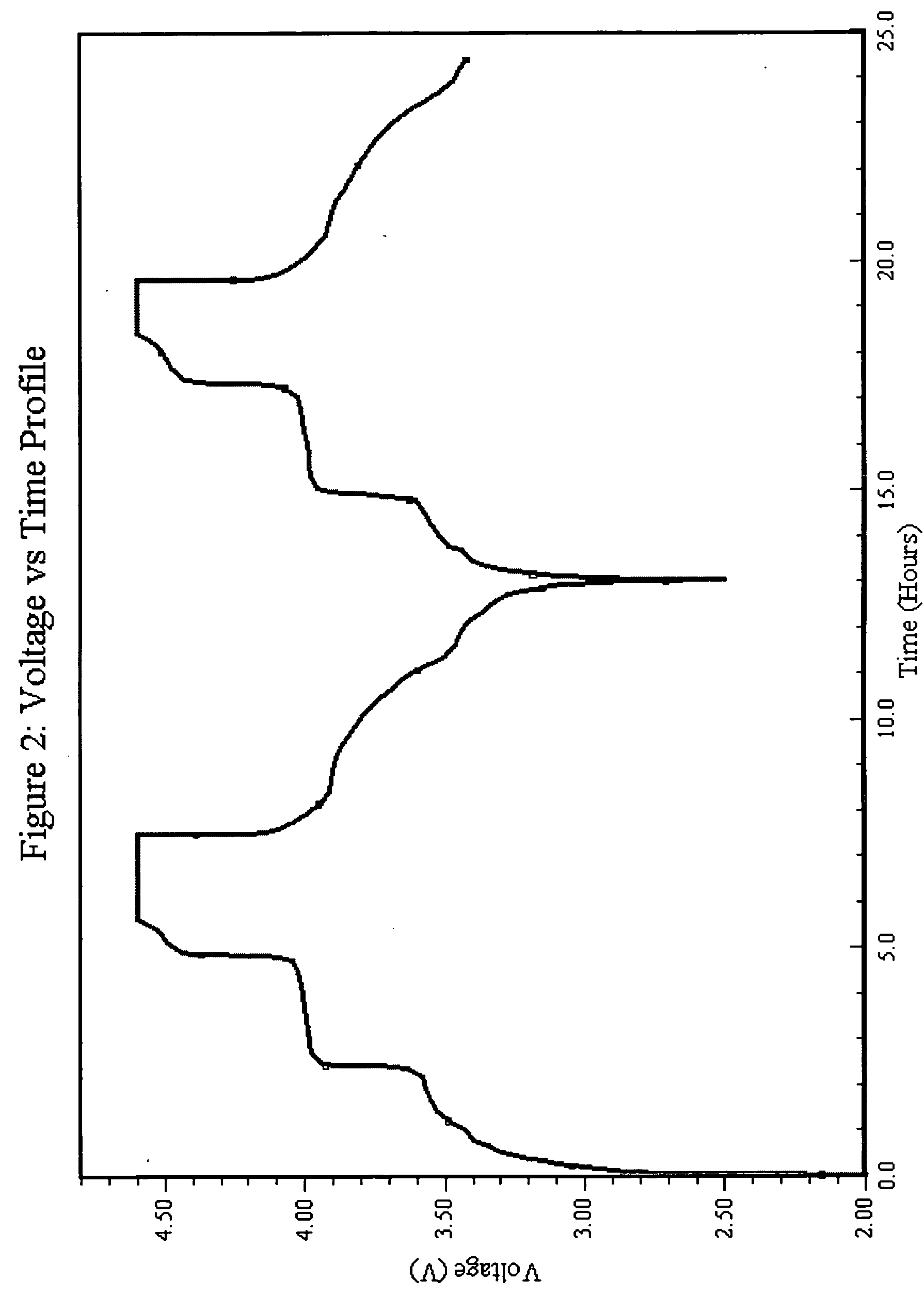
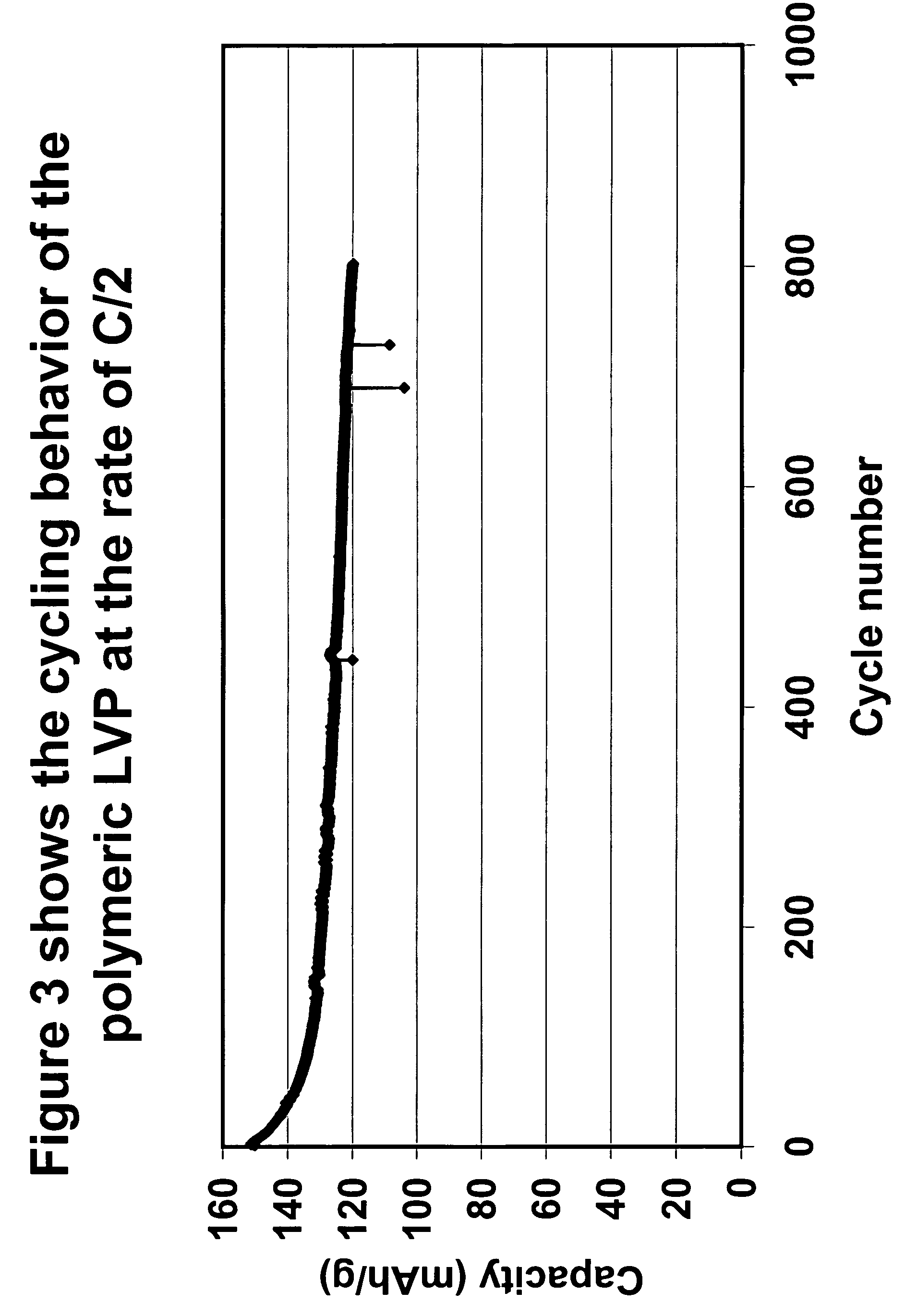
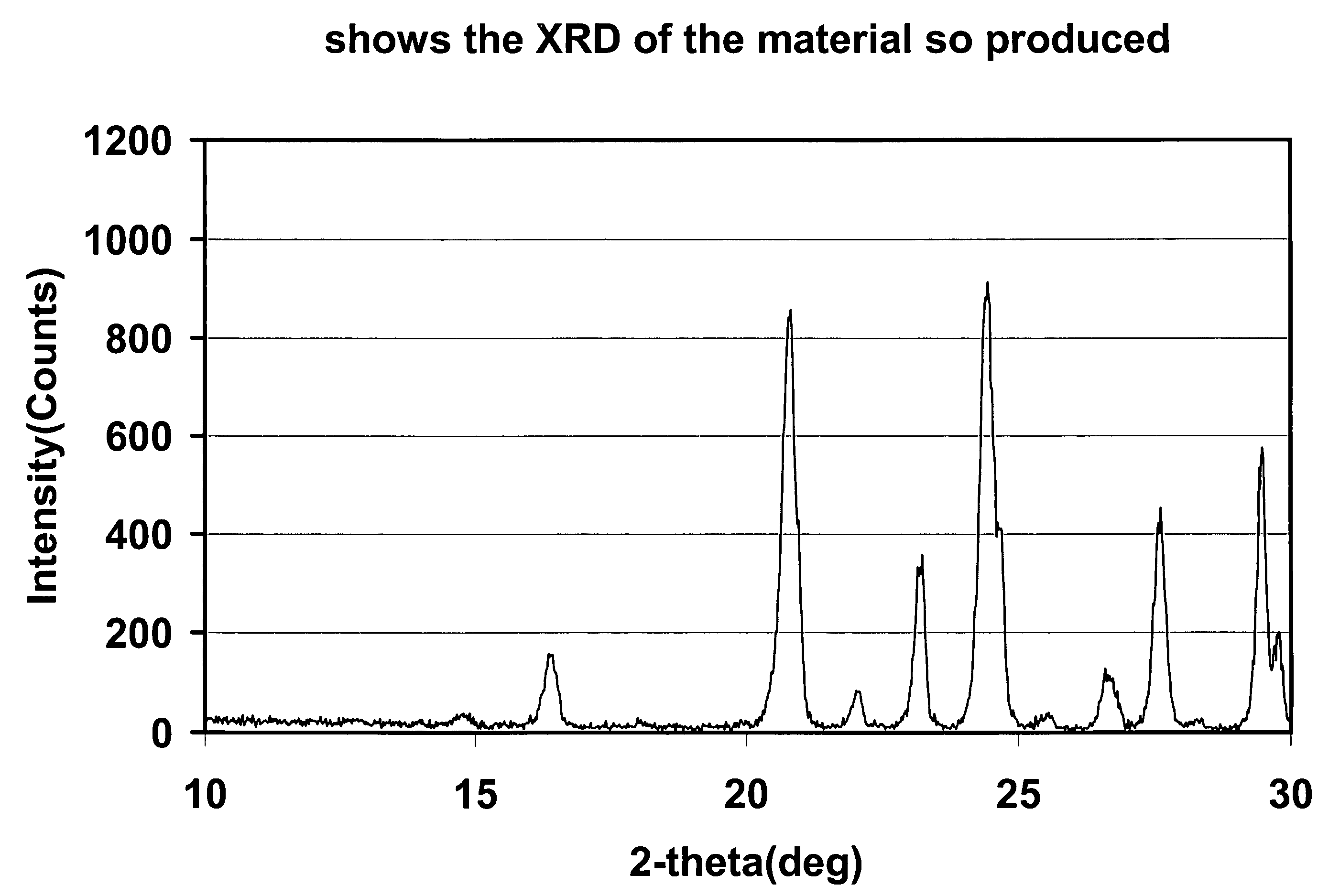
The present invention relates to a method for preparing an electroactive metal polyanion or a mixed metal polyanion comprising forming a slurry comprising a polymeric material, a solvent, a polyanion source or alkali metal polyanion source and at least one metal ion source; heating said slurry at a temperature and for a time sufficient to remove the solvent and form an essentially dried mixture; and heating said mixture at a temperature and for a time sufficient to produce an electroactive metal polyanion or electroactive mixed metal polyanion. In an alternative embodiment the present invention relates to a method for preparing a metal polyanion or a mixed metal polyanion which comprises mixing a polymeric material with a polyanion source or alternatively an alkali metal polyanion source and a source of at least one metal ion to produce a fine mixture and heating the mixture to a temperature higher than the melting point of the polymeric material, milling the resulting material and then heating the milled material. It is another object of the invention to provide electrochemically active materials produced by said methods. The electrochemically active materials so produced are useful in making electrodes and batteries.
Owner:LITHIUM WERKS TECH BV +1
Synthesis of cathode active materials
Owner:LITHIUM WERKS TECH BV +1
Novel Heteropolyanions With Late Transition Metal Addenda Atoms And Process For Their Preparation
The invention relates to polyoxometalates represented by the formula (An)m+ [M13X8RqOy]m− or solvates thereof, wherein A represents a cation, n is the number of the cations, m is the charge of the polyanion, M represents a transition metal selected from Pd, Pt, Au, Rh, Ir and mixtures thereof, X represents a heteroatom selected from As, Sb, Bi, P, Si, Ge, B, Al, Ga, S, Se, Te and mixtures thereof, and y is the number of oxygen atoms ranging from 32 to 40, a process for their preparation and their use for the catalytic oxidation of organic molecules.
Owner:EXXONMOBIL CHEM PAT INC
Method for treating arsenic-containing smoke dust
ActiveCN101817553ASolve the redissolution problemSolve the open circuit problemArsenites/arsenatesSulfur preparation/purificationPyriteWastewater
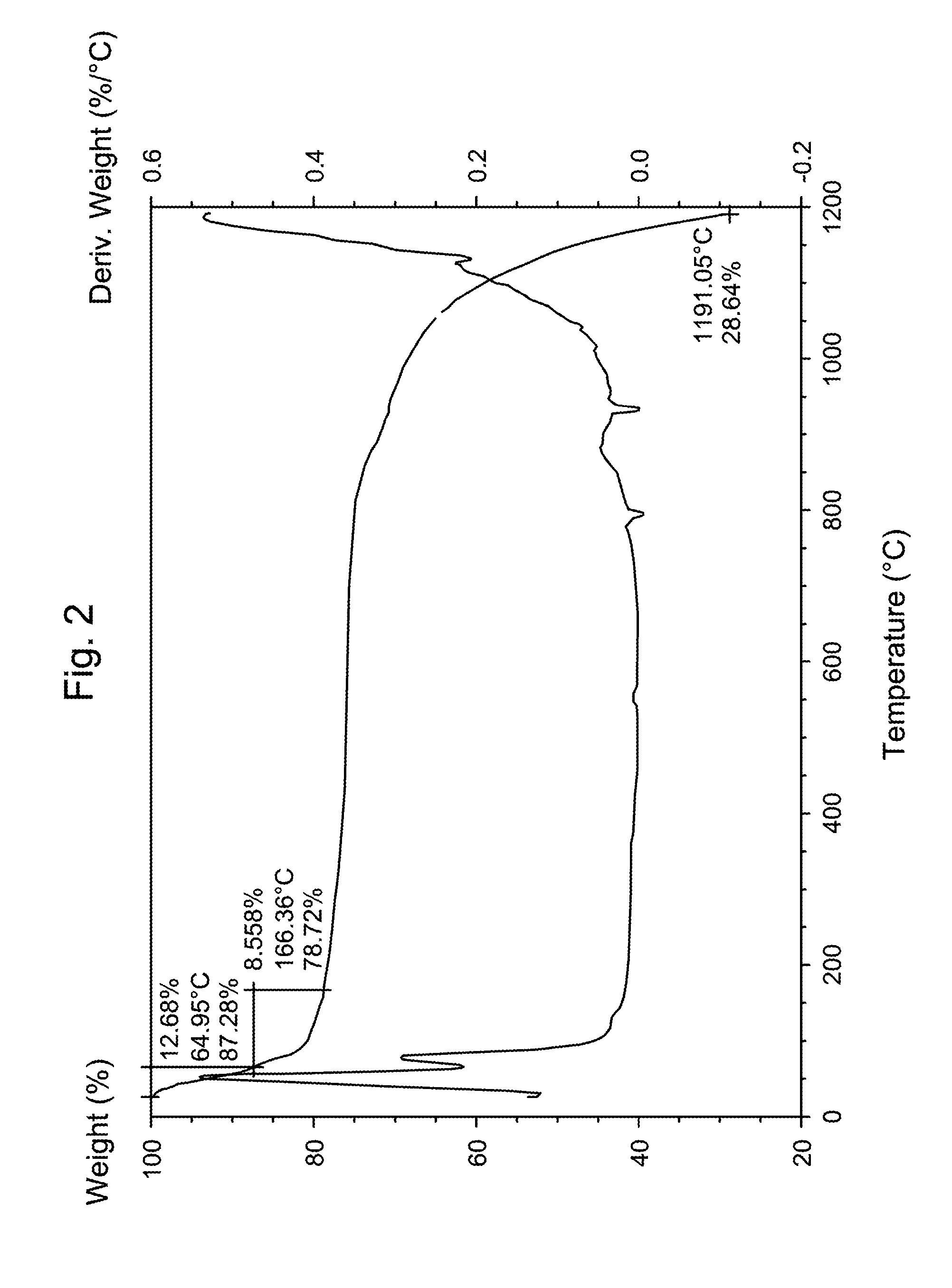
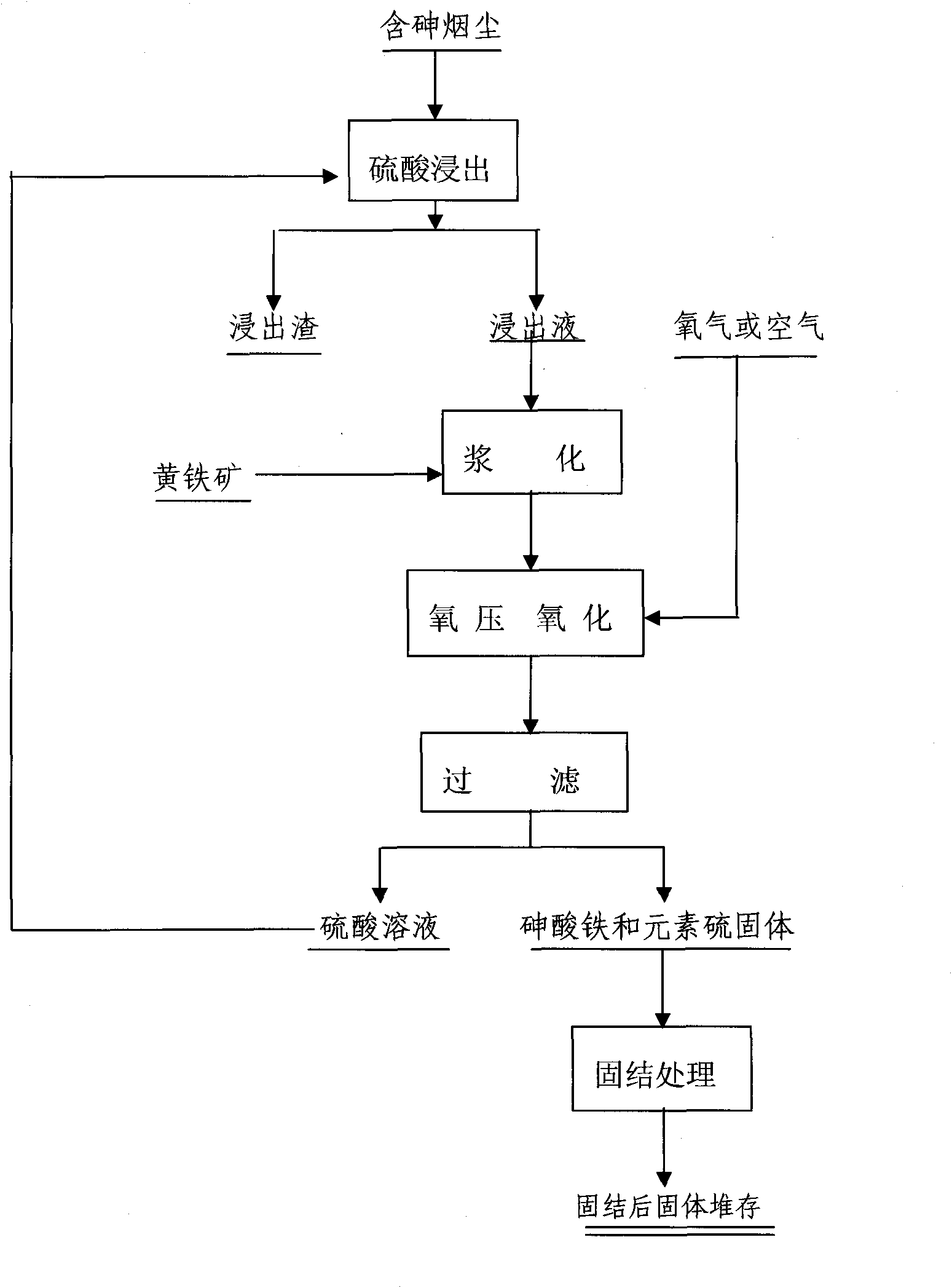
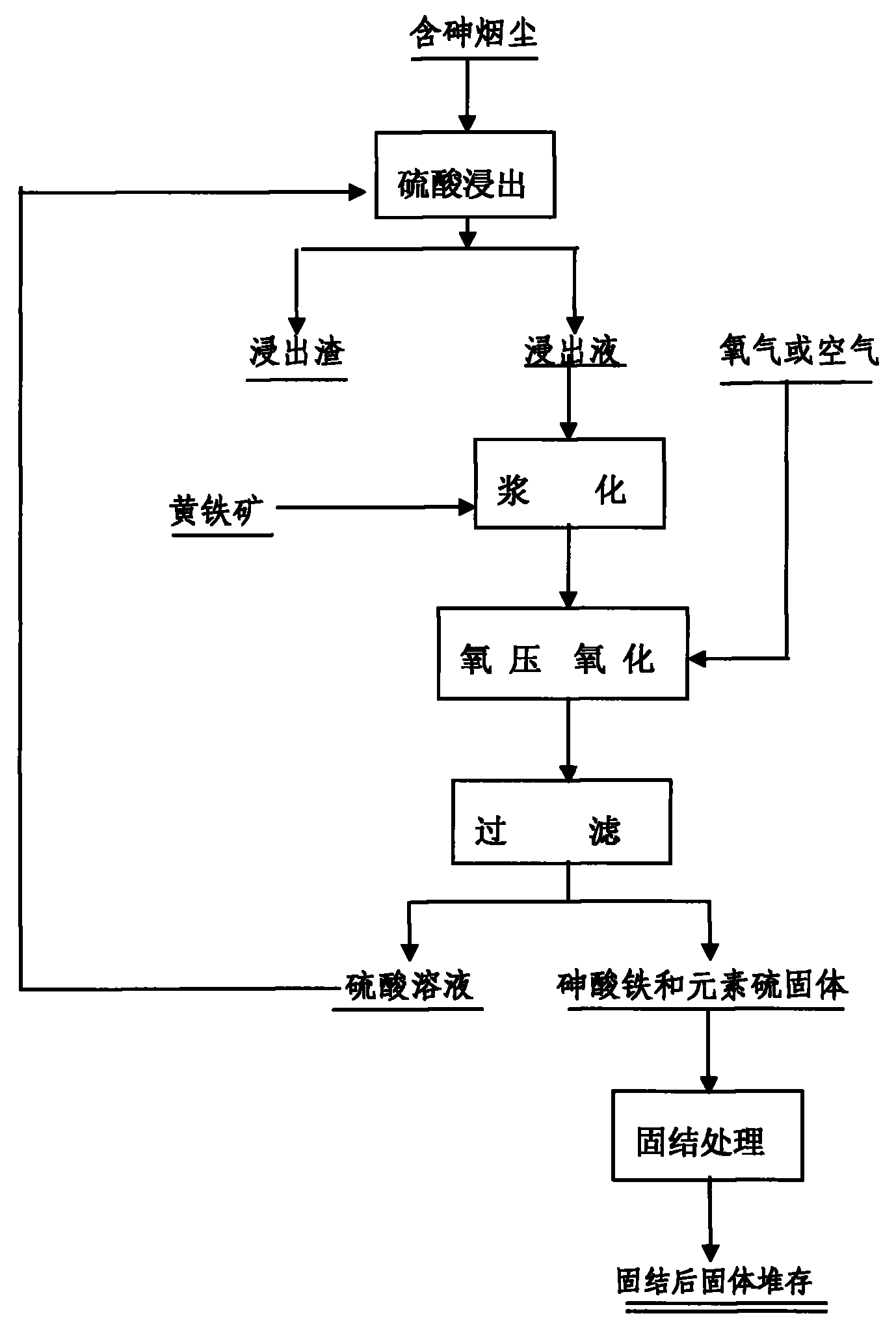
The invention provides a method for synthesizing ferricarsenate from arsenic-containing smoke dust by leaching, pressure oxidation and conversion, and consolidating the ferricarsenate. The method comprises the following steps of: leaching the arsenic-containing smoke dust by using 10 to 30g / L of dilute sulfuric acid solution; mixing and stirring the smoke dust and powdered pyrite; adding the mixture into a closed stirring reactor; introducing oxygen into the reactor until the oxygen partial pressure is between 0.6 and 1.5MPa; heating the reactor to the temperature of between 100 and 160 DEG C, stirring the mixture to perform reaction for 2 to 4 hours; filtering the reaction product to obtain mixed solid of ferricarsenate and sulphur, and 10 to 30g / L of dilute sulfuric acid solution which is returned and leached; and solidifying the mixed solid to obtain environment-friendly hydrophobic solid with high strength. The method has no emission of waste gas or waste water in the treatment process, can solve the opening problem of arsenic in various arsenic-containing smoke dusts, and eliminates pollution of the arsenic-containing smoke dust to the environment.
Owner:红河砷业有限责任公司
Method for leaching and simultaneously stabilizing arsenic sulfide slag
InactiveCN105967232AImprove stabilityFacilitate solid-liquid separationArsenites/arsenatesSlagCulture mediums
The invention relates to a method for leaching and simultaneously stabilizing arsenic sulfide slag. The method comprises the following steps: preparing ore pulp from arsenic sulfide slag under the acid condition, adding ferrous sulfate, thermophilic iron oxide microbial strains and microbial culture medium salt, filling air and stirring, oxidizing and leaching arsenic sulfide slag under the temperature of 70-95 DEG C and a pH value of 0-2.0 by taking trivalent iron generated by microbial ferrous oxide, simultaneously adsorbing iron and arsenic by using microbial cell surface and accelerating generation of scorodite crystals. The method is simple in process flow and low in cost; the concentration of arsenic leached by the generated scorodite crystals meets the stipulation of solid waste identification standard which is leaching toxicity identification (GB5085.3-2007); the arsenic can be safely piled and stored; the leaching solution can be reused.
Owner:SCI RES ACADEMY OF GUANGXI ENVIRONMENTAL PROTECTION
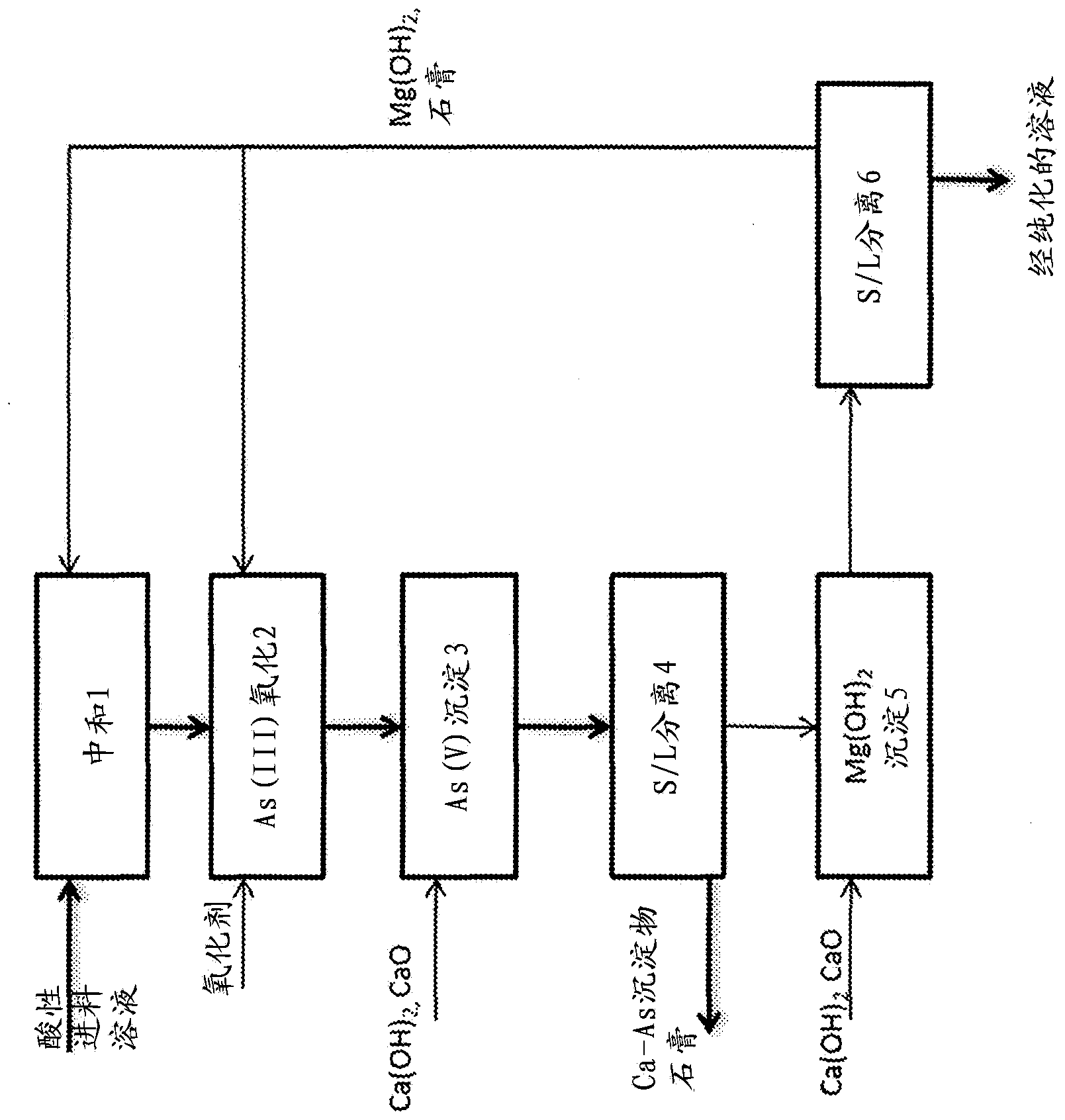
Stabilization of Arsenic-Containing Wastes Generated During Treatment of Sulfide Ores
InactiveUS20120164041A1Safe disposalEffective pressureArsenites/arsenatesAntimony compoundsChemical reactionIodide
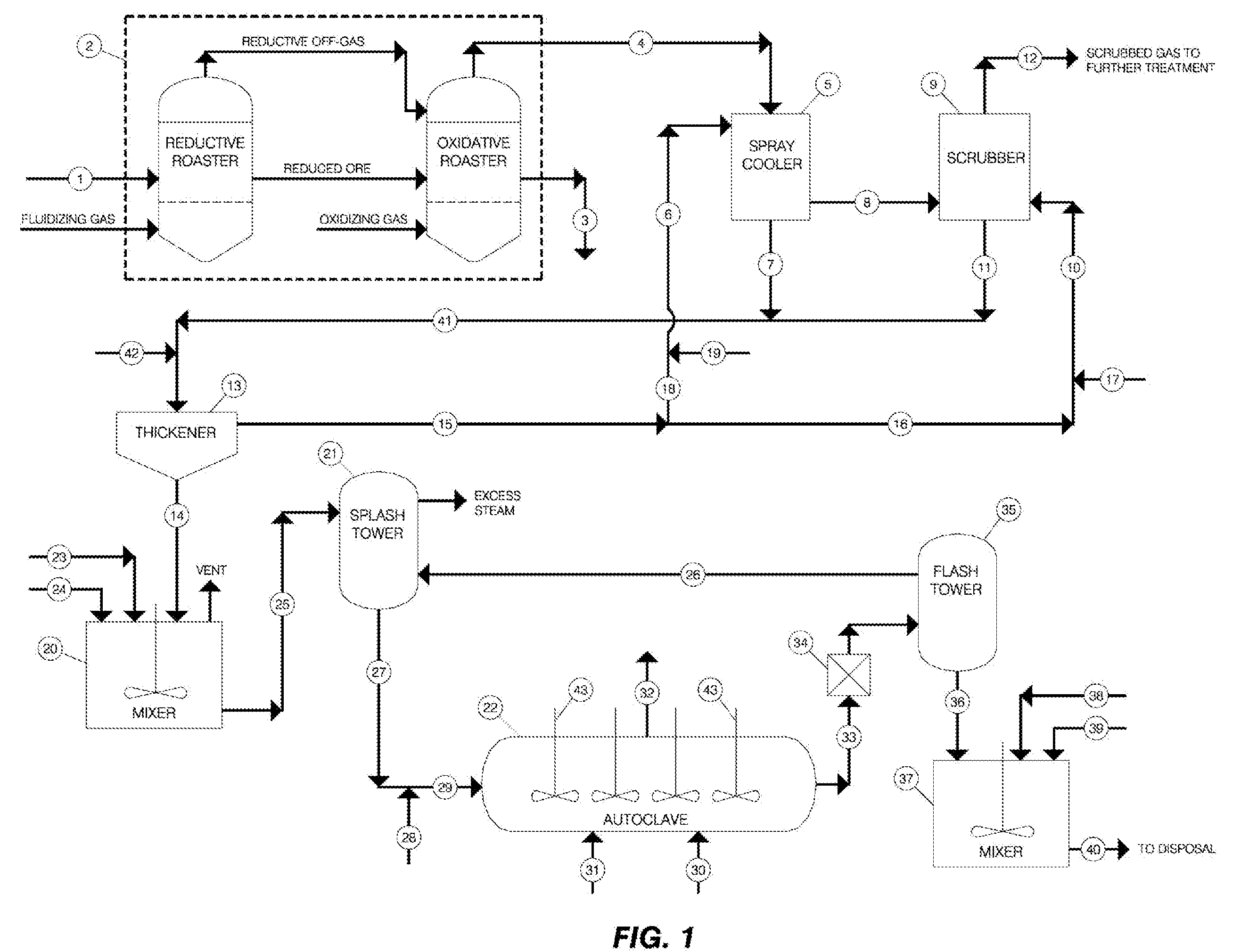
A method is provided for the efficient stabilization, removal and disposal of arsenic-containing wastes generated in metal recovery processes that employ roasting techniques and the like. The conversion of the mostly trivalent arsenite compounds in the wastes to mostly pentavalent solid arsenate precipitates is accomplished by mixing the wastes with water and a ground iron-containing mineral, such as goethite, to form an aqueous slurry of wastes and ground iron-containing mineral, acidifying the slurry to a pH of less than about 1.0, treating the acidified slurry with oxygen gas in a pressurized vessel at a temperature higher than about 120° C. and providing an oxidation catalyst comprised of a water-soluble nitrate and a water-soluble iodide. The overall efficiency of the controlling chemical reactions is improved by the addition and use of the catalyst. The resulting solid arsenate precipitates, in the form of scorodite, are ideally suited for safe disposal with minimum or no further treatment. Unconverted soluble trivalent arsenic compounds remaining in solution may be converted and precipitated as additional scorodite by mixing and agitating the slurry with soluble iron salts under controlled conditions. The resulting precipitates meet or exceed environmental requirements for impoundment and safe disposal.
Owner:ALTYNALMAS GOLD A CANADA CORP
Method for removing arsenic hydride in tail gas of iron making blast furnace by wet process
InactiveCN102389697AGood removal effectImprove purification effectArsenites/arsenatesDispersed particle separationProduct gasTower
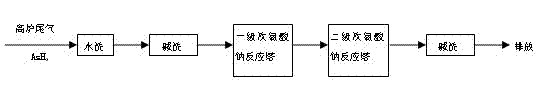
The invention discloses a method for removing arsenic hydride in tail gas of an iron making blast furnace by a wet process. In the method, the tail gas of the iron making blast furnace is subjected to water washing and alkali washing to remove dust and acidic gas, then the tail gas is introduced into a reaction tower containing sodium hypochlorite, the arsenic hydride in the tail gas is removed by the strong oxidation action of sodium hypochlorite, and the thus, the tail gas is purified. With simple process and low treatment cost, the method is not limited by field, can treat complexly composed tail gas containing arsenic hydride and is suitable for industrial application.
Owner:KUNMING UNIV OF SCI & TECH
Method for synthesizing calcium arsenate by oxygen pressure conversion
InactiveCN101817554ANo pollution in the processEliminate pollutionArsenites/arsenatesDispersed particle separationWater insolubleWastewater
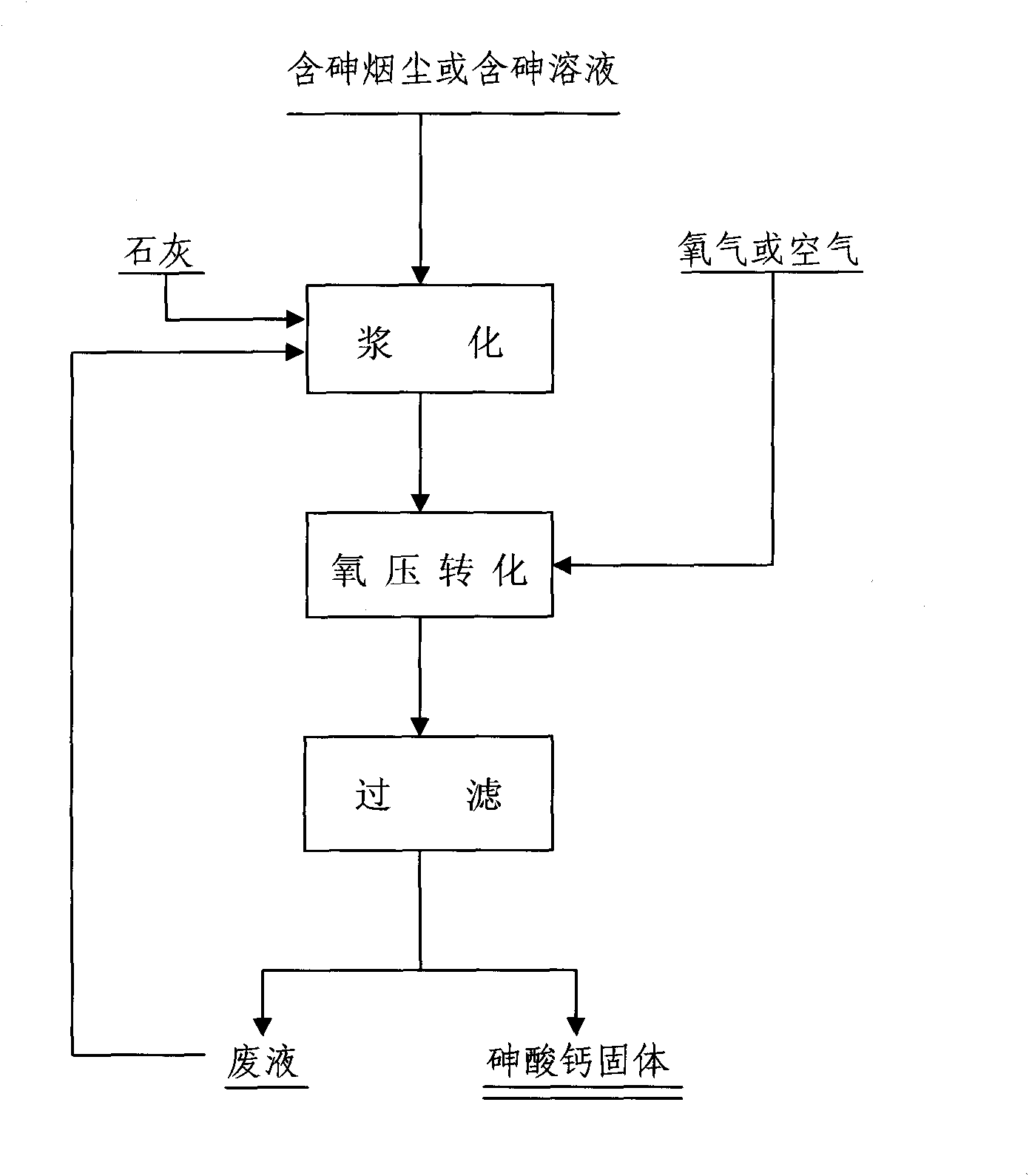
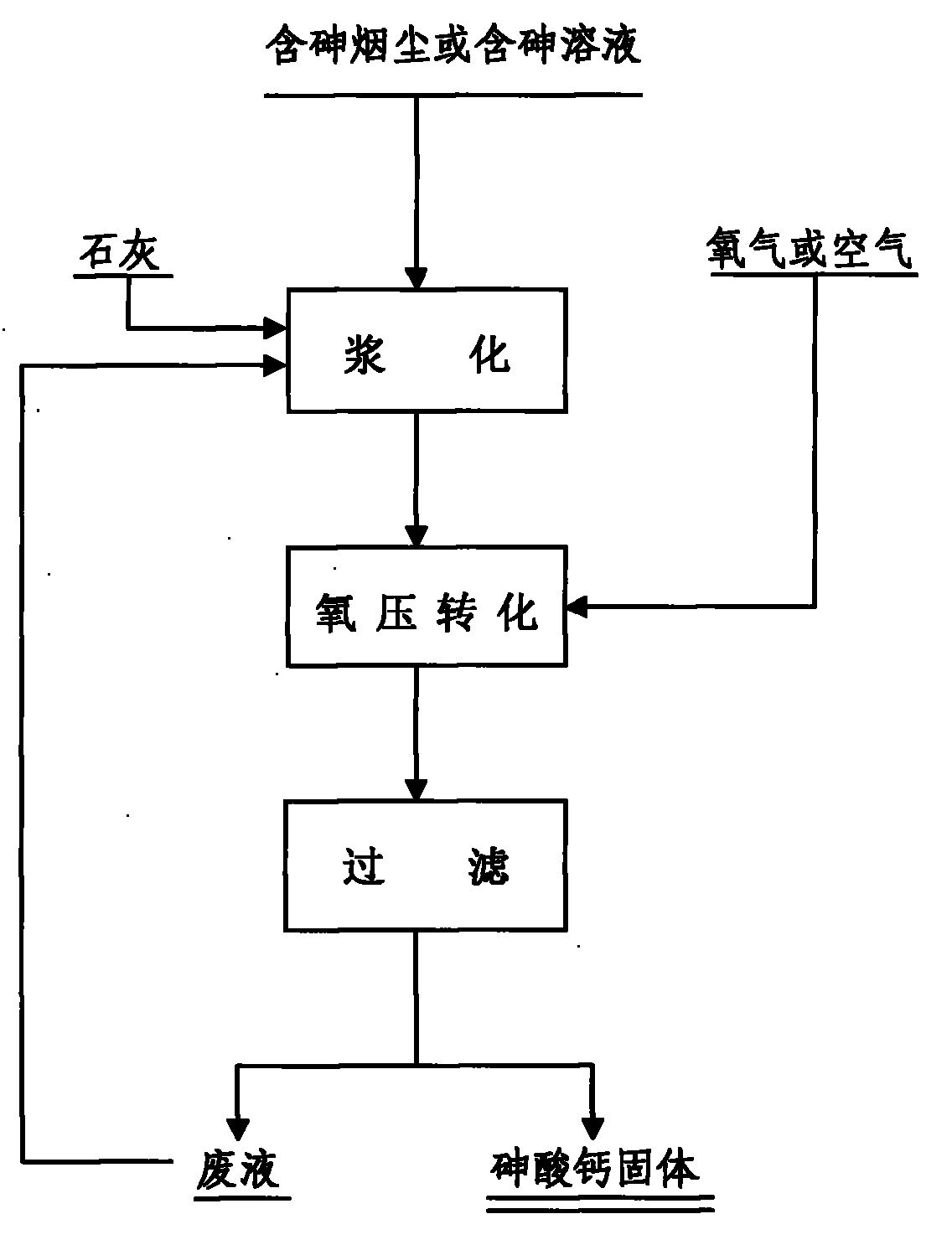
The invention relates to a method for synthesizing water insoluble calcium arsenate by oxidizing arsenic in arsenic-containing smoke dust or solution and belongs to the technical field of metallurgy. The method comprises the following steps of: (1) mixing and stirring the arsenic-containing smoke dust or solution with weighed lime to make slurry; and (2) adding the slurry material into a closed stirring reactor, introducing the air or oxygen into the closed container till the oxygen partial pressure in the closed container reach 0.6 to 1.5MPa, stirring the slurry material under a closed condition to perform an reaction to oxide trivalent arsenic in the slurry material into pentavalent arsenic and convert the pentavalent arsenic into water insoluble calcium arsenate, cooling the reaction solution, and filtering the reaction solution to obtain solid calcium arsenate. In the method, the arsenic in the arsenic-containing smoke dust or solution is oxidized into the pentavalent arsenic and the pentavalent arsenic is converted into the water insoluble calcium arsenate, neither waste gases nor waste water is produced in the process, the problem of opening a way for the arsenic in various kinds of arsenic-containing smoke dust is solved, and the environmental pollution caused by arsenic is eliminated.
Owner:YUNNAN TIN GROUP HLDG
Method for manufacturing scorodite
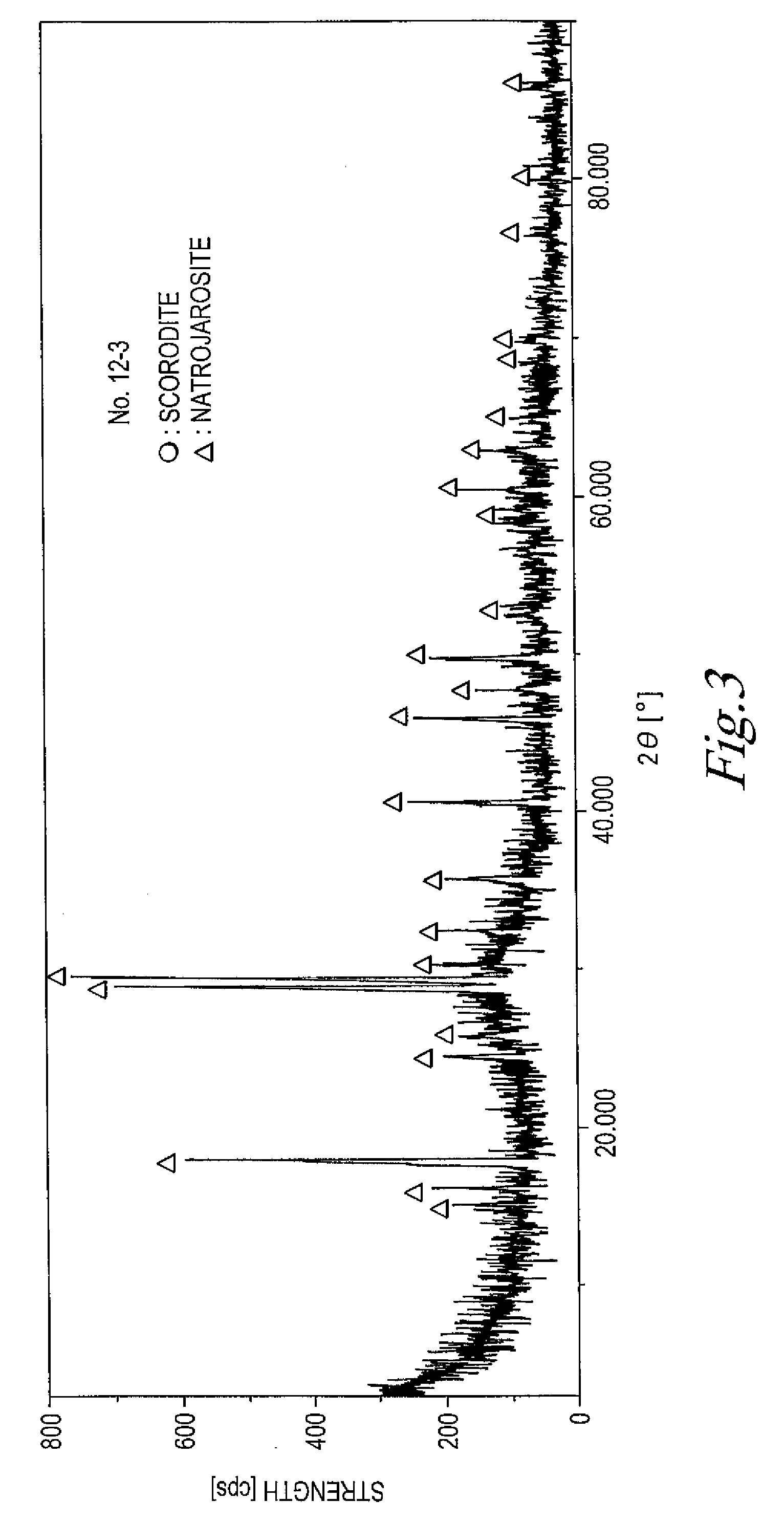
InactiveUS20080233023A1Inhibition formationIncrease the concentration ratioArsenites/arsenatesAntimony compoundsTM compoundAqueous solution
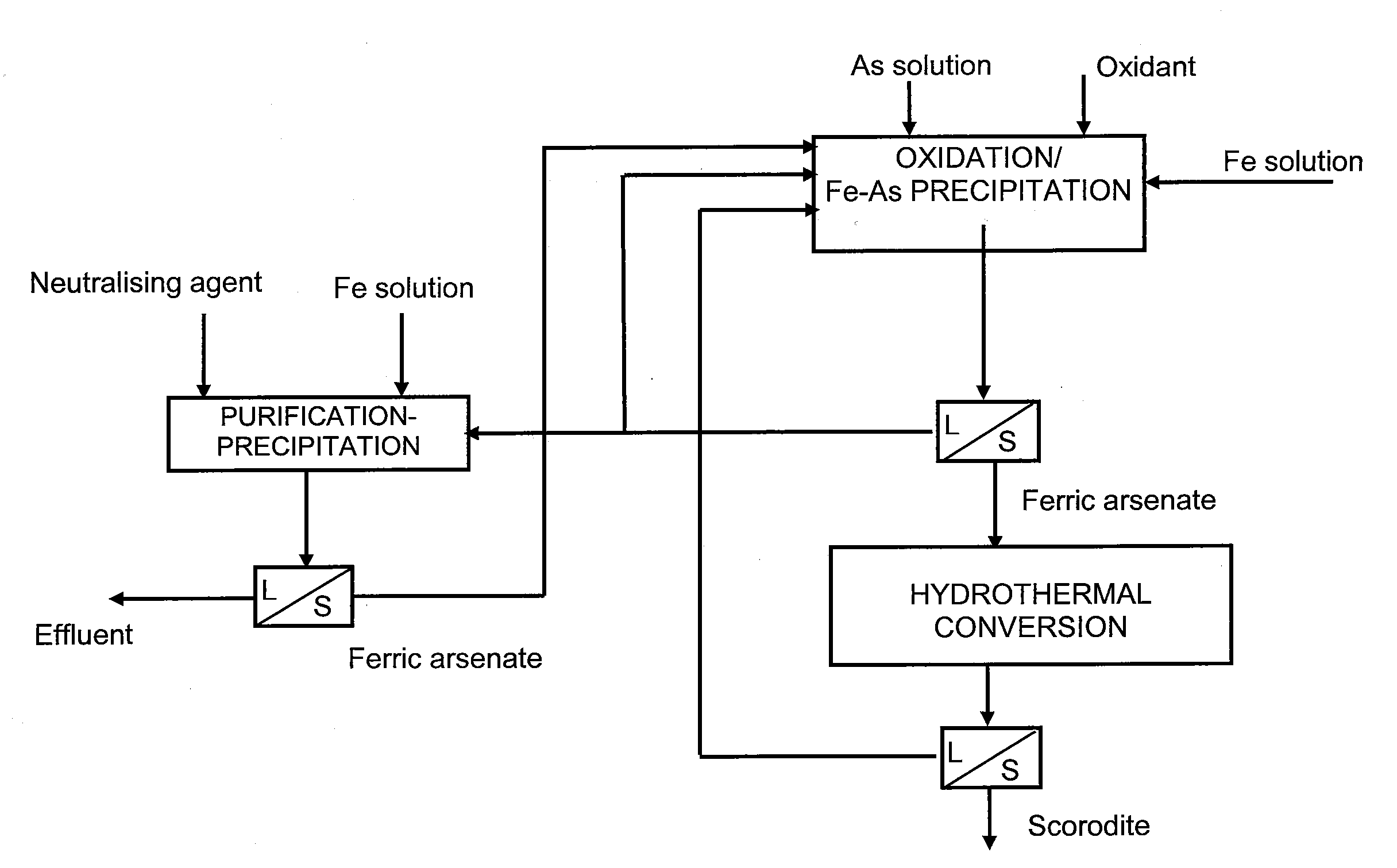
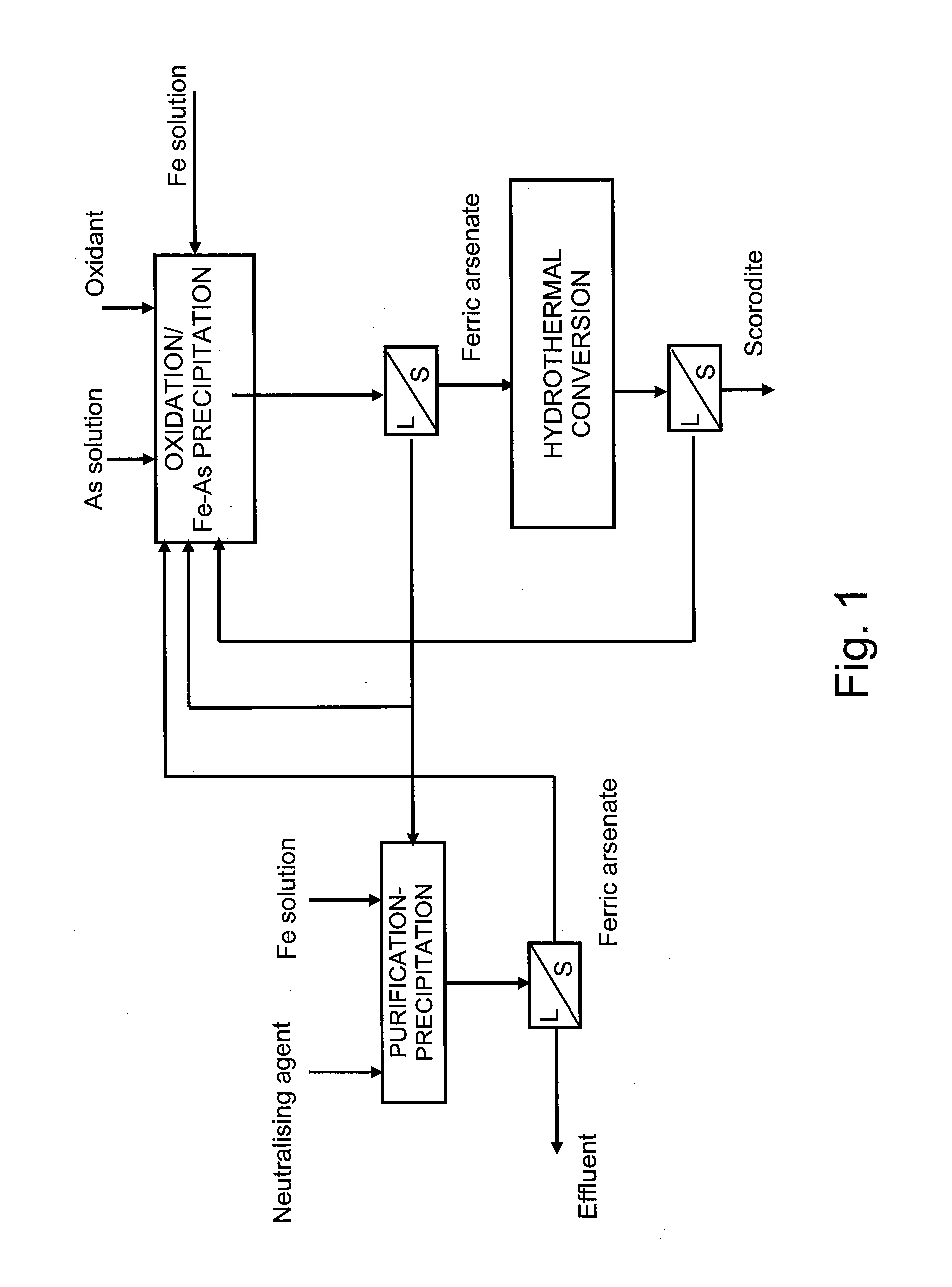
The present invention provides a method for manufacturing scorodite in which scorodite may be obtained at high production efficiency and a high As concentration ratio. The present invention provides a method for manufacturing crystalline scorodite from acidic aqueous solution containing pentavalent As and trivalent Fe, the method comprising a step for adding a basic sodium compound to the acidic aqueous solution such that the sodium concentration in the acidic aqueous solution becomes larger than 0 g / L and equal to or less than 4 g / L.
Owner:JX NIPPON MINING& METALS CORP
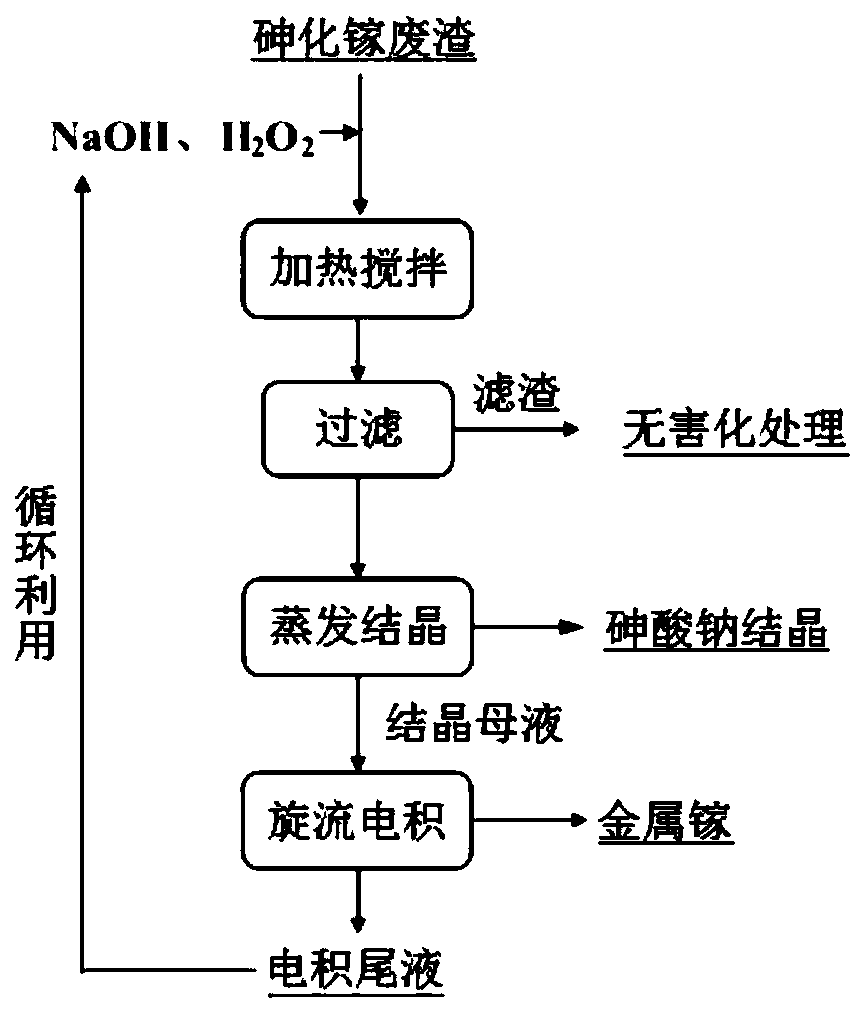
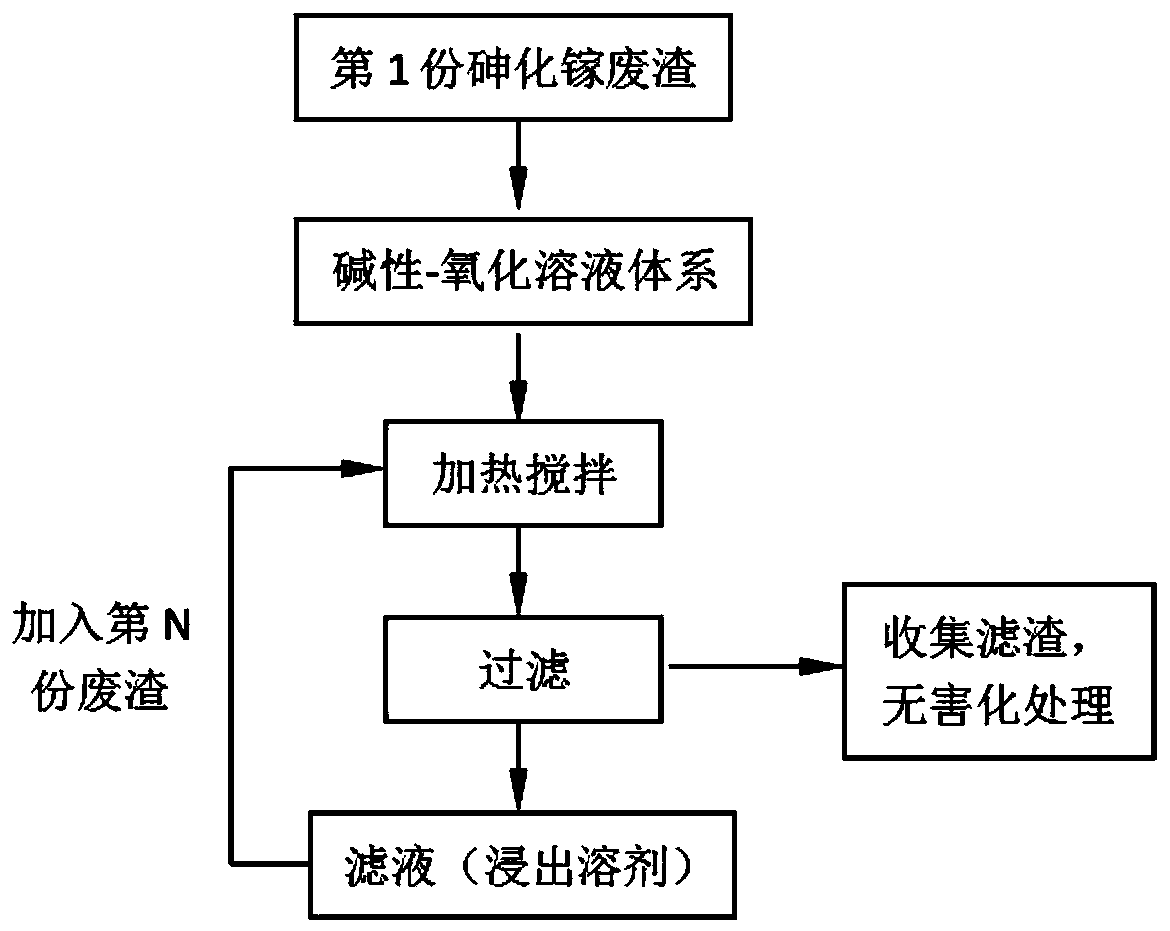
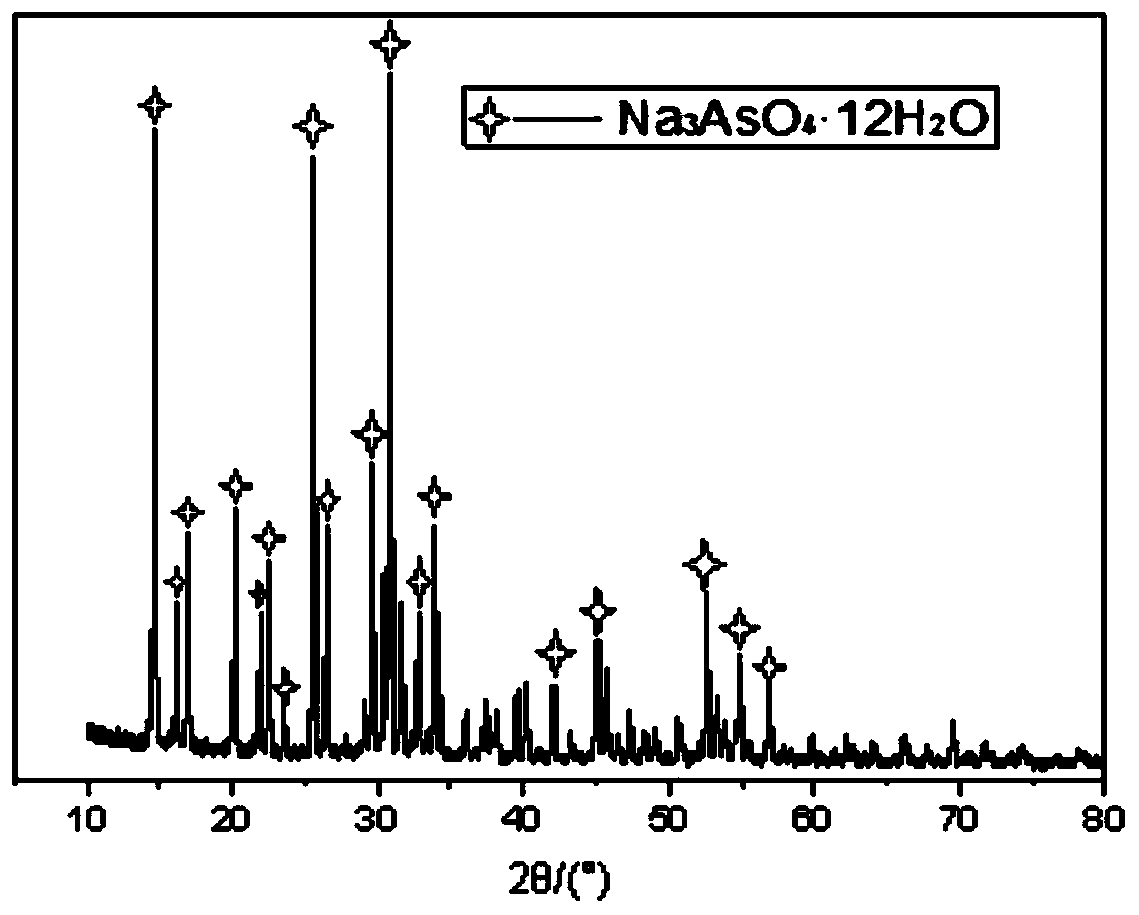
Method for recycling and preparing sodium arsenate and gallium metal from gallium arsenide waste residue
ActiveCN110938742AEasy to separateHigh purityPhotography auxillary processesArsenites/arsenatesGallium arsenateGallium
The invention relates to a method for recycling and preparing sodium arsenate and gallium metal from gallium arsenide waste residue. The method comprises the following steps of leaching the gallium arsenide waste residue in an alkaline-oxidized solution system after the gallium arsenide waste residue is dried, crushed and screened, wherein the conditions of the NaOH concentration, the oxidant concentration, the time, the temperature and the like during leaching are controlled so that arsenic and gallium are simultaneously quickly dissolved in a leaching solution to the great extent; performingevaporative crystallization to make the sodium arsenate crystallize preferentially to realize quick effective separation of the arsenic and the gallium; performing recrystallization to improve the purity of the sodium arsenate; and performing cyclone electrowinning to obtain high-purity gallium metal. The raw material gallium arsenide waste residue is from solid waste landfill and is sampled without cost; according to the method provided by the invention, the sodium arsenate and gallium metal are recycled and prepared from the waste residue; the purity of the prepared sodium arsenate is at least 95 percent; the purity of the prepared gallium metal is at least 3N; the prepared sodium arsenate and gallium metal have great utilization value; the recycled and purified sodium arsenate can serve as a chemical raw material; and the method disclosed by the invention effectively solves the problems of environment pollution and resource waste and meets the requirements of sustainable development society.
Owner:YANGZHOU NINGDA NOBLE METAL CO LTD
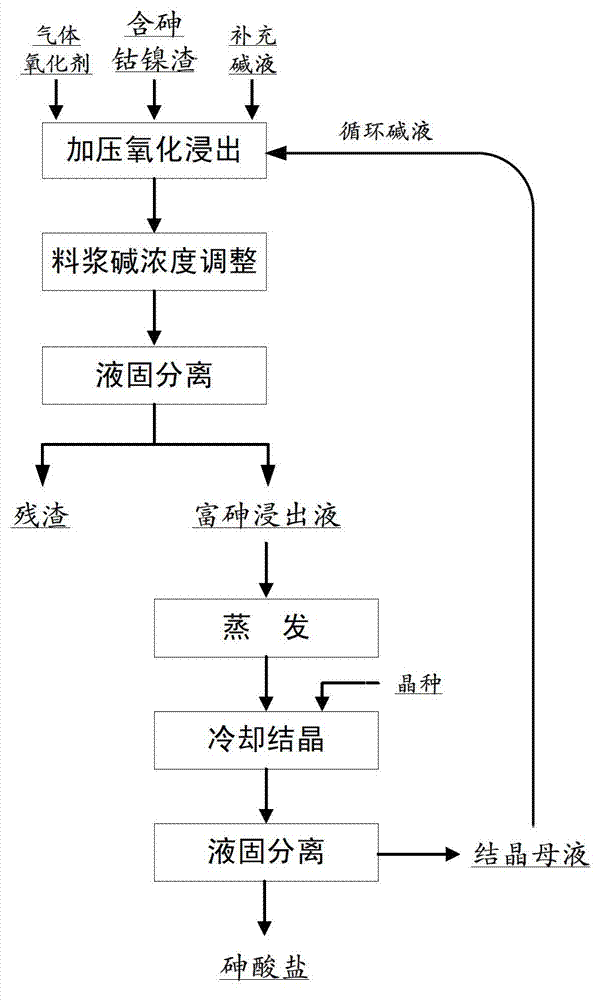
Method using wet alkaline process of cobalt-nickel (Co-Ni) residue containing arsenic to prepare arsenate
InactiveCN102925701ACrystallize fastAchieve separationArsenites/arsenatesProcess efficiency improvementPotassium hydroxideSlurry
The invention relates to a method using wet alkaline process of cobalt-nickel (Co-Ni) residue containing arsenic to prepare arsenate, and then preparing an arsenate product by using evaporative cooling and crystallizing method. The method comprises the steps of evenly mixing the Co-Ni residue containing the arsenic with sodium hydroxide or potassium hydroxide solution of certain density, placing mixture in a alkali-resistant high-pressure reaction kettle, introducing oxygen or oxygen-enriched gas of certain pressure as an oxidant, and reacting at a certain temperature, adjusting alkali density of slurry after reaction to less than 150g / L with water or low-density alkali liquid when the reaction is over, carrying out liquid-solid separation, obtaining arsenic-enriched liquid, evaporating the arsenic-enriched liquid, adding little seed crystal to cool and crystallize, and obtaining qualified arsenate crystal products after the liquid-solid separation. Crystallized mother liquid can be returned to be used for material distribution of the Co-Ni residue containing the arsenic. Recycling rate of the arsenic is over 99%, the method can prevent the arsenic from volatilizing during baking process and arsenic hydride gas from being generated during acid leaching. The method is simple in process, and can achieve recycle and reuse of the arsenic of the Co-Ni residue containing the arsenic.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
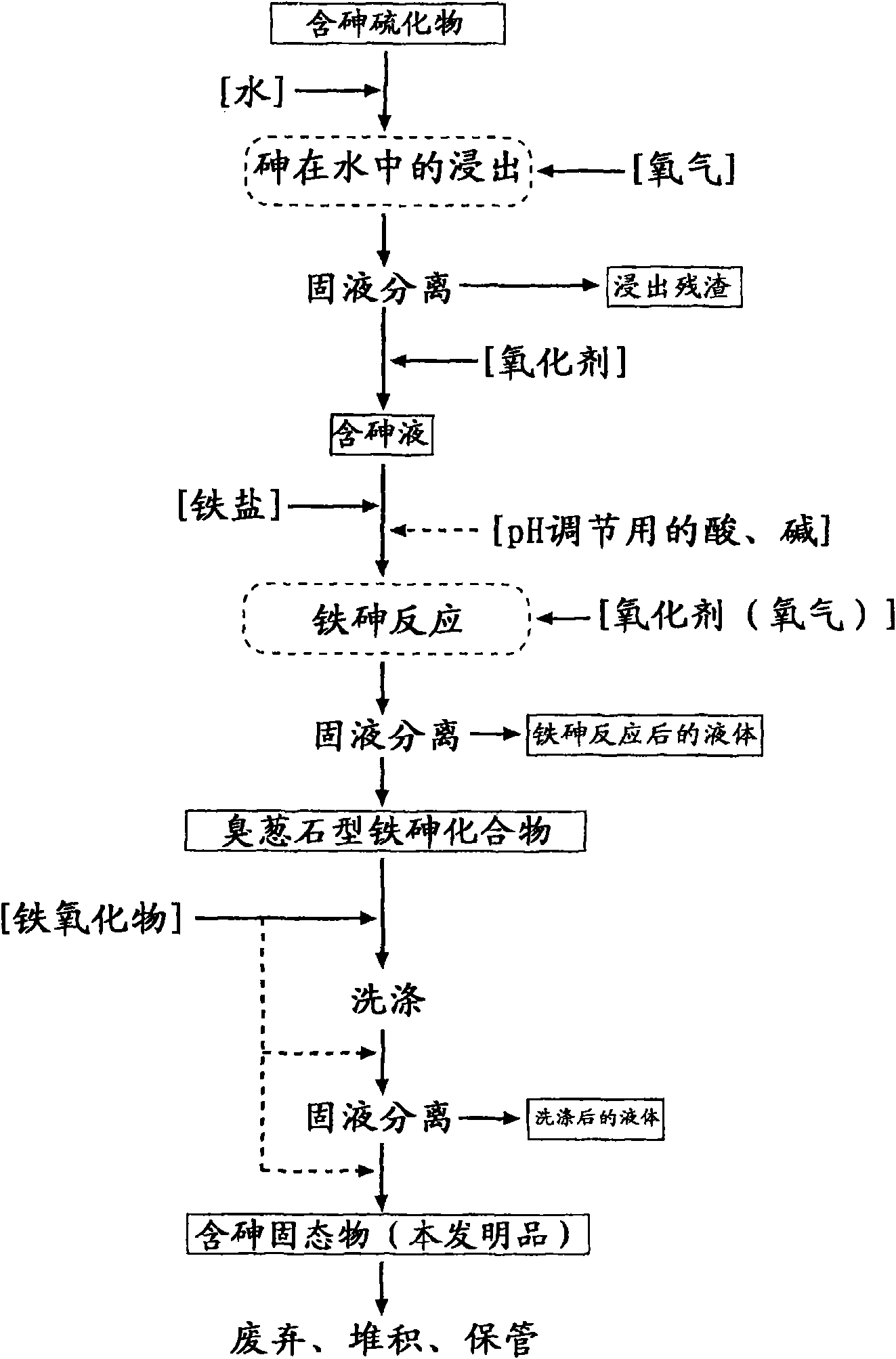
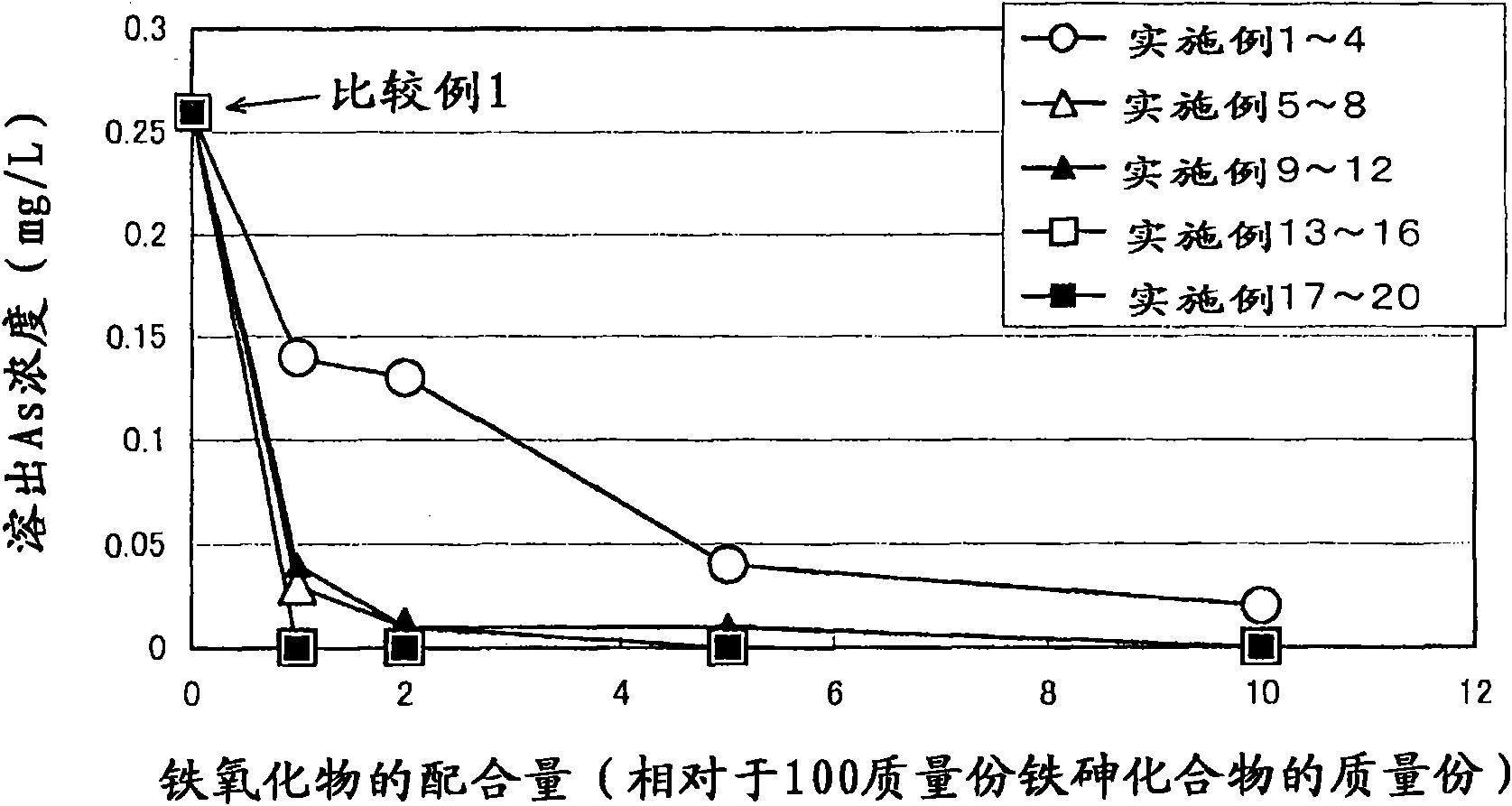
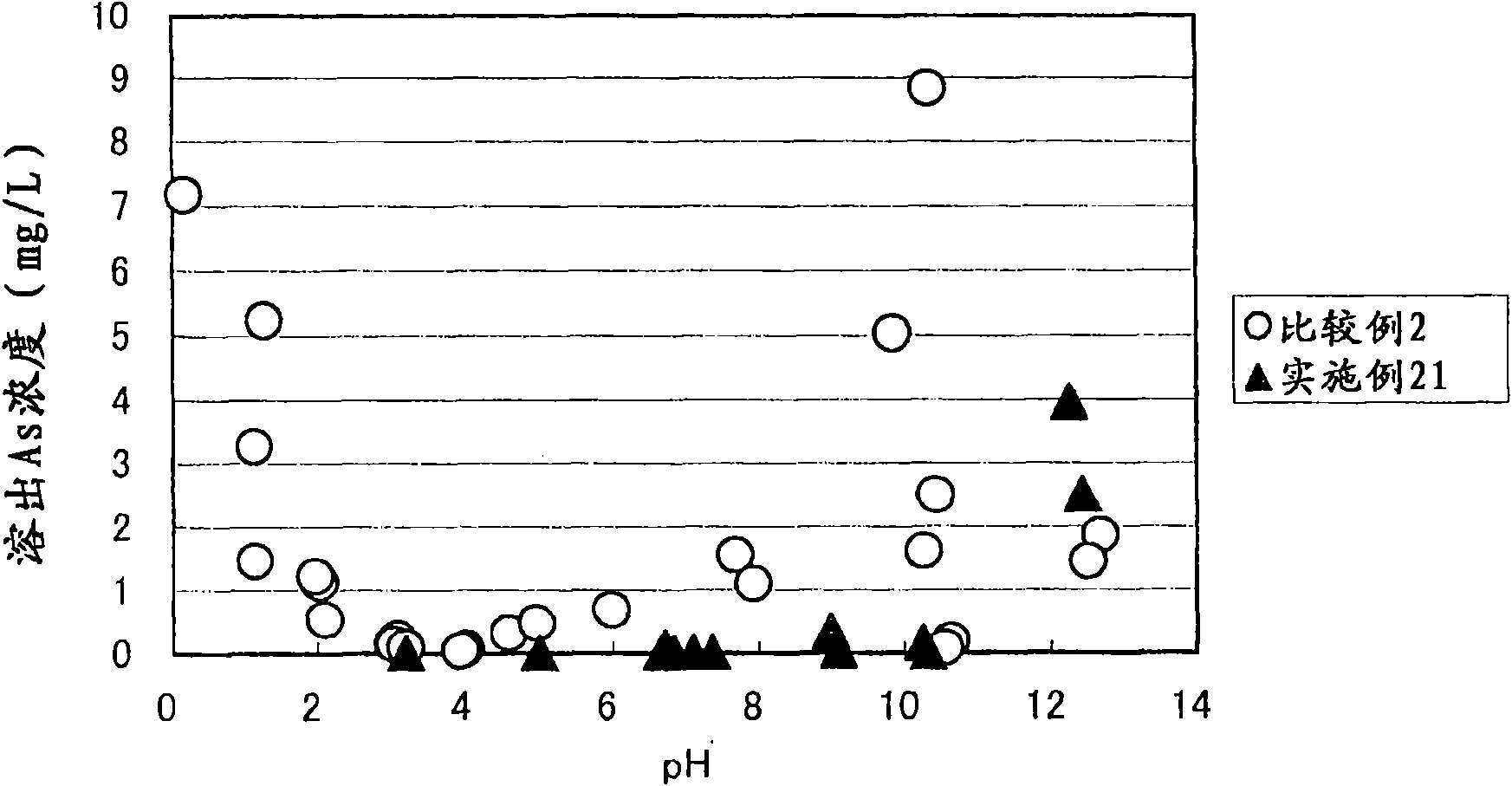
Arsenic-containing solid material and method for production thereof
InactiveCN101636352AStable and excellent dissolution prevention effectShort reaction timeArsenites/arsenatesSolution crystallizationGoethiteAqueous solution
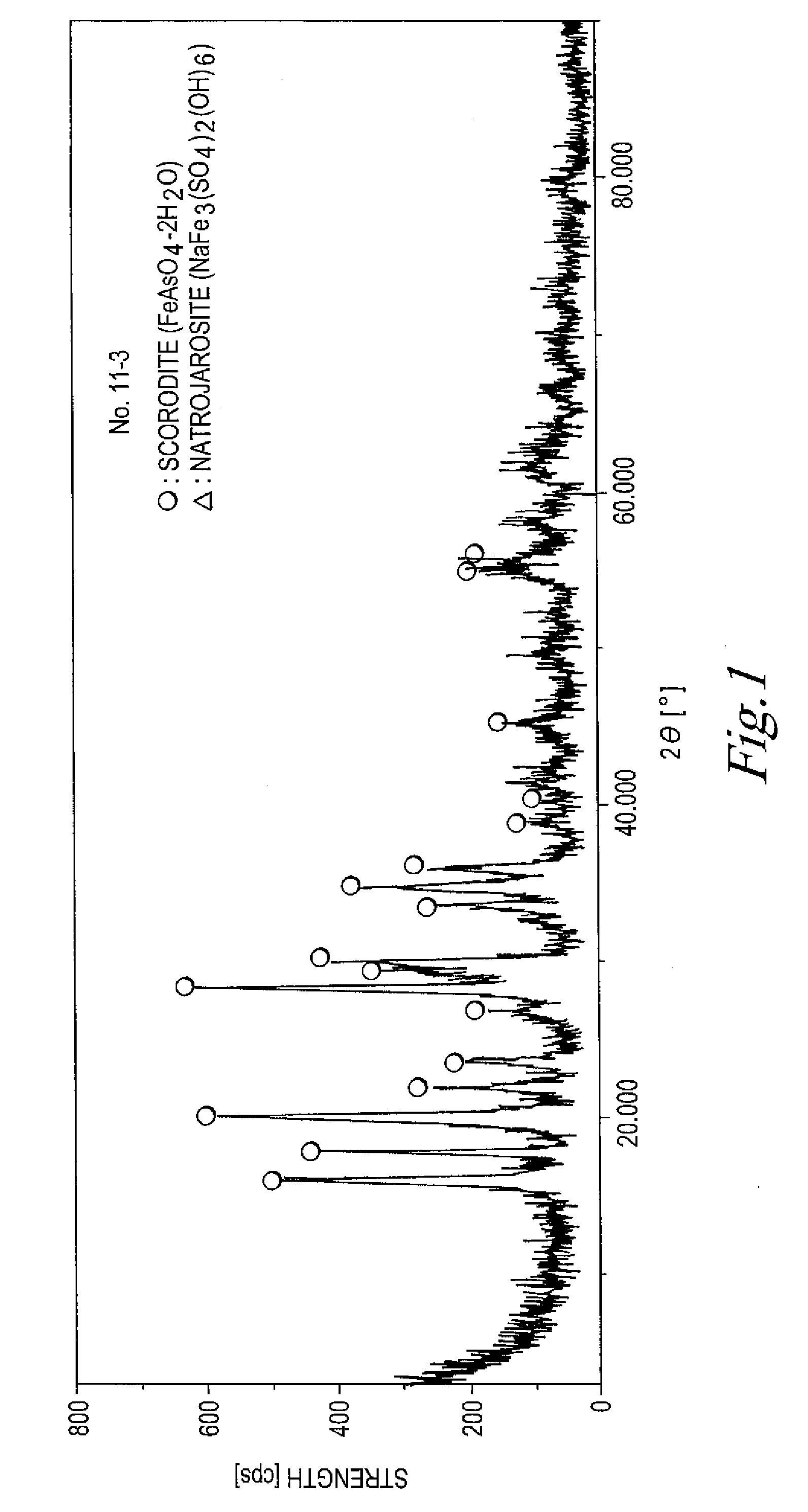
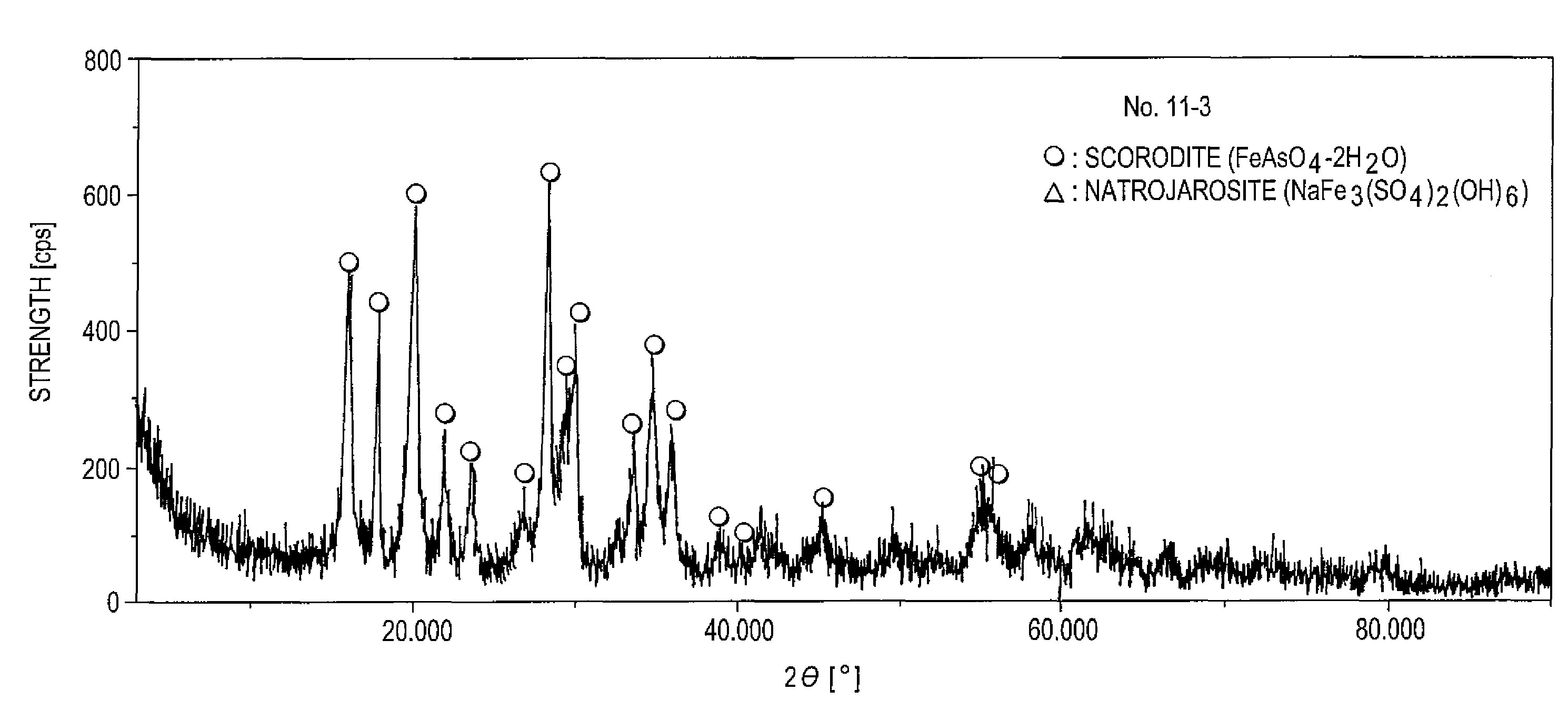
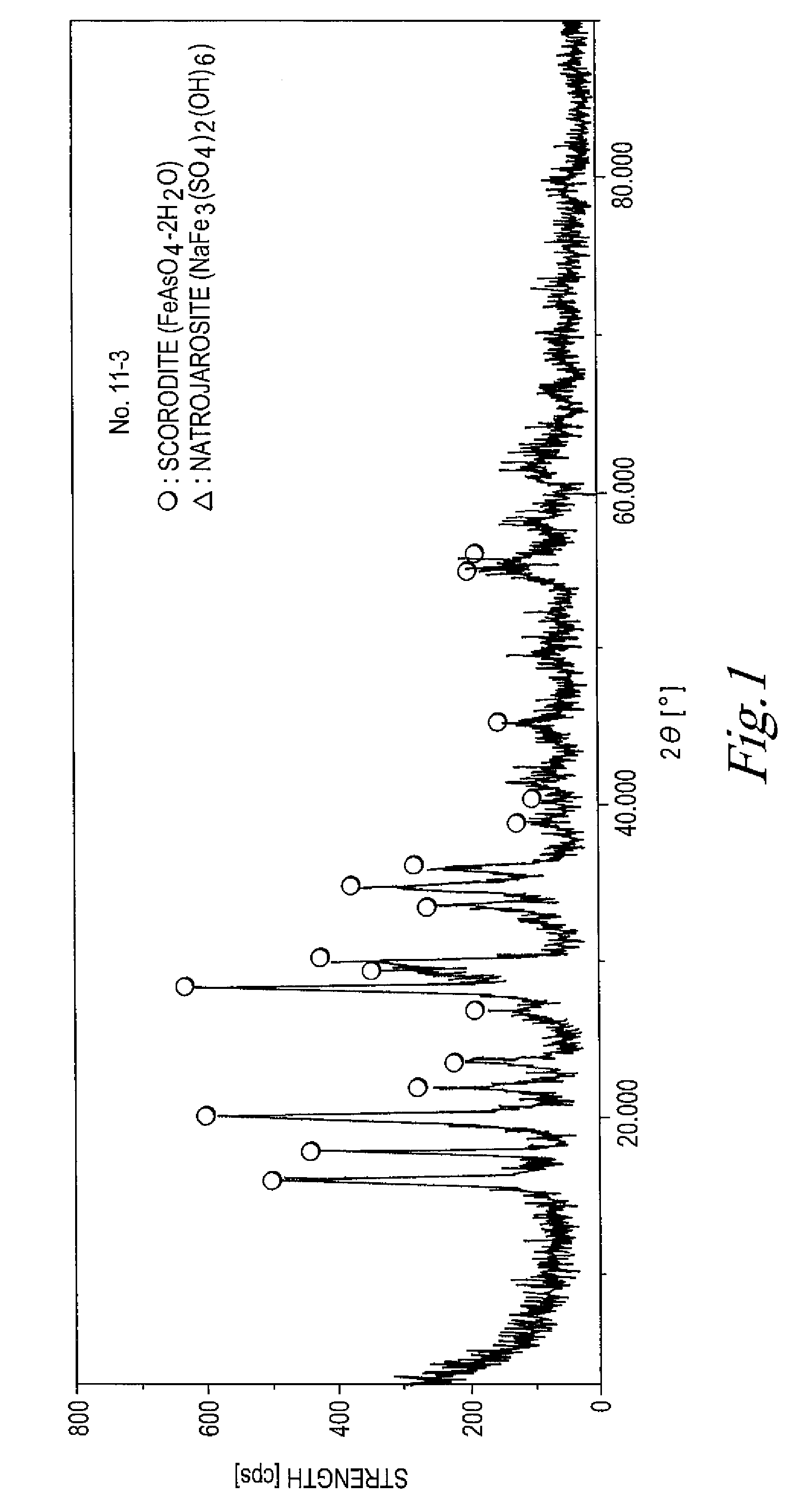
The invention provides an arsenic-containing solid material comprising 100 parts by mass of a scorodite-type iron-arsenic compound and 1 part by mass or more of an iron oxidation compound, wherein the scorodite-type iron-arsenic compound is synthesized by adding an oxidizing agent to an acidic aqueous solution containing a pentavalent arsenic ion and a bivalent arsenic ion to cause the precipitation of the iron-arsenic compound to proceed while agitating the solution, and terminating the precipitation at a pH value of 1.2 or lower. The iron oxidation compound may be goethite, hematite, or a mixture thereof. It is preferred to use an iron oxidation compound having a BET specific surface area of 3 m<2> / g or more, preferably 20 m<2> / g or more.
Owner:DOWA METALS & MINING CO LTD
Process for producing scorodite and recycling the post-scorodite-synthesis solution
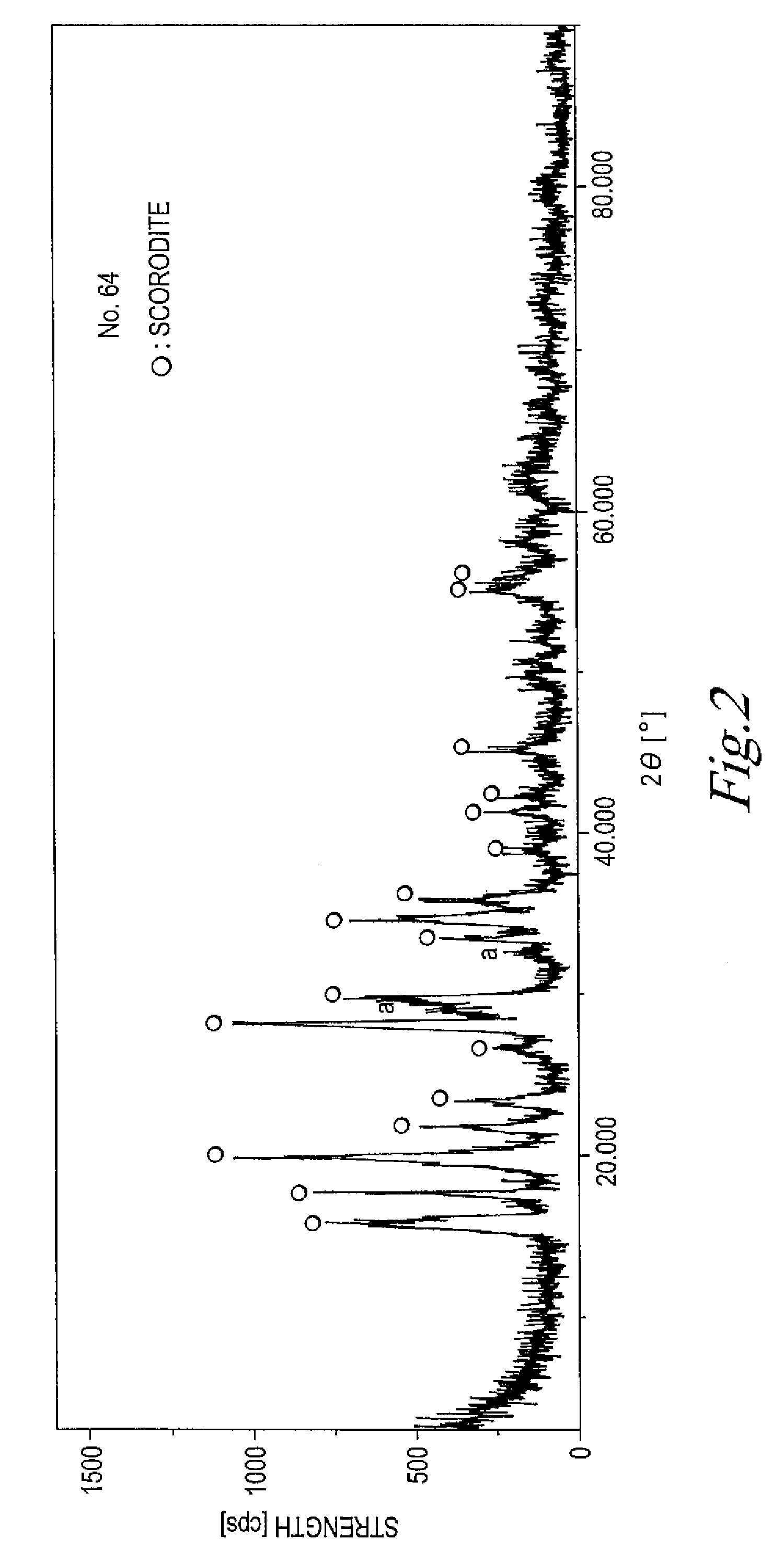
InactiveUS20090078584A1Shorten the timeIncrease productionElectrolysis componentsPhotography auxillary processesAqueous solutionFerric
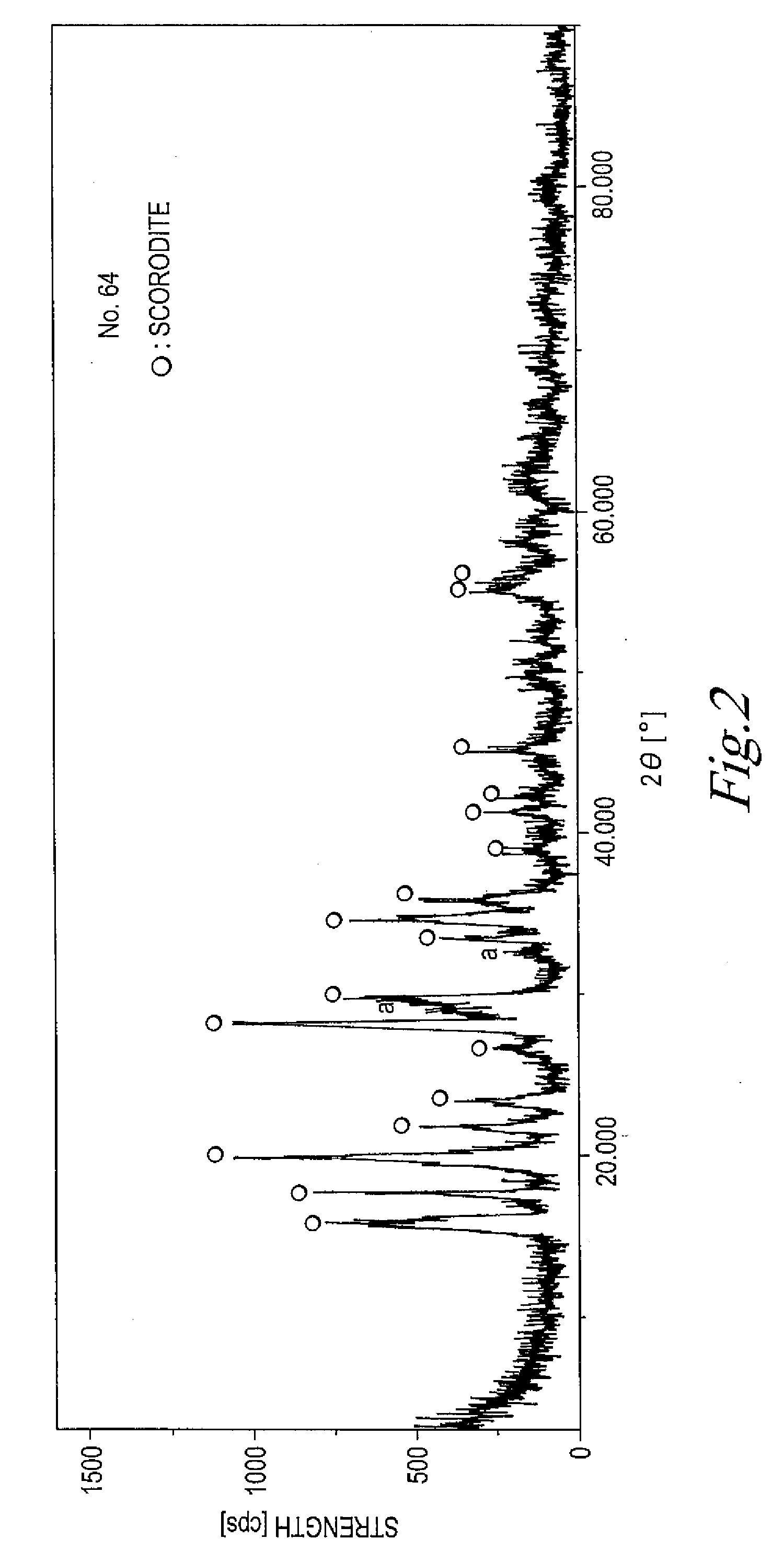
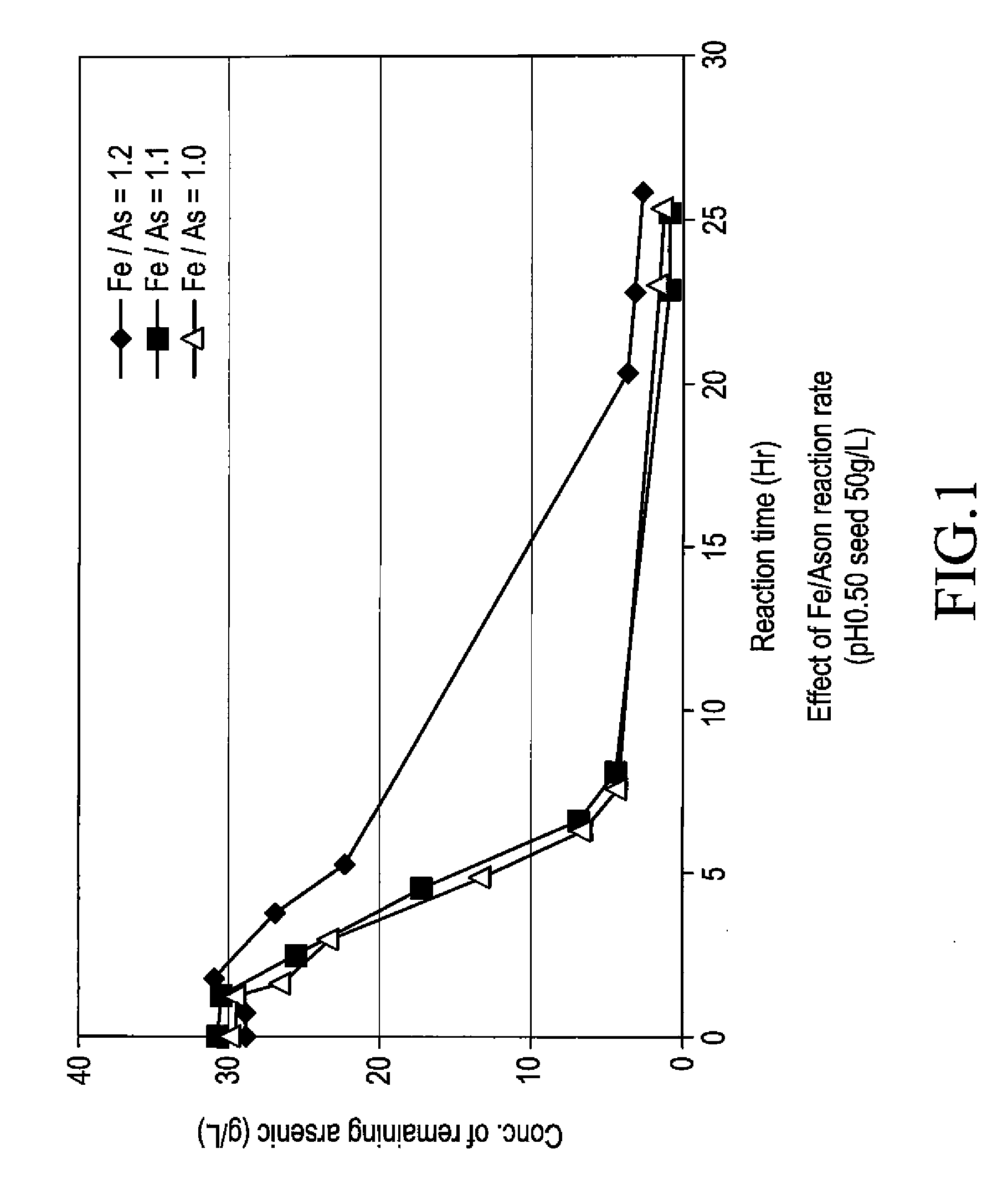
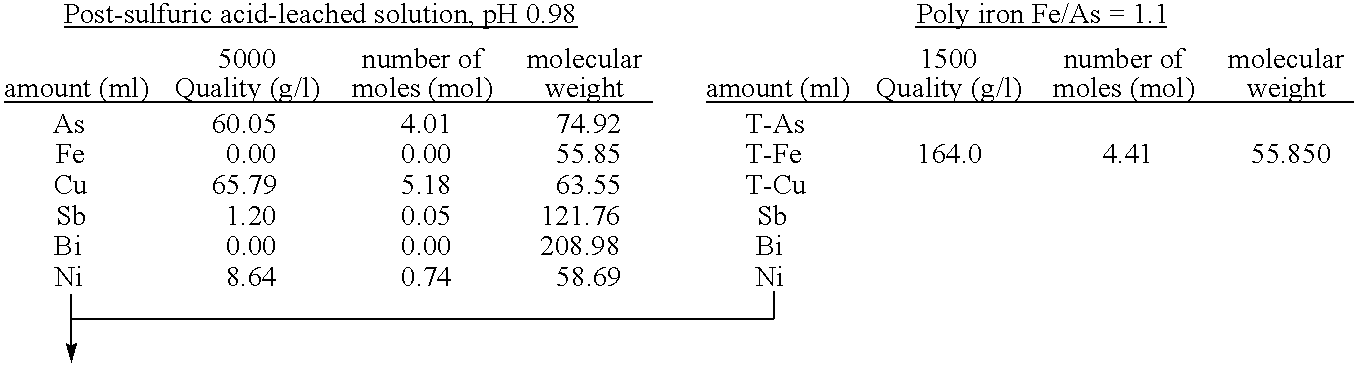
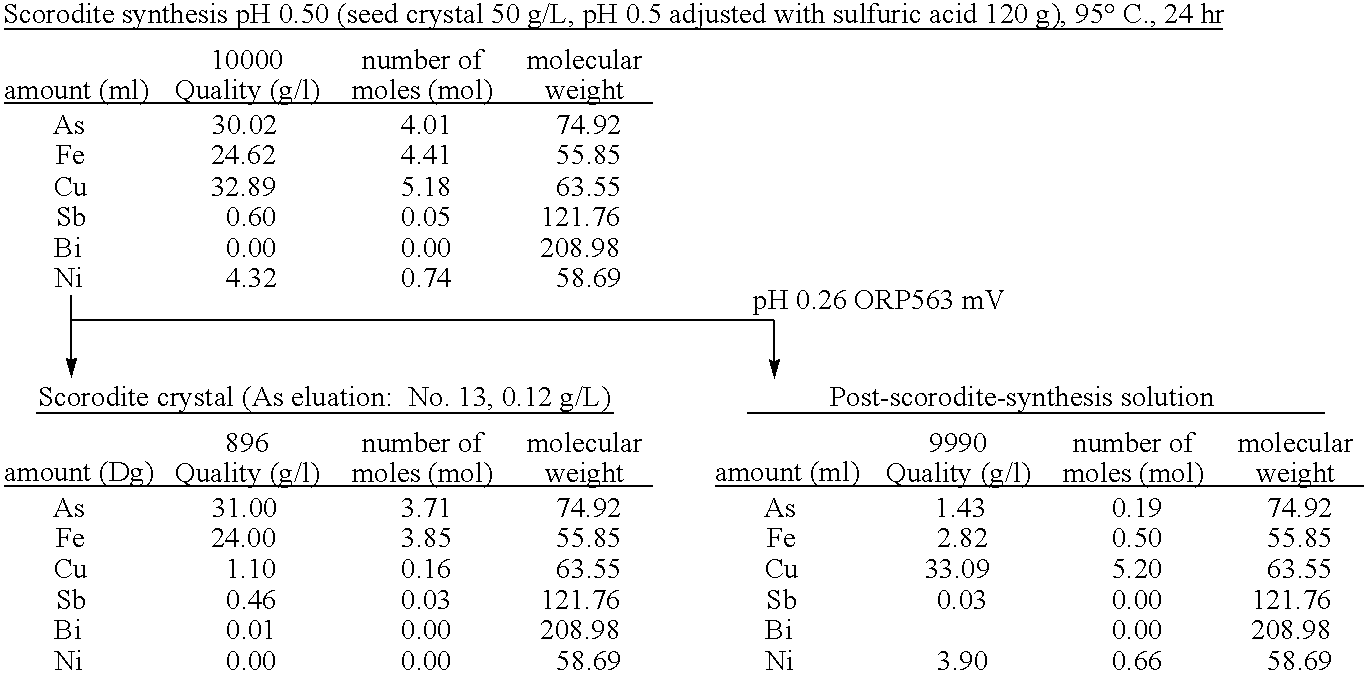
The present invention is to provide a process for producing a scorodite that can shorten the time required for synthesizing the scorodite, and further can improve the yield of arsenic and iron into the scorodite. Accordingly, a process for producing a crystalline scorodite from an acidic aqueous solution containing pentavalent As and trivalent Fe, wherein the synthesis of the crystalline scorodite is performed after the molar ratio of trivalent Fe to pentavalent As contained in the acidic aqueous solution is adjusted to be equal to or more than 0.9 and equal to or less than 1.1 is provided.
Owner:JX NIPPON MINING& METALS CORP
Eulytite solid acid electrolytes for electrochemical devices
Improved solid acid electrolyte materials, methods of synthesizing such materials, and electrochemical devices incorporating such materials are provided. The stable electrolyte material comprises a solid acid in a eulytine structure capable of undergoing rotational disorder of oxyanion groups and capable of extended operation at elevated temperatures, that is, solid acids having hydrogen bonded anion groups; a superprotonic disordered phase; and capable of operating at temperatures of ˜100° C. and higher.
Owner:CALIFORNIA INST OF TECH
Method for producing a poorly soluble calcium-arsenic compound
InactiveCN103415472AArsenites/arsenatesCalcium/strontium/barium sulfatesCalcium arseniteArsenic Compound
The invention relates to a method for precipitating pentavalent calcium arsenate from an acidic solution, in which arsenic is at least partially in trivalent form. The acidic solution is neutralised before being routed to an arsenic oxidation stage,and a poorly soluble calcium-arsenic compound is precipitated from the solution, in which all the arsenic is pentavalent.
Owner:OUTOTEC OYJ
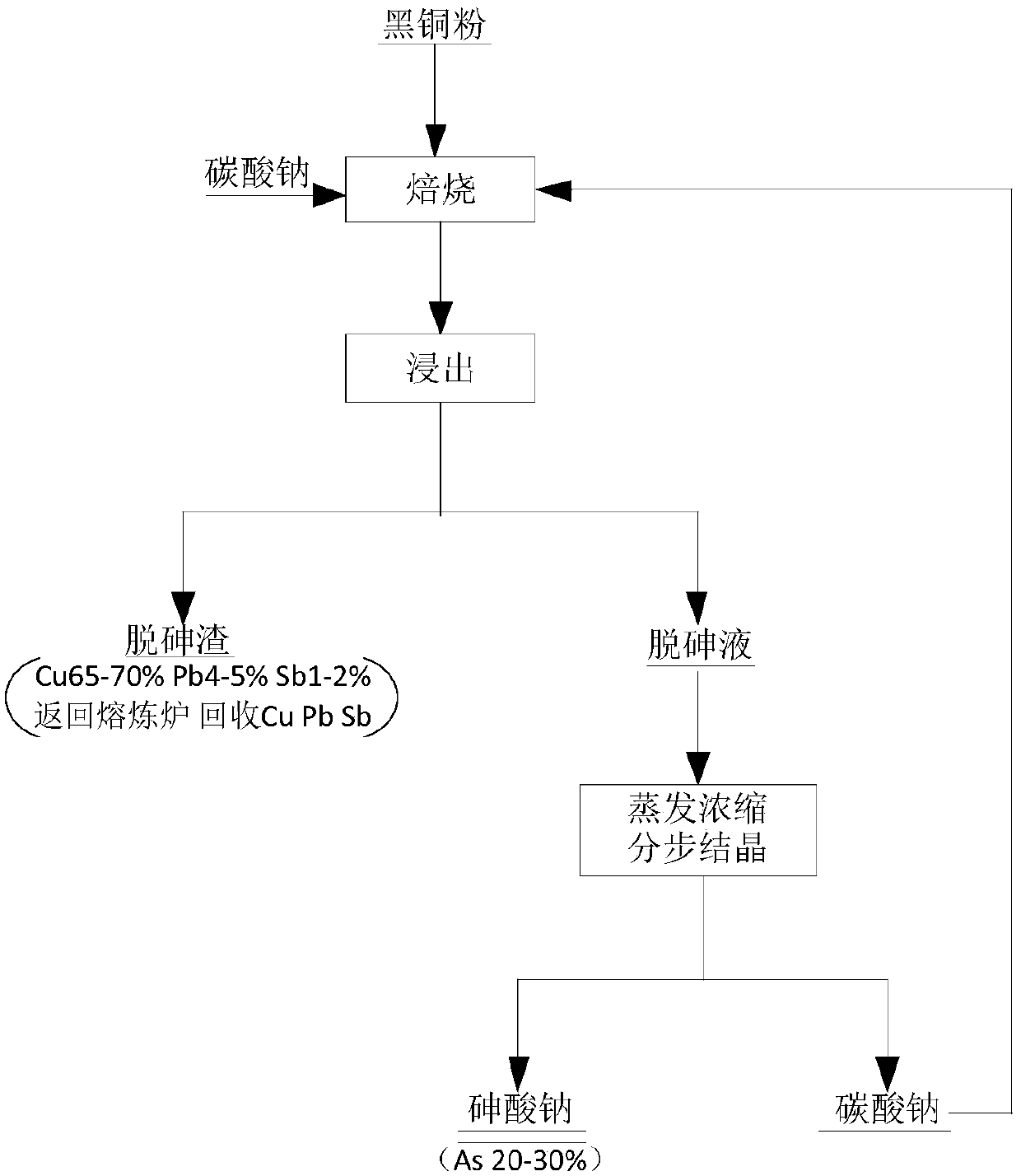
Method and application for removing arsenic from black copper sludge
InactiveCN108048664ALow impurity contentReduce pollutionArsenites/arsenatesProcess efficiency improvementEnvironmental resistanceSludge
The invention provides a method and application for removing arsenic from black copper sludge, and relates to the technical field of arsenic removal. The method for removing the arsenic from the blackcopper sludge comprises the steps that the black copper sludge and alkali are mixed and roasted; and after a roasted product and water are mixed, infiltrated and filtered, arsenic removal liquid is subjected to fractional crystallization to obtain a sodium arsenate product and alkali. The method is easy and convenient to operate and high in controllability, alkali can be cyclically utilized, resource regeneration is achieved, and environment protection and high efficiency are achieved.
Owner:GUANGDONG POLYTECHNIC OF ENVIRONMENTAL PROTECTION ENG
Method for producing magnesium arsenate by using arsenic-containing waste liquid
ActiveCN106495215AHigh purityEasy to purifyArsenites/arsenatesWater contaminantsLiquid wasteArsenate
The invention relates to a method for producing magnesium arsenate by using arsenic-containing waste liquid. The arsenic-containing waste liquid produced in the acid-making process of non-ferrous metal smelting enterprises by adopting a 'pyrogenic-process smelting-smoke acid-making system' process is used as a raw material to produce the magnesium arsenate. Firstly, iron, zinc and copper are removed in sequence, then As<3+> in the liquid is oxidized into As<5+> and Mg<2+>, full compounding is performed to produce a Mg3(AsO4)2 crude product, and finally the Mg3(AsO4)2 crude product is purified to produce a magnesium arsenate product. The arsenic-containing waste liquid is treated while high-purity magnesium arsenate is produced, and the effects of saving energy, reducing emissions and recycling resources are achieved.
Owner:林子柯
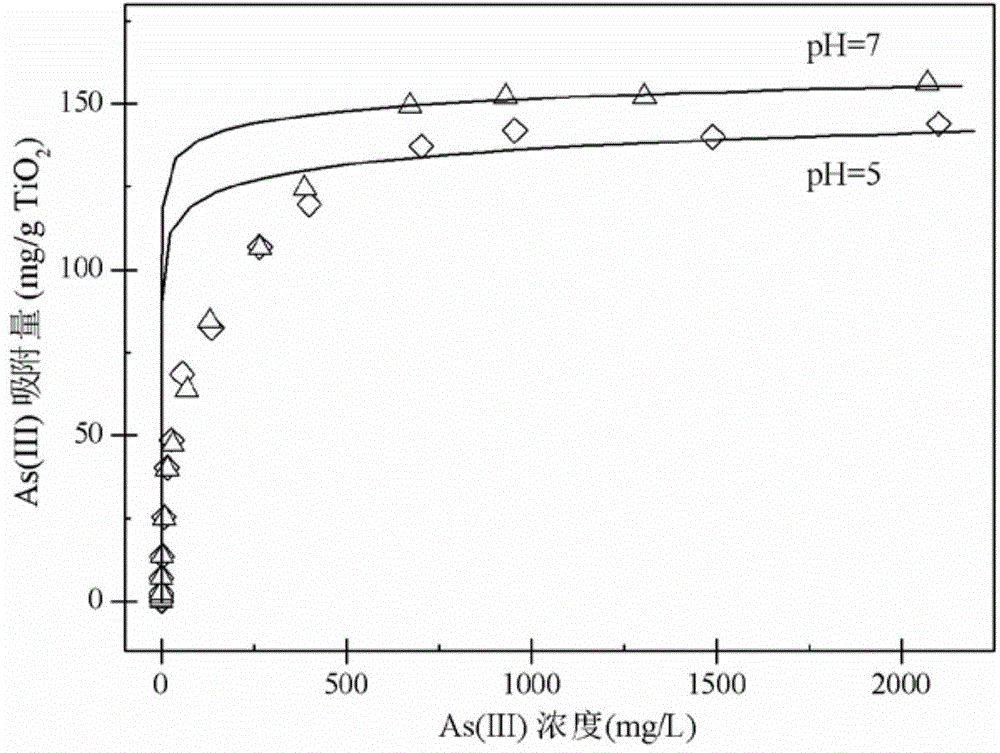
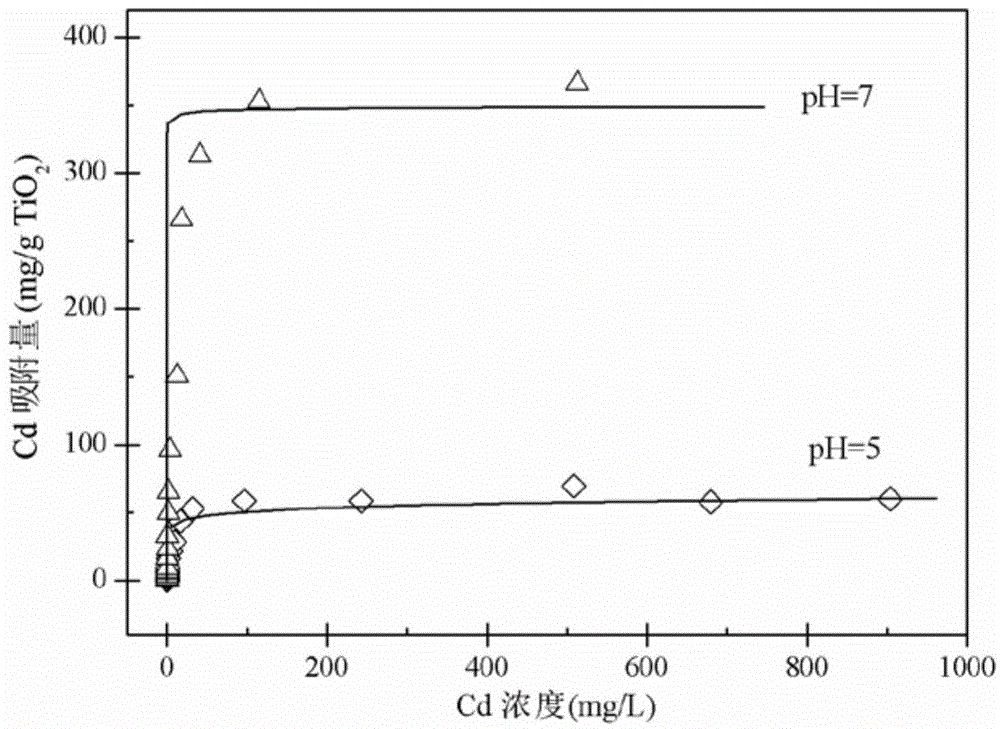
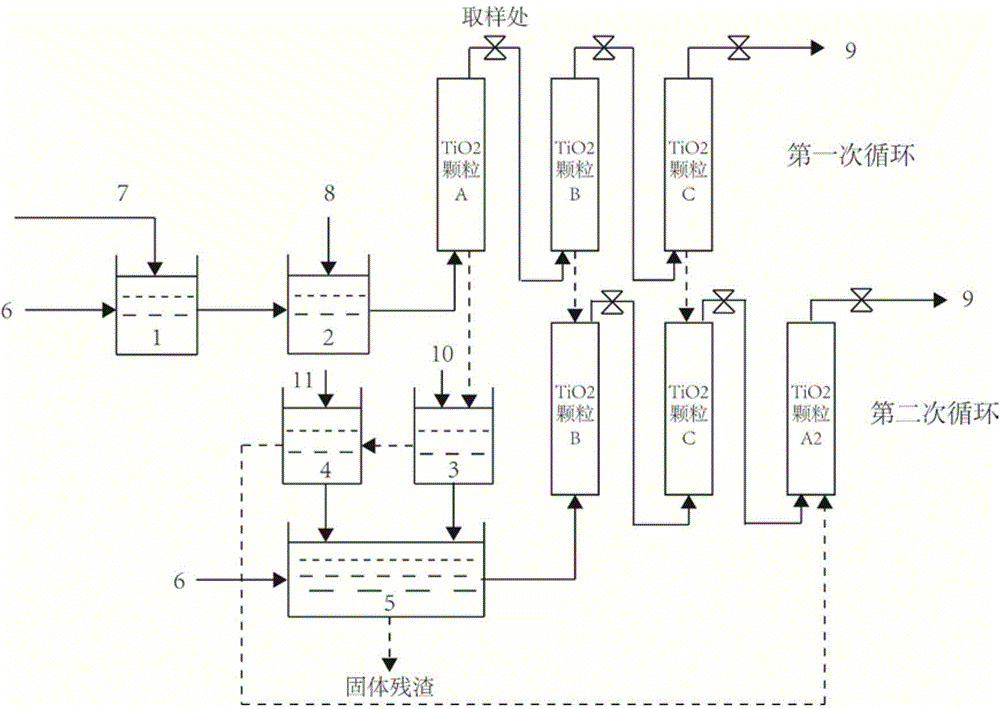
Method of treating high-concentration arsenic and cadmium in acidic waste water with granular titanium dioxide
InactiveCN104973710AImprove processing efficiencyImprove adsorption capacityArsenites/arsenatesMultistage water/sewage treatmentHigh concentrationEmission standard
The invention provides a method of treating high-concentration arsenic and cadmium in acidic waste water with granular titanium dioxide. The method includes following steps: regulating the pH of the waste water to 7 with lime milk and industrial-grade NaOH, passing the waste water through three in-series filter columns filled with the granular titanium dioxide continuously; and performing three-time continuous adsorption, so that the concentration of arsenic and cadmium in discharged water can both reach national sewage emission standard. The method can effectively remove high-concentration trivalent arsenic and heavy metal ion cadmium in the waste water, wherein the adsorbent can be recycled and reused. Meanwhile, the method also can recycle arsenic. The whole process is almost free of generation of waste residue so that the method is environment-friendly and can generate economic benefit.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Carbon combustion synthesis of oxides
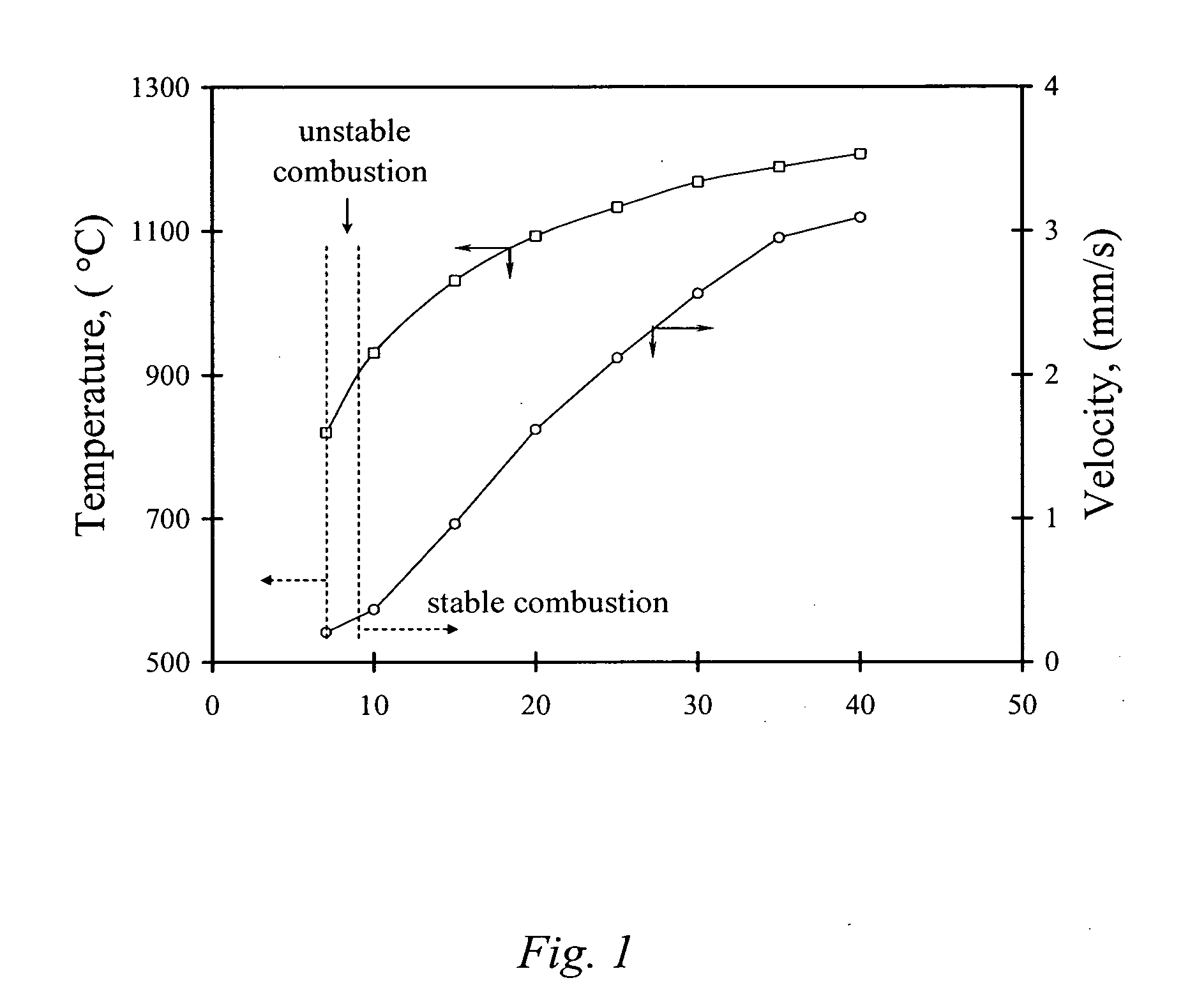
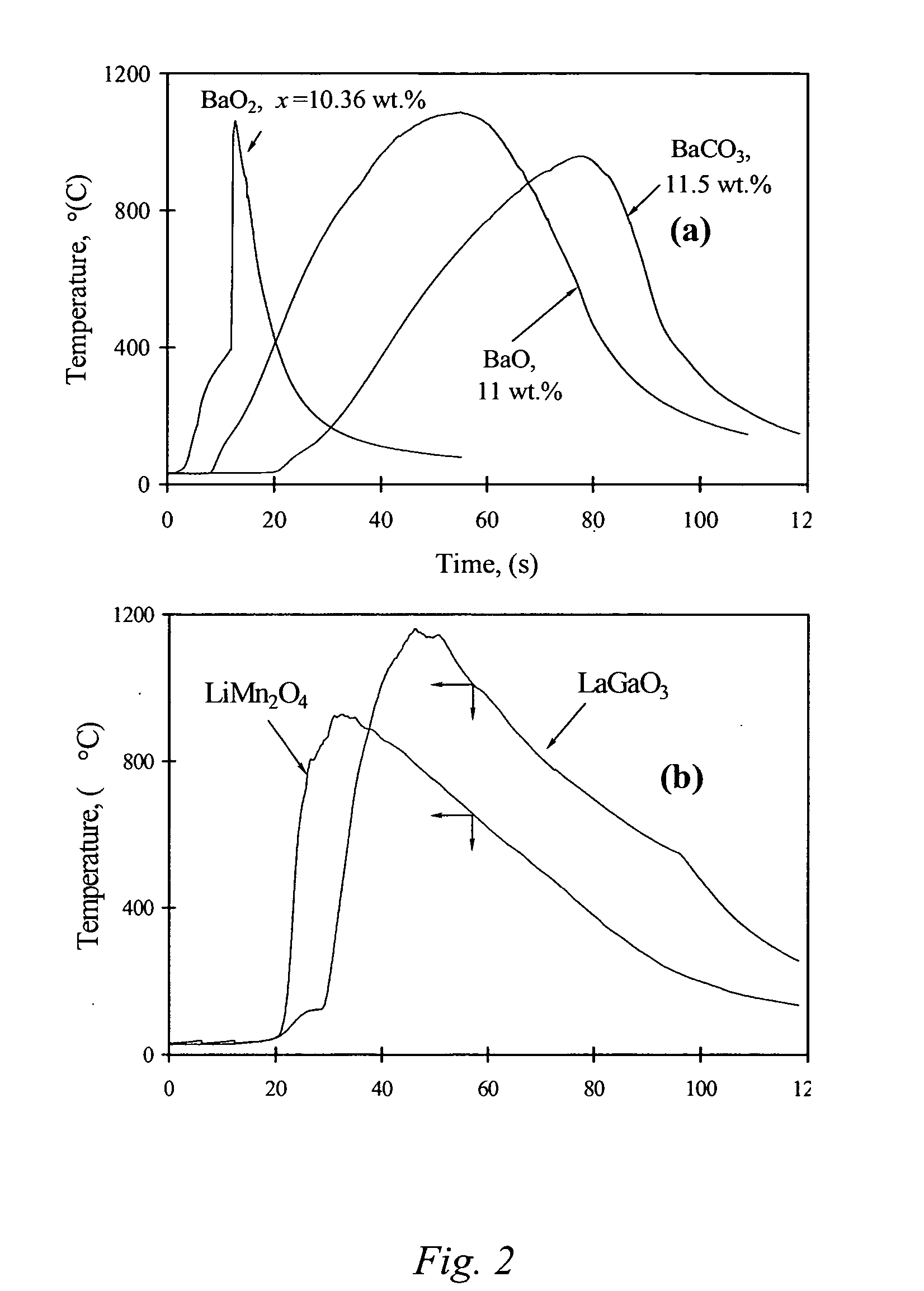
ActiveUS20060097419A1Low production costWell mixedPigmenting treatmentAlkaline earth titanatesOxide ceramicSelf-propagating high-temperature synthesis
The present invention is generally directed to a novel, economic synthesis of oxide ceramic composites. Methods of the present invention, referred to as carbon combustion synthesis of oxides (CCSO), are a modification of self-propagating high-temperature synthesis (SHS) methods in which the heat needed for the synthesis is generated by combustion of carbon in oxygen rather than that of a pure metal. This enables a more economic production of the ceramic material and minimizes the presence of intermediate metal oxides in the product. The reactant mixture generally comprises at least one oxide precursor (e.g., a metal or non metal oxide, or super oxide, or nitride, or carbonate, or chloride, or oxalate, or halides) as a reactant, but no pure metal. Pure carbon in the form of graphite or soot is added to the reactant mixture to generate the desired heat (upon ignition). The mixture is placed in a reactor and exposed to gaseous oxygen. The high-temperature exothermic reaction between the carbon and oxygen generates a self-sustaining reaction in the form of a propagating temperature wave that causes a reaction among the reactants. The reaction proceeds rapidly following ignition, and the final product comprises simple and / or complex oxides of elements present in the oxide precursor(s). CCSO also enables synthesis of oxides that cannot be produced by conventional SHS, such as when the pure metal is pyrophoric (such as Li or La) or such as when it melts at room temperature (e.g., Ga) or such as the combustion heat of the metal is relatively low.
Owner:UNIV HOUSTON SYST
Catalytic oxide anodes for high temperature fuel cells
InactiveUS20080124265A1High activityImprove conductivityArsenites/arsenatesCell electrodesCelsius DegreeLanthanide
An anode in a Direct Carbon Fuel Cell (DCFC) operating in a temperature range between 500 and 1200 degrees Celsius is provided. The anode material has high catalytic activity and selectivity for carbon oxidation, sufficient oxygen non-stoichiometry, rapid oxygen chemical diffusion, wide thermodynamic stability window to withstand reducing environment, sufficient electronic conductivity and tolerance to sulfur and CO2 environments. The anode has doped ruthenate compositions A1−xA′xRuO3, AB1−yRuyO3, or A1−xA′xB1−yRuyO3. A and A′ may be divalent, trivalent, or tetravalent cation, and B is a multivalent cation. A is among lanthanide series elements La, Ce, Pr, Nd, Sm, Eu, Gd, Dy, Er or Yb, and dopant A′ is from Group IIA, IIIB, or IVB elements. The doped ruthenates can also be a (AB1−yRuyO3) structure or an ordered Ruddlesden-Popper series ((A1−xAx′)n+1(B1−yRuy)nO3n+1) structure where n=1 or 2. The dopant B is among Group IVB, VB, VIB, VIII, IB, and IIB elements.
Owner:DIRECT CARBON TECH
Harmlessness method for treating arsenic in antimony oxide
InactiveCN103484693AWide adaptabilityAchieve separationArsenites/arsenatesProcess efficiency improvementArsenateNonferrous metal
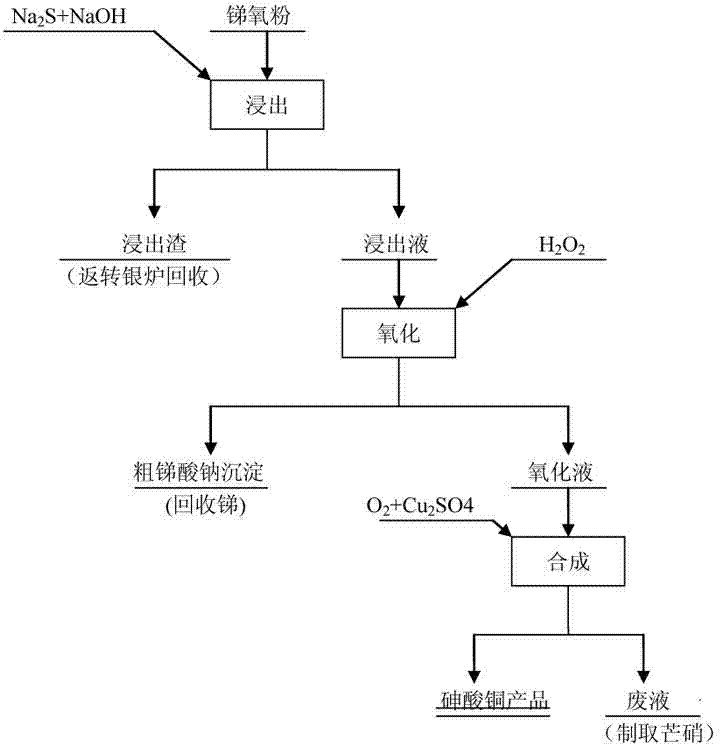
The invention relates to a harmlessness method for treating arsenic in antimony oxide, and belongs to the fields of non ferrous metal wet method metallurgy and chemical engineering. The method comprises carrying out selective leaching on antimony oxide and oxidizing to obtain an oxidation-fluid containing arsenic, and adding oxygen and copper sulphate to synthesize copper arsenate. The synthesized copper arsenate can be used for preparation of a wood preservative. The method has the advantages of simpleness, explicitness, strong adaptability of raw materials, small equipment requirement, low production cost and solution of arsenic harm.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
Pyrogenic copper smelting white smoke resource comprehensive utilization method
InactiveCN106834705AMeet the feed requirementsReduce manufacturing costArsenites/arsenatesProcess efficiency improvementElectrolysisLead smelting
The invention discloses a pyrogenic copper smelting white smoke resource comprehensive utilization method. The method comprises the following specific steps: white smoke and caustic soda are mixed in proportion; sodium sulfate is added, so that solution has a certain SO42- concentration; air is fed at 80-95 DEG C for oxidation leaching; then, the filtration is performed; and a proper amount of water is used for washing filter cakes. Filtrate has the main component of sodium arsenate, and is evaporated, condensed, cooled, crystallized, filtered and separated to obtain a sodium arsenate double salt product; and mother liquor after separation of sodium arsenate is returned to the white smoke for alkali leaching. The filter cakes in alkali leaching are pulped; sulfuric acid is added for acid leaching at 80-95 DEG C; then, the filtration is performed; the filter cakes have the main component of lead sulfate; and lead sulfate serves as a lead concentrate for lead smelting. Filtrate is copper sulfate solution; iron powder is added for replacement to obtain sponge copper; and the sponge copper is returned to a pyrogenic copper smelting system for refining as electrolyzed copper. The leaching rate of arsenic in the white smoke can reach above 95%; such precious metals as copper, lead, gold and silver are nearly not leached; the leaching rate of copper is higher than 98%; the content of lead in acid leaching slag is higher than 50%; and such precious metals as gold and silver are further enriched.
Owner:HENAN ZHONGYUAN GOLD SMELTERY
Eulytite solid acid electrolytes for electrochemical devices
Improved solid acid electrolyte materials, methods of synthesizing such materials, and electrochemical devices incorporating such materials are provided. The stable electrolyte material comprises a solid acid in a eulytine structure capable of undergoing rotational disorder of oxyanion groups and capable of extended operation at elevated temperatures, that is, solid acids having hydrogen bonded anion groups; a superprotonic disordered phase; and capable of operating at temperatures of ˜100° C. and higher.
Owner:CALIFORNIA INST OF TECH
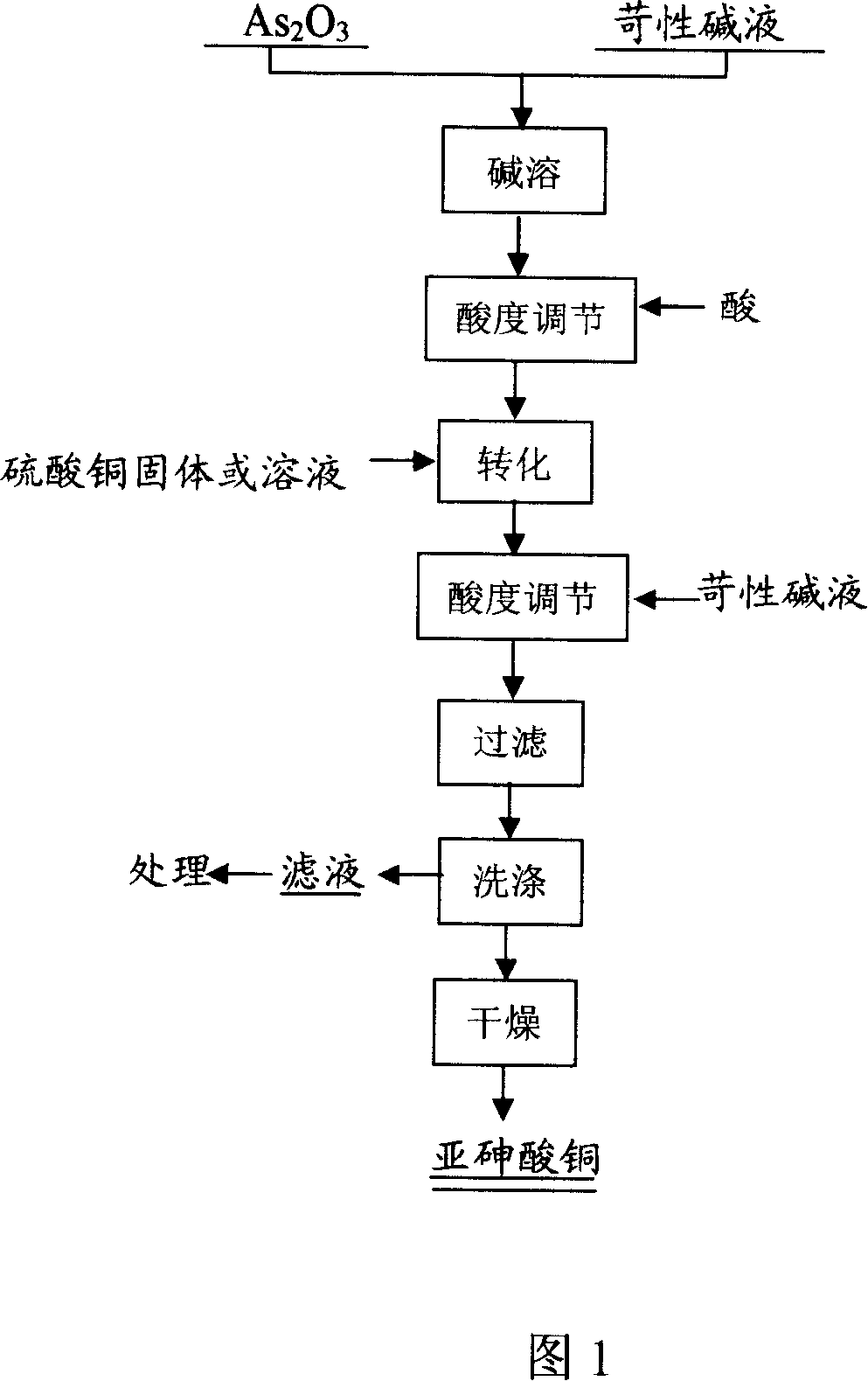
Manufacture of copper arsenite and application of the same
ActiveCN101108744AHigh removal rateEliminate long particlesArsenites/arsenatesPhotography auxillary processesPotassium hydroxideVolume concentration
A method for preparing the cupric arsenite is provided. Water is firstly added in the reactor, and sodium hydroxide or potassium hydroxide lye is added in accordance with the 0.5mol / L to 5mol / L quantity volume concentration of matters and is stirred to dissolve. The arsenic trioxide is added in accordance with the mol ratio of 3 : 2 to 3 : 1 between the hydroxyl and the arsenic of the arsenic-alkali and the pH value of the solution is adjusted to 6.0 to 10.. Under 0 DEG C. to 40 DEG C., the copper sulfate solid is added in accordance with the mol ratio of 1 : 1 to 2 : 1 between the copper and the arsenic. The caustic lye is added and the pH value of the solution is adjusted to 6.0 to 10. reversely. The cupric arsenite is gained after filtering and washing and is repeatedly washed by clear water and is added in the copper electrolyte or the decoppering final solution; the sedimentation containing As, Sb and Bi produced after reaction is filtered and removed and the removal rate of the Sb and Bi impurities in the copper electrolyte highly reaches 50 per cent to 70 per cent. The cupric arsenite added in the copper electrolyte is characterized by strong purification capacity, simple process and low cost.
Owner:CENT SOUTH UNIV +1
Novel cocrystalline metallic compounds and electrochemical redox active material employing the same
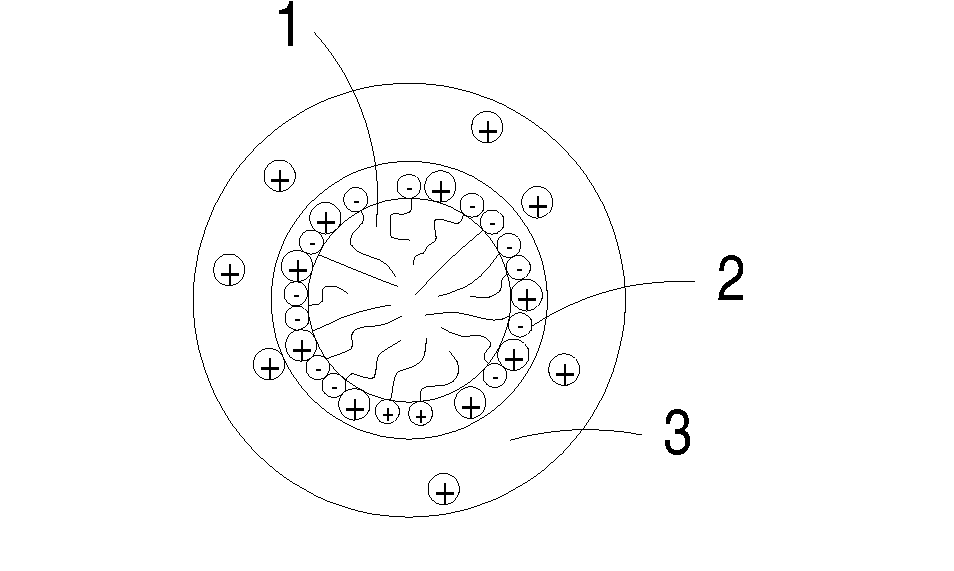
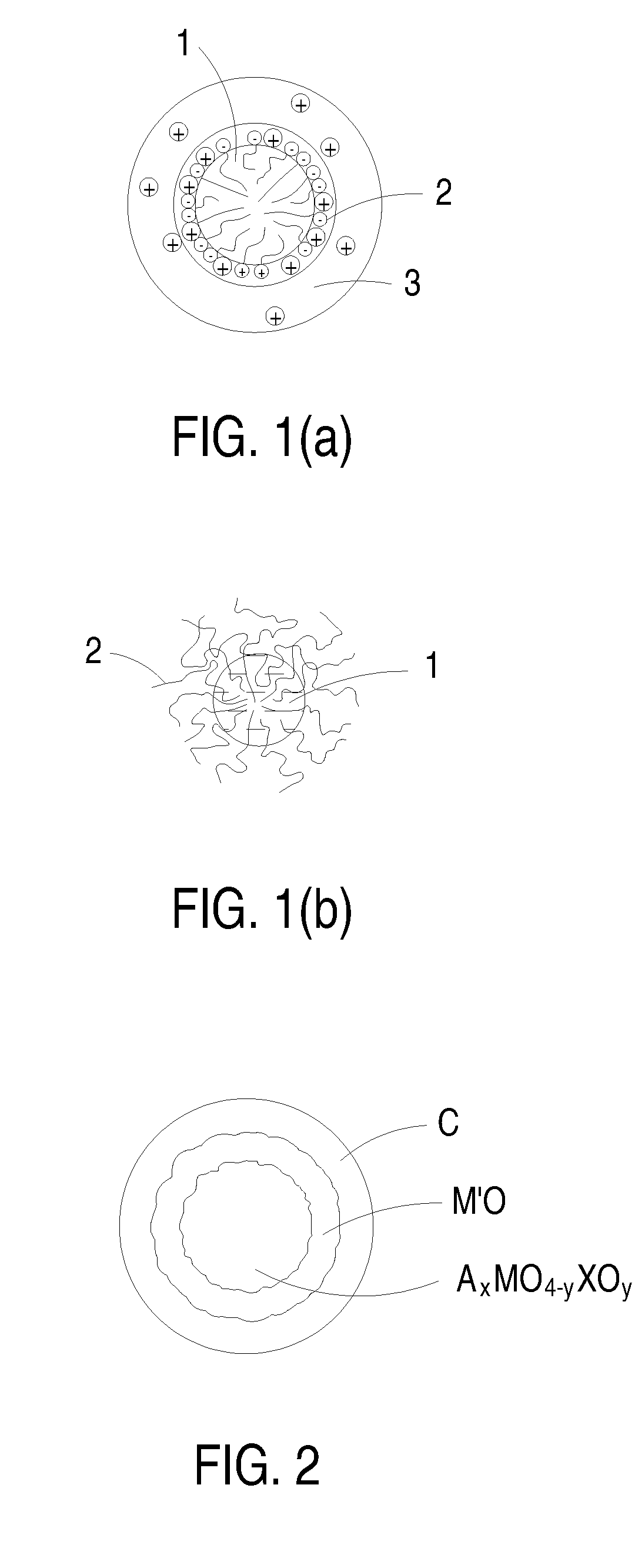
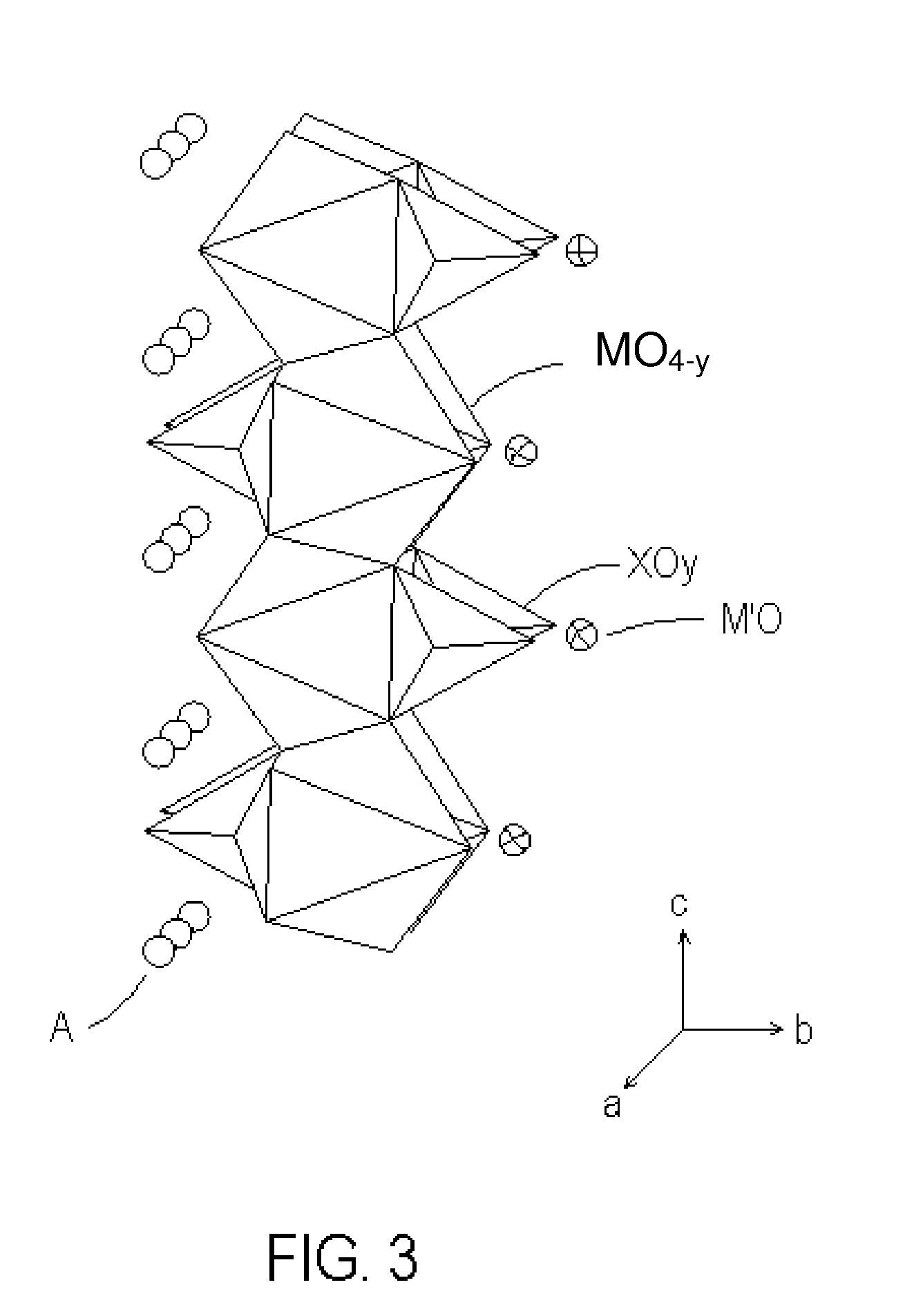
ActiveUS20080308773A1Enhanced ion diffusibilityImprove electronic conductivityMaterial nanotechnologyArsenites/arsenatesSemimetalAlkali metal
The present invention includes an electrochemical redox active material. The electrochemical redox active material includes a cocrystalline metallic compound having a general formula AxMO4-yXOy.M′O, where A is at least one metallic element selected from a group consisting of alkali metals, M and M′ may be identical or different and independently of one another at least one selected from a group consisting of transition metals and semimetals, X is P or As, 0.9≦x≦1.1, and 0<y<4.
Owner:ADVANCED LITHIUM ELECTROCHEMISTRY CO LTD
Method for manufacturing scorodite
InactiveUS7935328B2Inhibition formationArsenites/arsenatesIron oxides/hydroxidesConcentration ratioAqueous solution
Owner:JX NIPPON MINING & METALS CORP
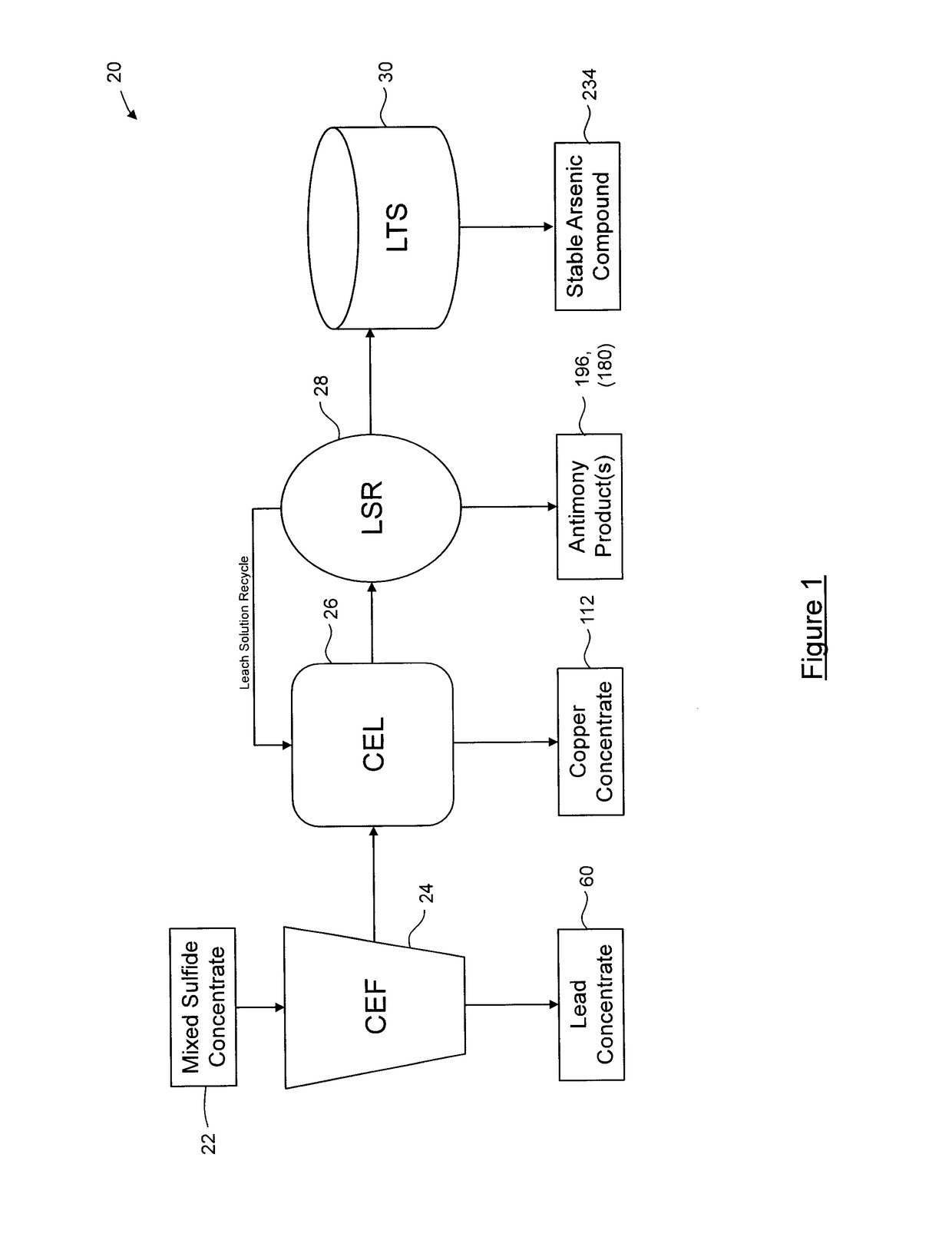
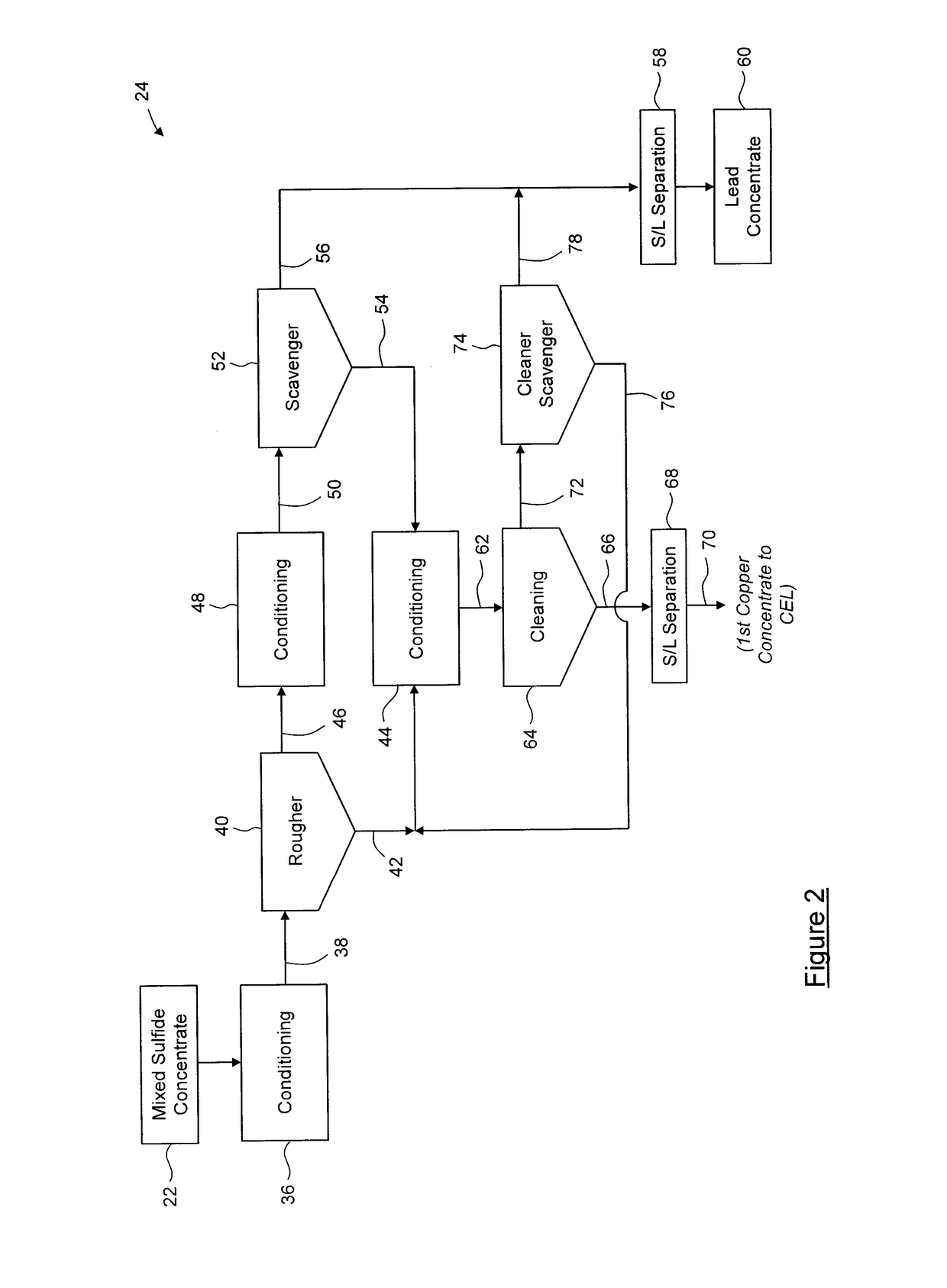
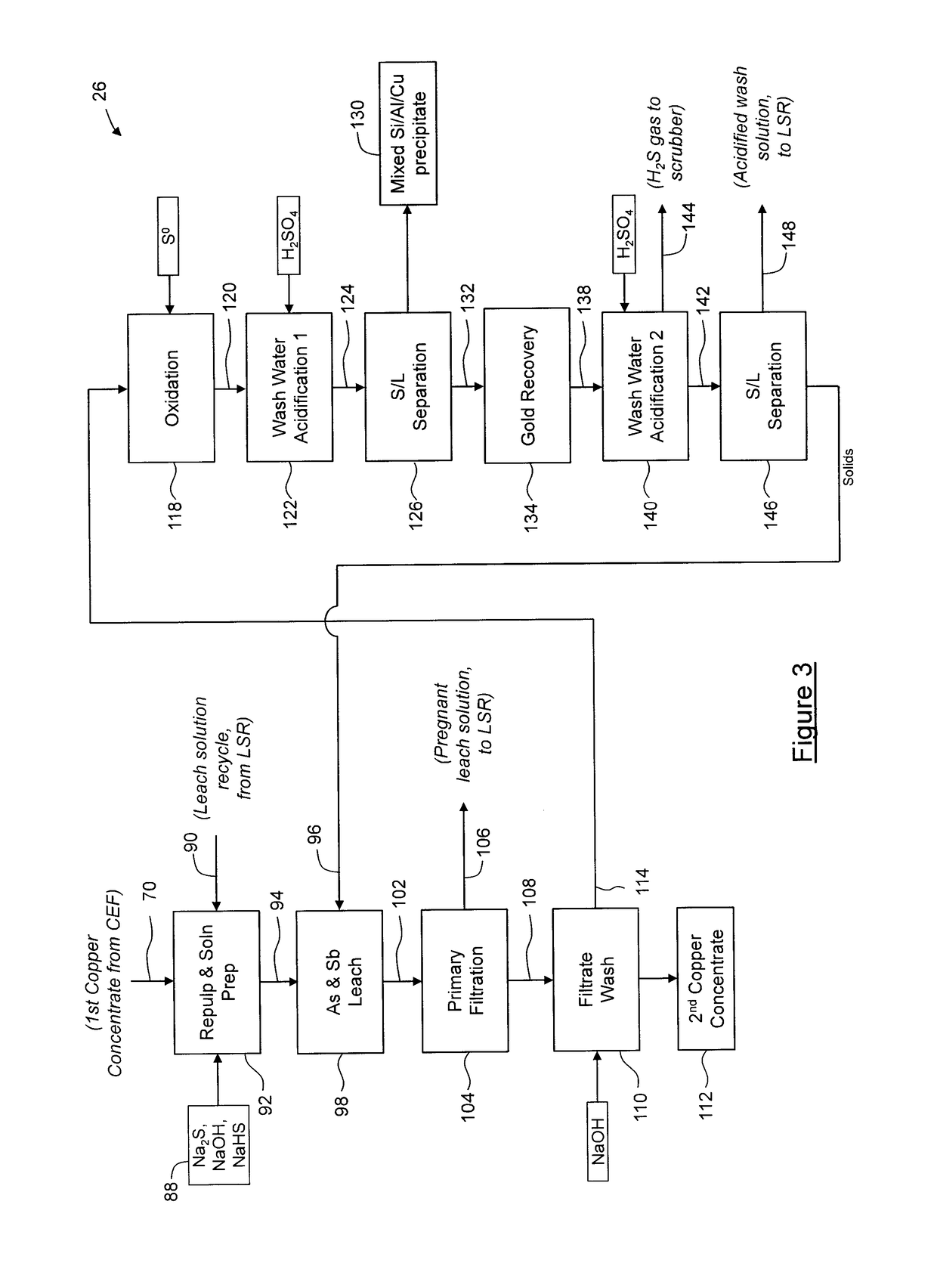
Process for separation of at least one metal sulfide from a mixed sulfide ore or concentrate
A stabilization process for an arsenic solution comprising thiosulfates, the process comprising: acidifying the arsenic solution to decompose the thiosulfates, to yield an acidified solution; oxidizing the acidified solution to oxidize residual As3+ to As5+ and reduced sulfur species to sulfates, to yield a slurry comprising elemental sulfur; separating elemental sulfur from the slurry to yield a liquid; oxidizing the liquid to oxidize residual reduced sulfur species, to yield an oxidized solution; and forming a stable arsenic compound from the oxidized solution.
Owner:GOLDCORP
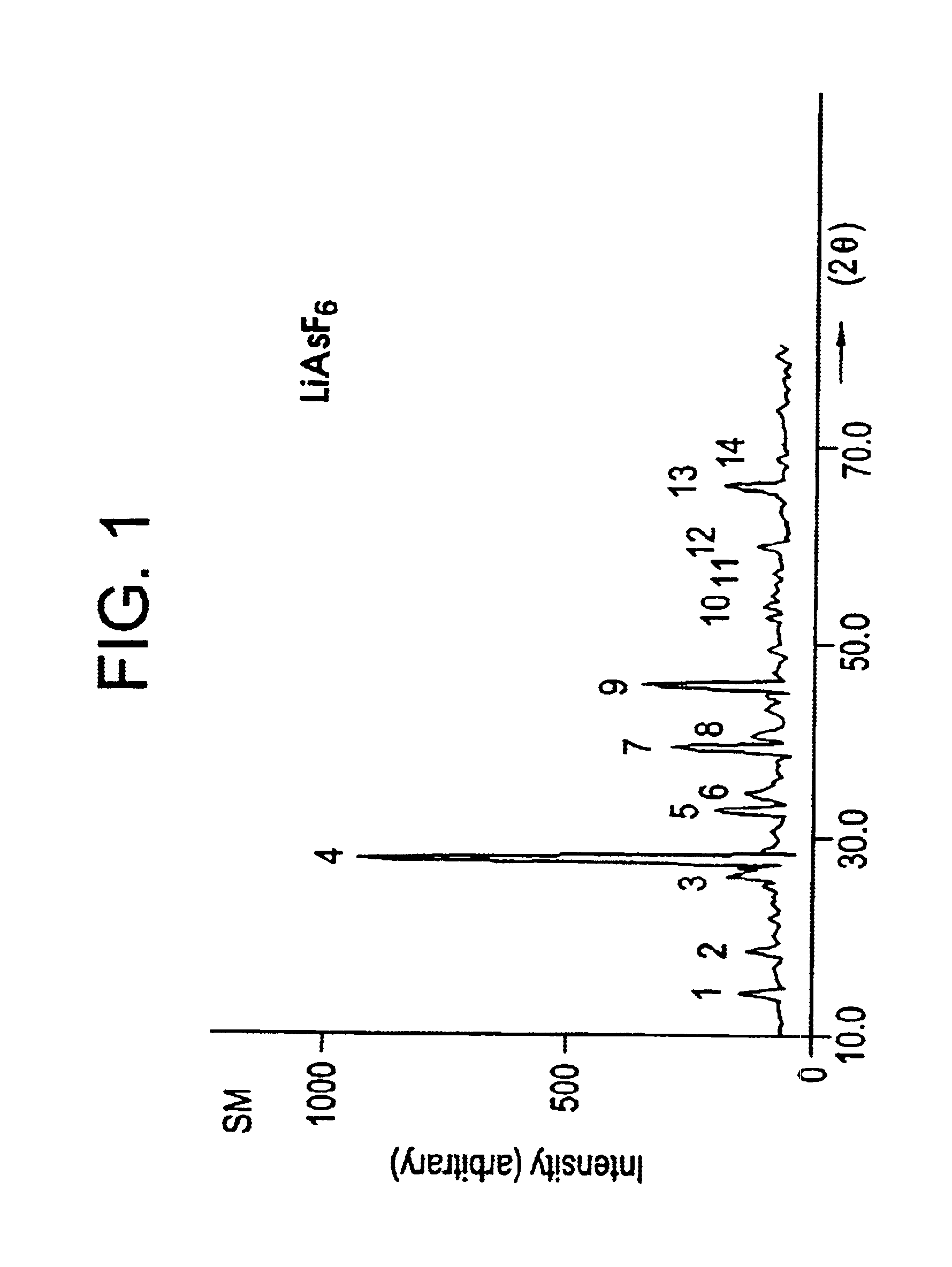
Process for the preparation of lithium hexafluoroarsenate
InactiveUS6682712B2Avoid costly equipmentArsenites/arsenatesFluoride preparationCombinatorial chemistryTwo step
The present invention provides a novel thermal solid state method for the synthesis of lithium hexfluoroarsenate either in a two step or a single step method, preferably at last substantially in the solid state.
Owner:COUNCIL OF SCI & IND RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com