Patents
Literature
84 results about "Sodium arsenate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
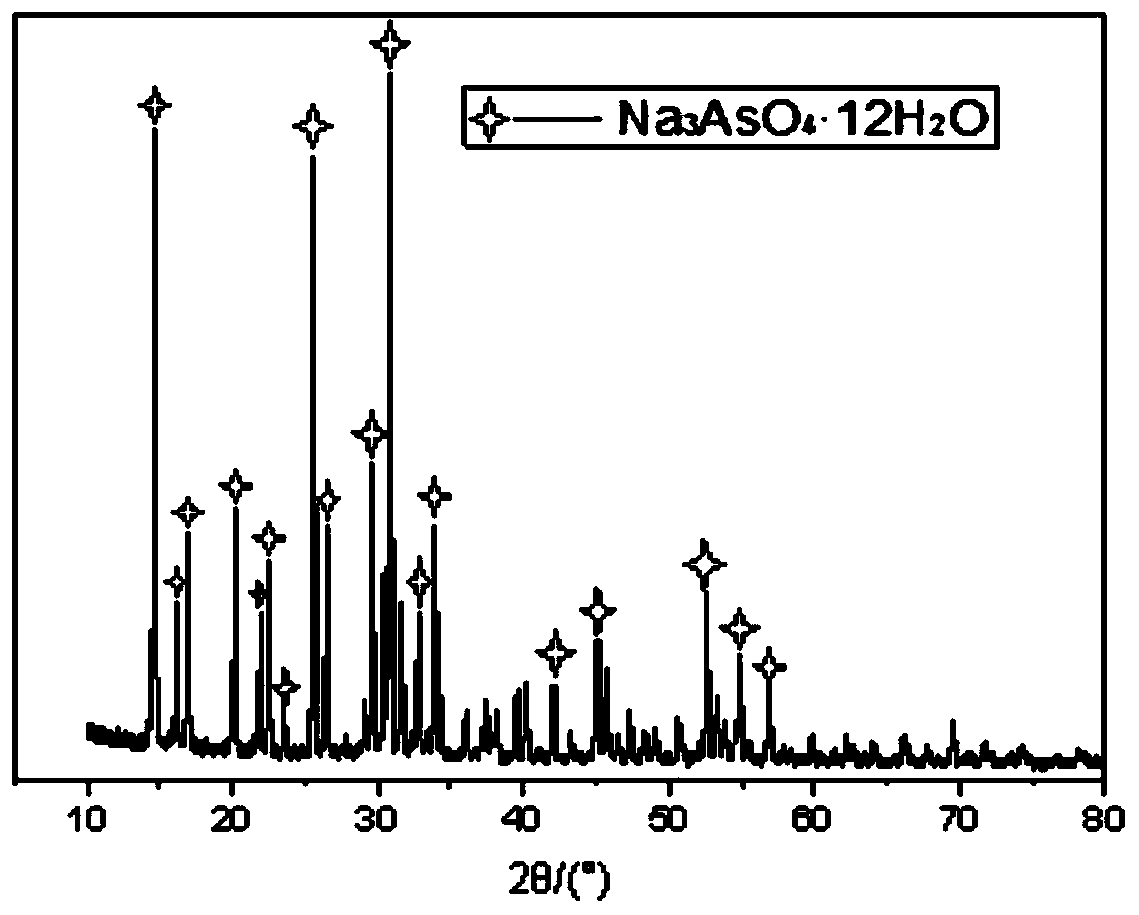
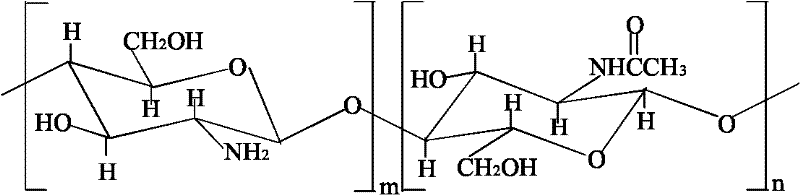
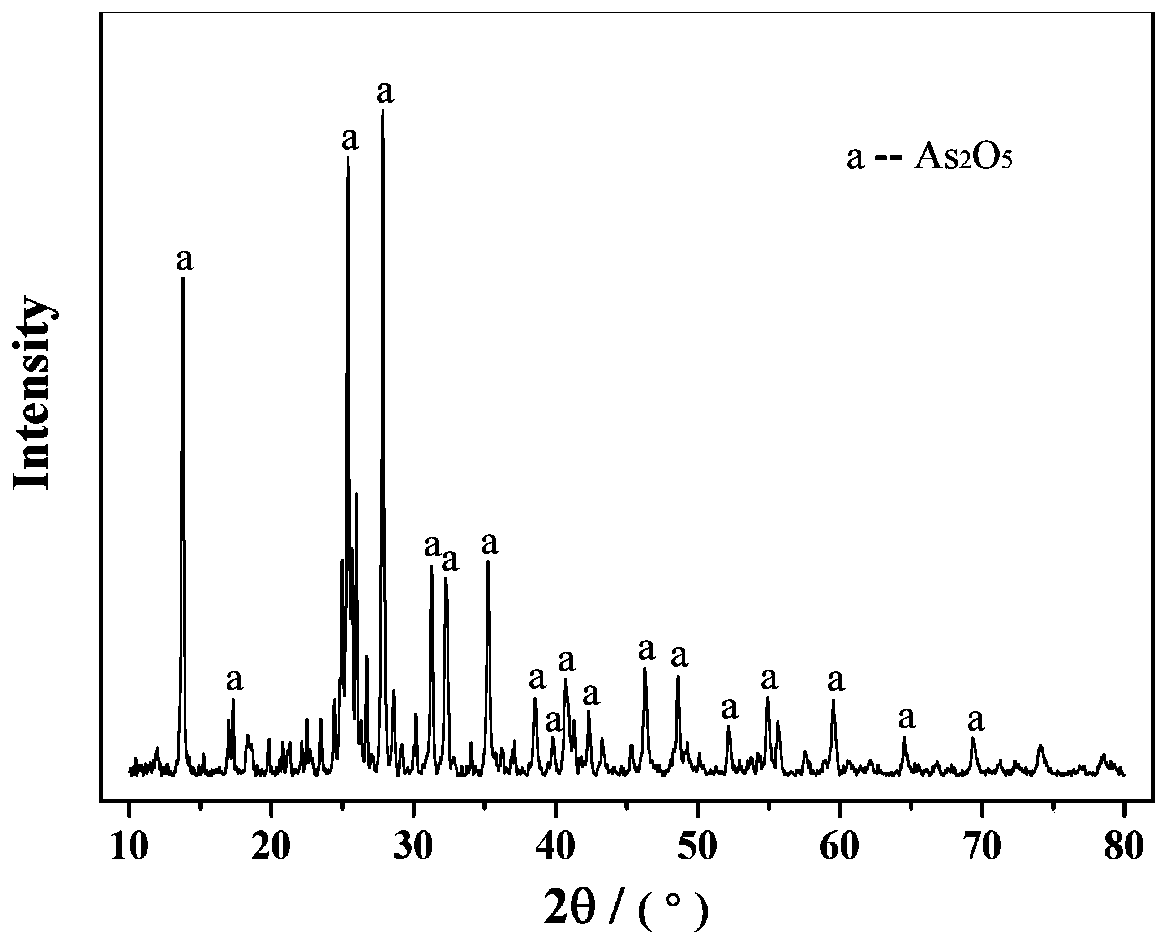
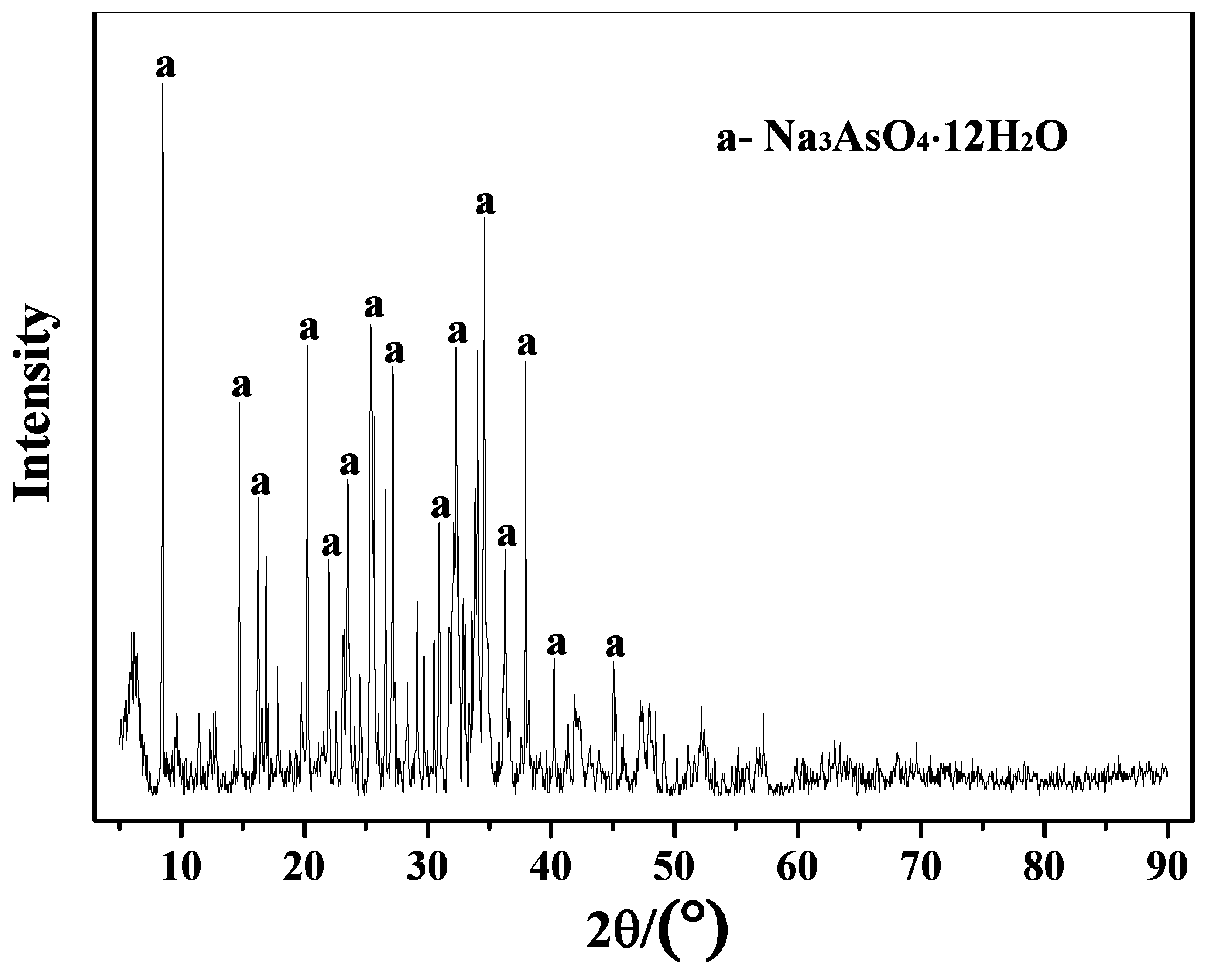
Sodium arsenate is the inorganic compound with the formula Na₃AsO₄. Related salts are also called sodium arsenate, including Na₂HAsO₄ (disodium hydrogen arsenate) and NaH₂AsO₄ (sodium dihydrogen arsenate). The trisodium salt is a white or colourless solid that is highly toxic. It is usually handled as the dodecahydrate Na₃AsO₄·12H₂O.
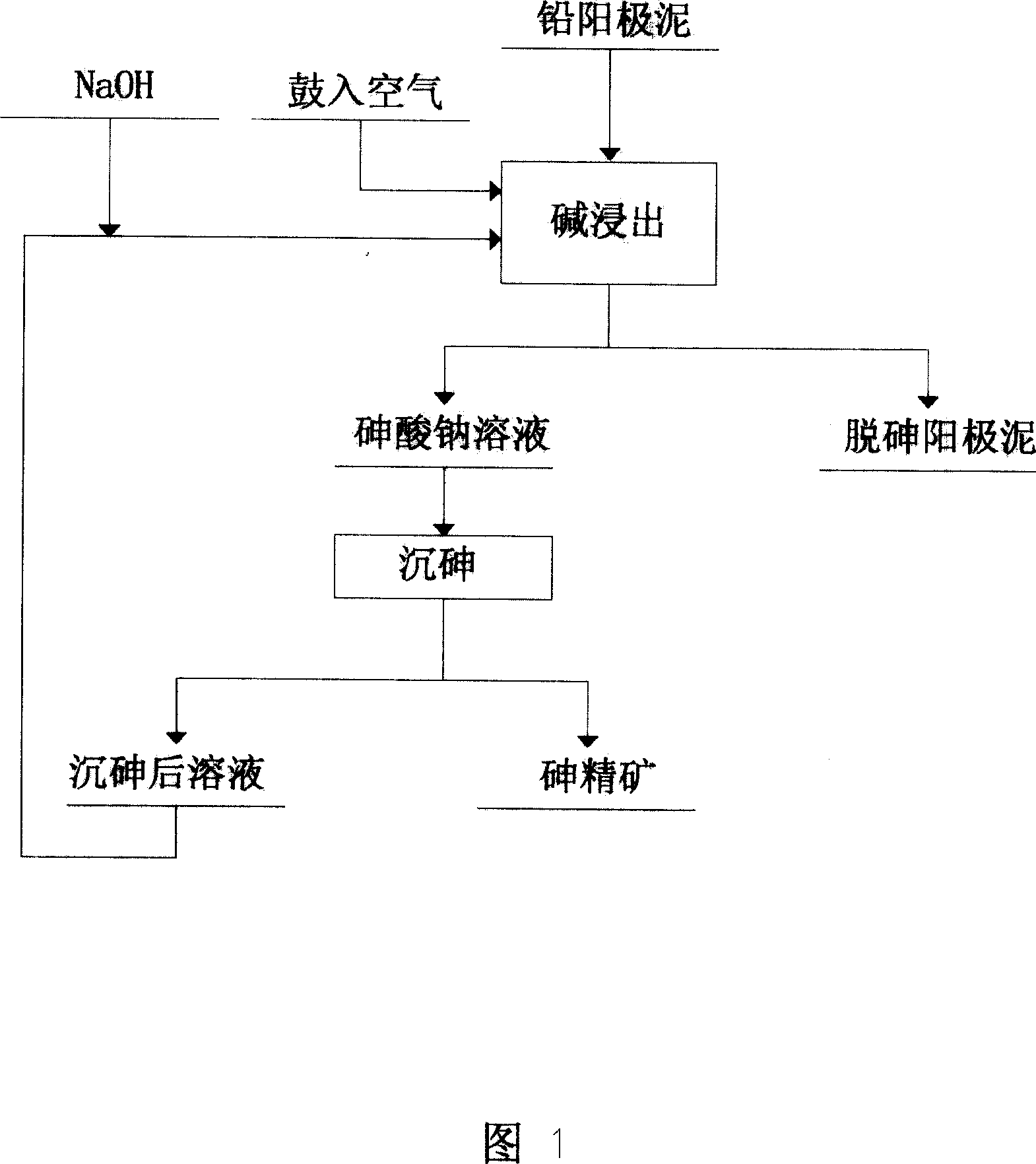
Method of removing arsenic for anode mud with high arsenic and lead content
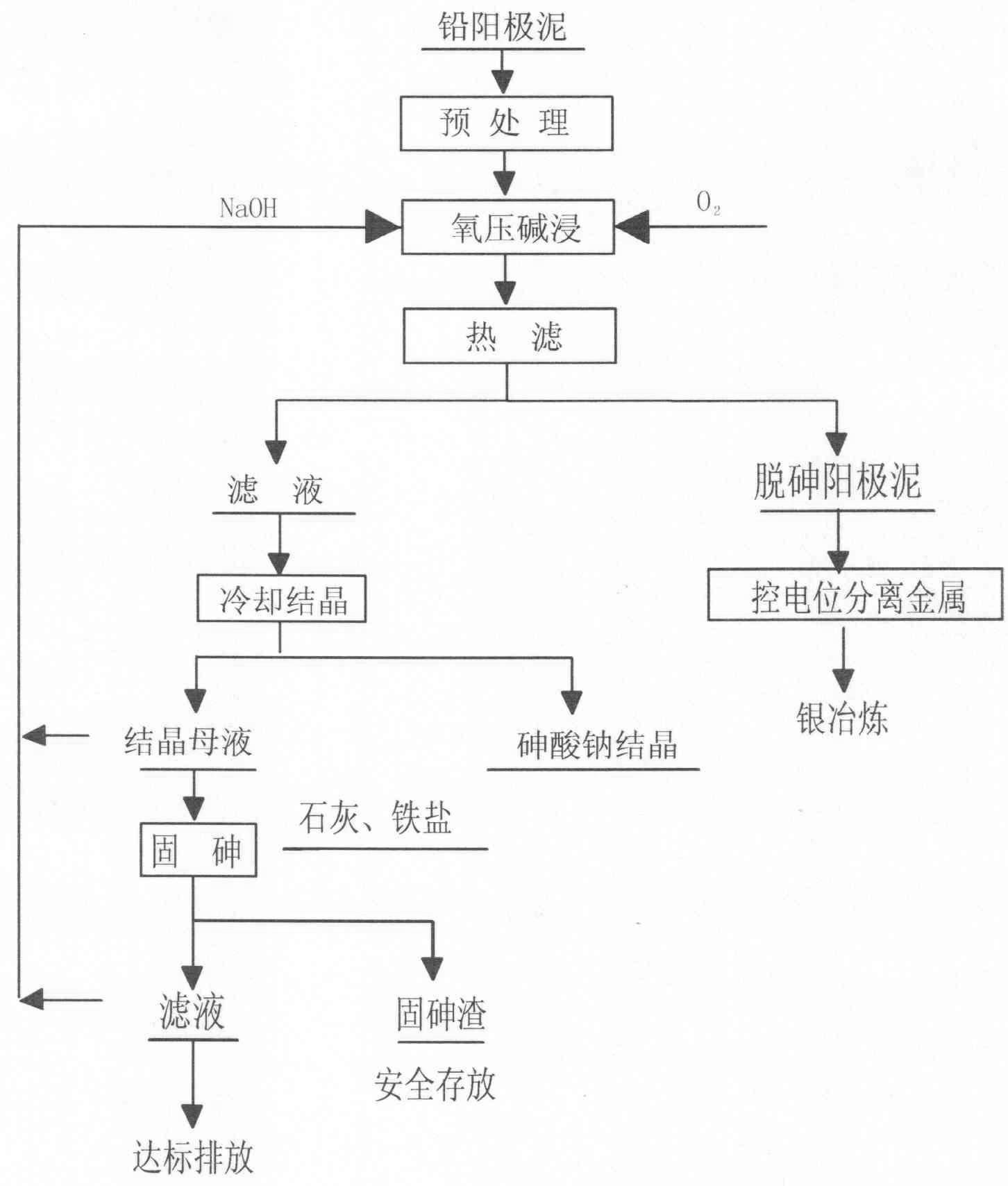
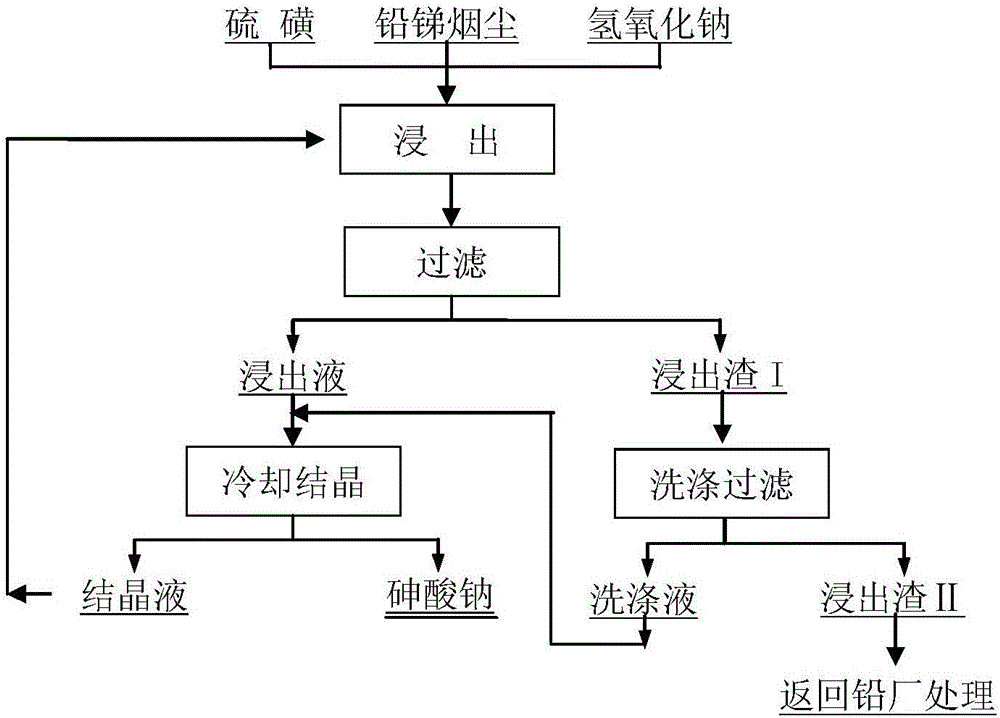
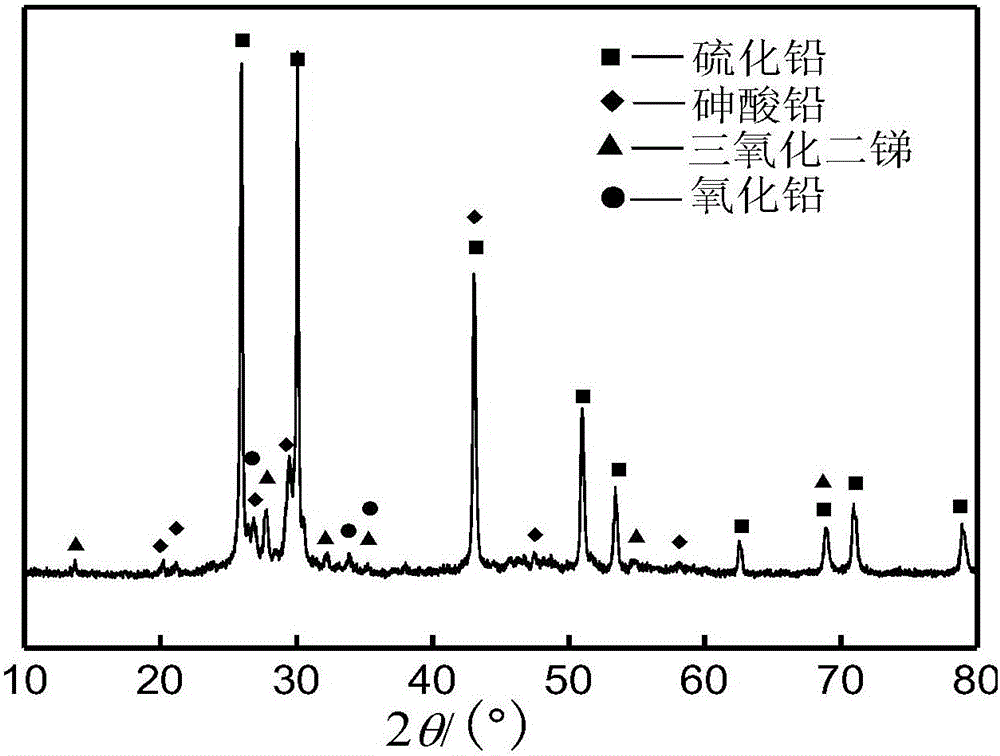
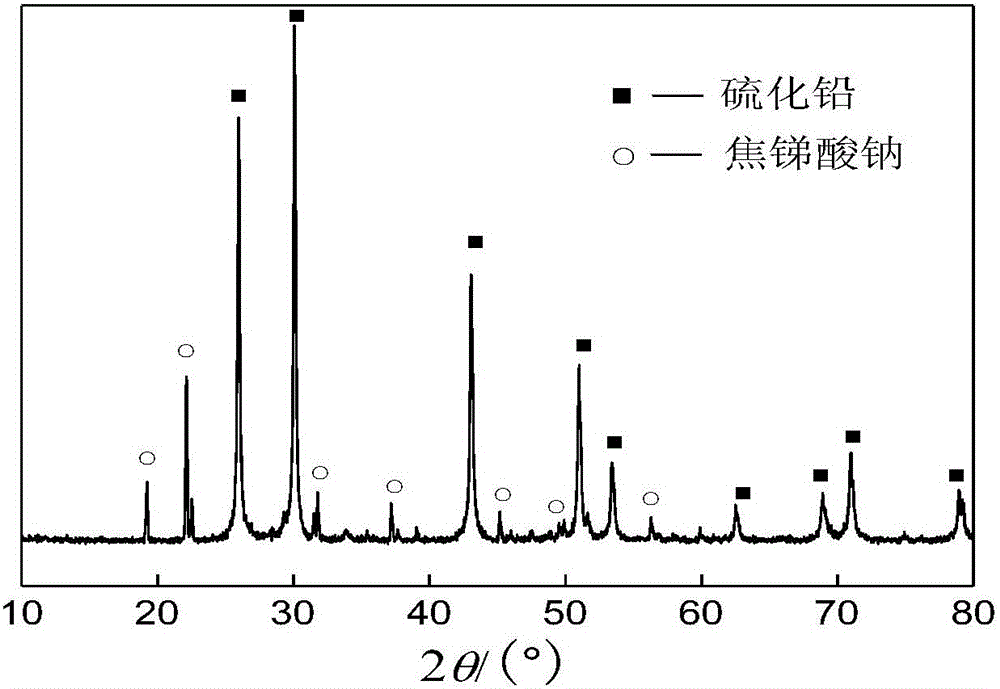
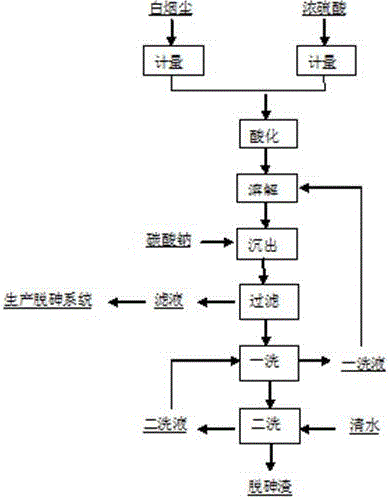
The invention discloses a method to remove arsenic with high arsenic lead anode mud in noble metal metallurgical technical domain, which comprises the following steps: adding alkali liquor into high arsenic lead anode mud; leaching with alkali under the condition of air-blowing and heating; removing the arsenic; getting lead anode mud; reclaiming god and silver and other valuable metal with known craft; incorporating sodium arsenate in the leachate; depositing arsenic with lime; reclaiming arsenic clean ore; supplementing alkali liquor for the solution after depositing arsenic; backing to alkaline leaching process. This invention can make arsenic of anode mud decrease to below 1% and does not need any alteration for original craft.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
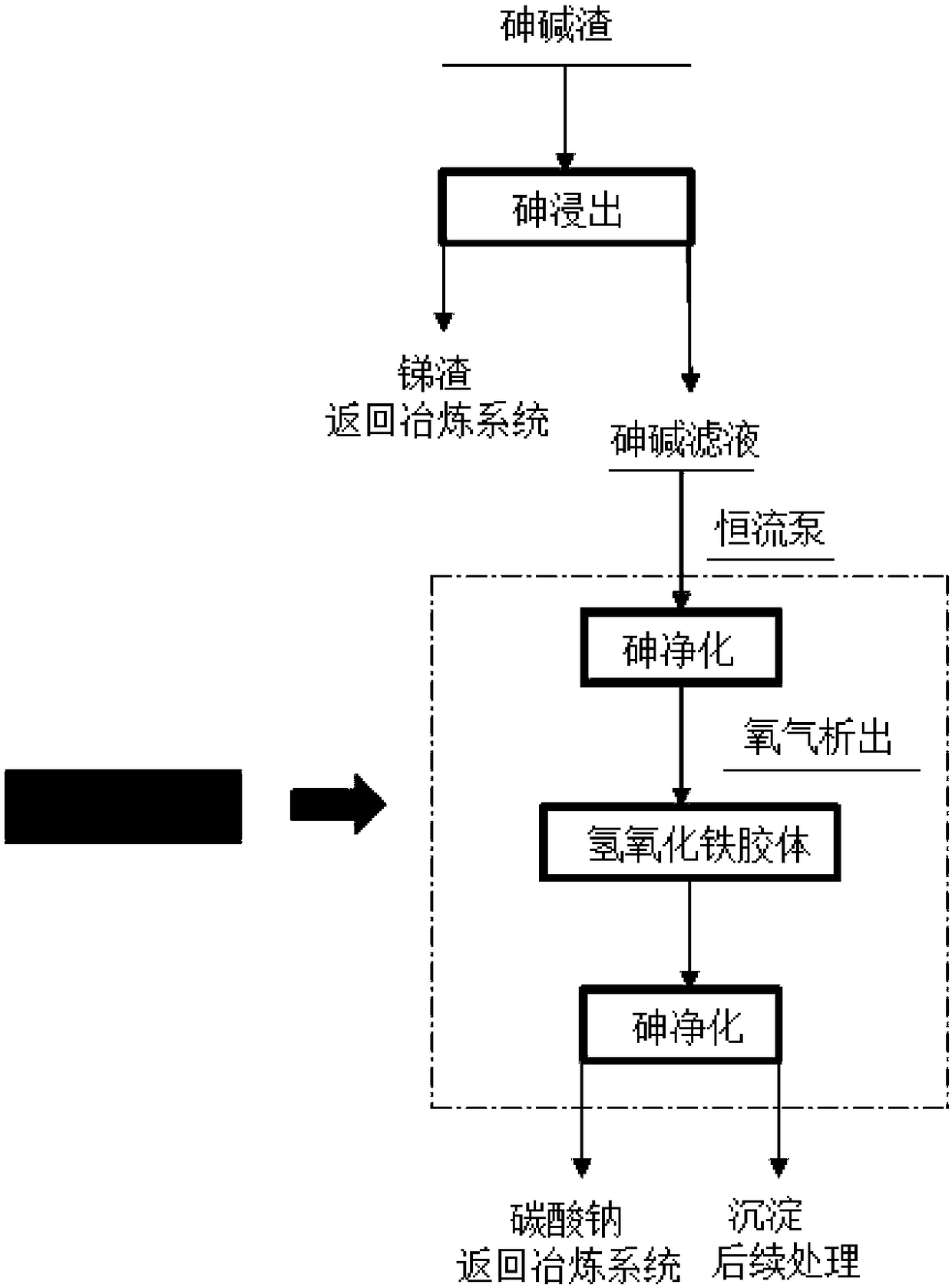
Treatment method for vitrifying arsenic-alkali residue
InactiveCN102965517ARecycling and recovery technologiesProcess efficiency improvementMolten stateIngot casting
The invention discloses a treatment method for vitrifying arsenic-alkali residue, aiming at solving the problem of pollution or potential menace of the existing arsenic-alkali residue treatment process on environment. The treatment method for vitrifying the arsenic-alkali residue comprises the following steps of: selectively reducing sodium antimonate in the arsenic-alkali residue to metallic antimony by a carbon reducing agent in a molten state and keeping arsenic in the residue in the form of sodium arsenate; and then adding a glass molten solvent in the arsenic-containing residue to form a low-temperature glass phase, discharging the glass phase, and then carrying out water-quenching on the glass phase into broken glass pieces or directly casting the glass phase into a glass ingot in an ingot casting mould, thus facilitating piling up or returning to a pit and land-filling. According to the invention, the recovery rate of antimony in the arsenic-alkali residue is greater than 95%, and the content of arsenic in the metallic antimony is less than 3%; and the arsenic-containing glass pieces belong to general solid wastes and can be directly piled up in the open air or land-filled.
Owner:CENT SOUTH UNIV
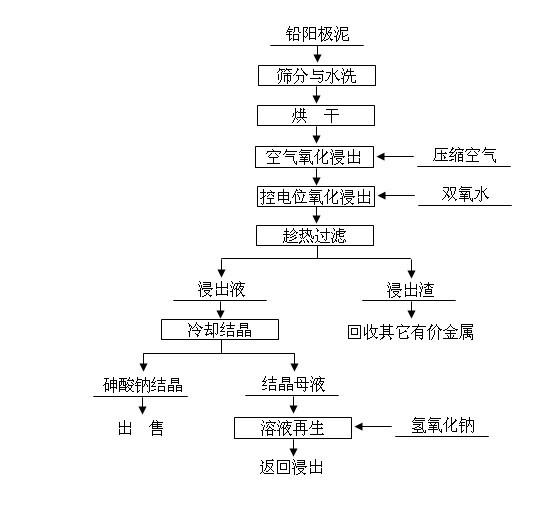
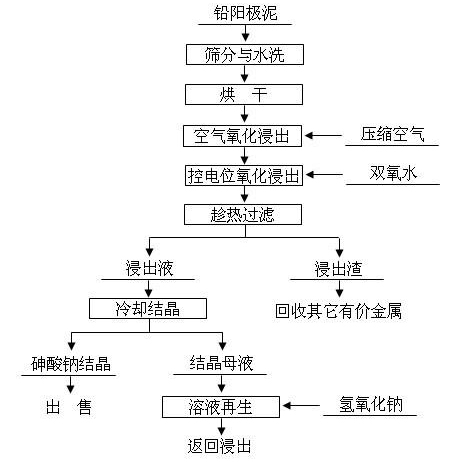
Method for removing and recovering arsenic from lead anode slime
ActiveCN101928838AHigh removal rateAvoid secondary pollutionProcess efficiency improvementArsenic pollutionSodium hydroxide
The invention discloses a method for removing and recovering arsenic from lead anode slime, which comprises the following steps of: performing screening, hot water washing and baking on the lead anode slime, performing oxidation leaching by controlling potential in sodium hydroxide solution, oxidizing the arsenic by using compressed air and hydrogen peroxide as oxidants respectively, adding the oxidized arsenic into alkaline leachate, and ensuring metals such as bismuth, lead, stibium and copper are oxidized and then enter alkaline leaching residue together with noble metals; and after alkaline oxidization leaching process is finished, filtering while hot, performing cooling crystallization on the leachate to generate sodium arsenate crystals, supplementing certain sodium hydroxide to crystallization mother liquor, returning the crystallization mother liquor to the leaching process, and separating and recovering the arsenic and other valuable metals from the lead anode slime. The arsenic leaching rate is over 98.0 percent and the secondary arsenic pollution is free; and the method has the advantages of low requirement on equipment materials, safe operation, low labor intensity, short treatment time and good operating environment.
Owner:YONGZHOU FUJIA NON FERROUS METALS
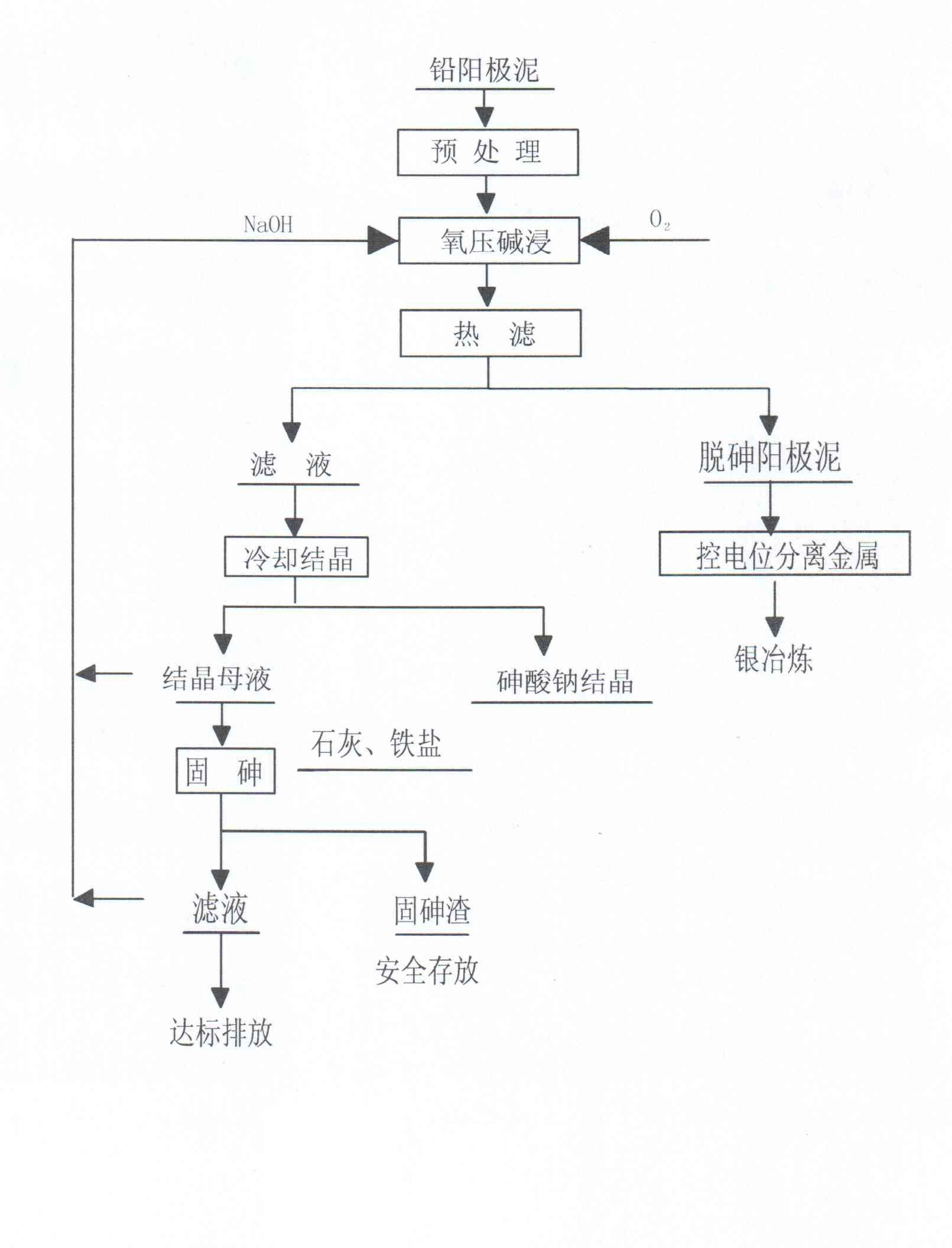
Method for dearsenicating fresh high-arsenium lead anode slime under oxygen pressure
InactiveCN102634666AEfficient removalSlag contains little arsenicProcess efficiency improvementFiltrationLow oxygen
The invention provides a method dearsenicating fresh high-arsenium lead anode slime under oxygen pressure, which comprises the following steps: pretreating lead anode slime, adding alkali, introducing oxygen, carrying out alkali dip under oxygen pressure, and carrying out hot filtration to form a filtrate and dearsenicated anode slime; after separating metals from the dearsenicated anode slime, smelting to acquire silver; and cooling to crystallize the filtrate, and separating sodium arsenate crystals and crystallization mother liquor, wherein the crystallization mother liquor is subjected toarsenium fixation to form arsenium fixed residues which are stored, and the filtration after arsenium fixation is subjected to environment-friendly emission. The method provided by the invention has the characteristics of strong selectivity in the reaction process, favorable dearsenication effect, high recovery rate of noble metals (gold and silver), low oxygen consumption and low energy consumption, and is simple to operate. The invention is suitable for dearsenicating lead anode slime in the hydrometallurgical process.
Owner:HULUDAO ZINC IND CO LTD
Method for recovering rhenium from arsenic filter cake
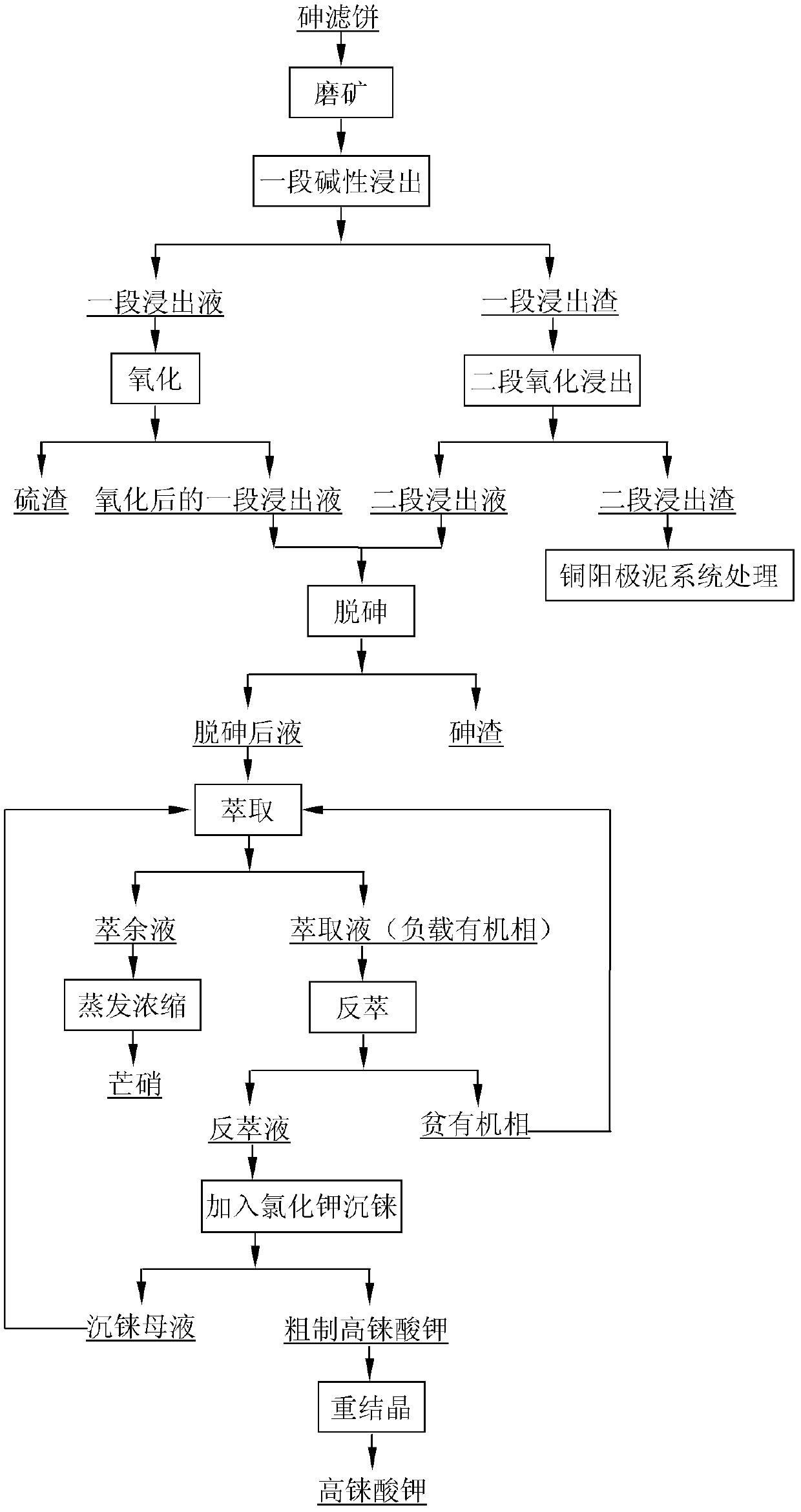
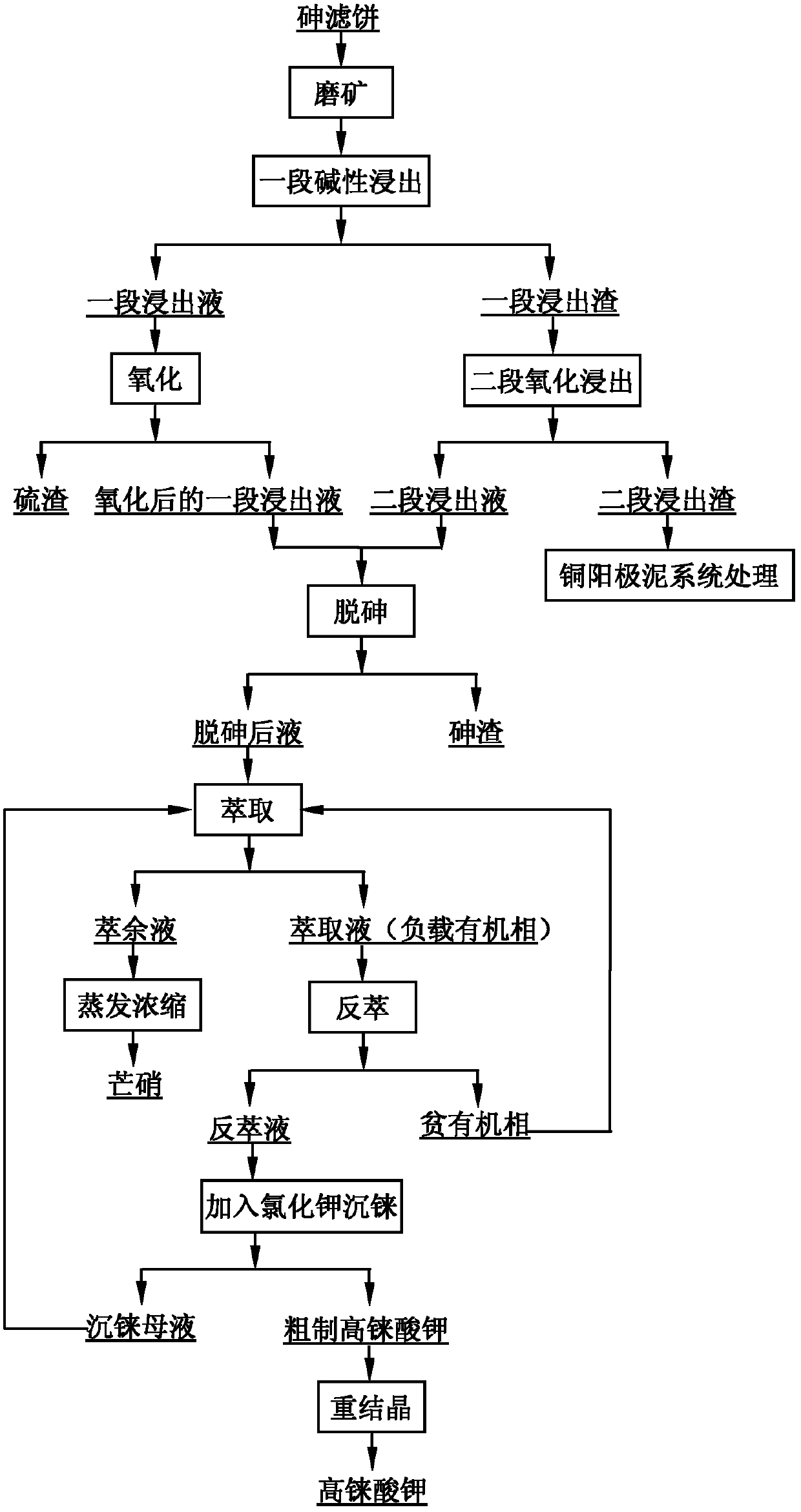
The invention provides a method for recovering rhenium from an arsenic filter cake. The method comprises the following steps: entering rhenium, arsenic and sulfur into a solution by adopting one-section alkali leaching and second-section oxidization and leaching, and simultaneously enriching lead, bismuth, copper and selenium in leaching slag; in the one-section leachate, converting rhenium into perrhenate in a form of sodium thioperrhenate by adopting an oxidization method, converting sodium thioarsenate into sodium arsenate, and separating out sulfur in a form of simple substance sulfur; removing arsenic from the solution obtained by oxidization conversion by using a sulfur dioxide reduction method or lime method; carrying out rhenium enrichment on the solution obtained after arsenic isremoved; treating a rhenium reextraction liquid by using a potassium chloride precipitation method to obtain crude potassium perrhenate; and refining crude potassium perrhenate to obtain potassium perrhenate. The method has short process flow, high rhenium recovery rate and low treatment cost; and by using the method, the comprehensive utilization of valuable metals in the arsenic filter cake canbe realized.
Owner:HUNAN RES INST FOR NONFERROUS METALS +1
Gradient arsenic removing method for high-arsenic metallurgical wastes
ActiveCN102409165AReduce manufacturing costChoose good dissolutionProcess efficiency improvementPotassiumOxidizing acid
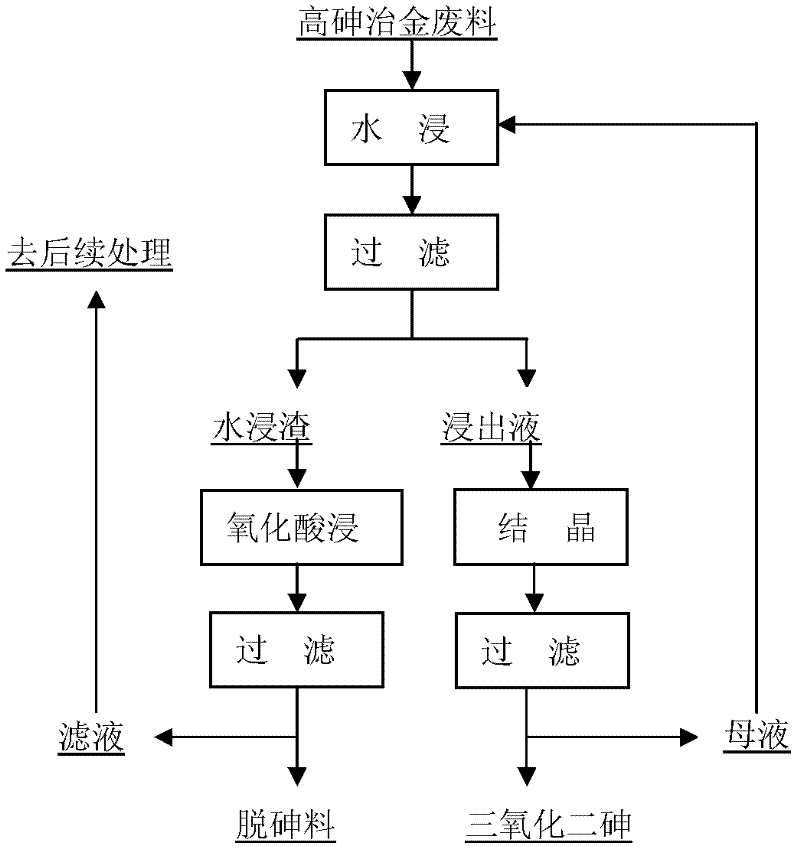
The invention provides a gradient arsenic removing method for high-arsenic metallurgical wastes, which is generally suitable for comprehensive arsenic removing treatments of high-arsenic smoke dust generated in the smelting process of lead, zinc, antimony, copper, tin and the like, high-arsenic anode mud generated in the electrolytic process of wet lead, silver, copper and the like and other metallurgical wastes. The method comprises two stages of water leaching arsenic removal and oxidizing acid leaching arsenic removal and specifically comprises the following steps of: first, selectively dissolving out free arsenic trioxide and water soluble arsenate (like sodium arsenate and potassium arsenate) through water leaching; and then further leaching out indissolvable arsenate and arsenic sulfide in the water leaching residue as well as little incomplete arsenic trioxide dissolved out by water by using a mixed leaching liquor of acid and water soluble oxidizing agent. The method provided by the invention has the advantages of low consumption of acid and alkali, high arsenic removing efficiency, safety, environment friendliness and suitability for arsenic removing treatment of various arsenic contained metallurgical wastes, in particular for arsenic removing treatment of the smoke dust with high content of free arsenic trioxide.
Owner:HUNAN ZHANTAI NON FERROUS METALS
Method for preferably removing arsenic in lead and antimony smoke
ActiveCN105039722AHigh removal rateAchieve selective removalProcess efficiency improvementPregnant leach solutionRoom temperature
The invention discloses a method for preferably removing arsenic in lead and antimony smoke. The method comprises the following steps that firstly, sulphur and the lead and antimony smoke are mixed according to the mass ratio of 0.01-0.5:1, then the mixture is added into a sodium hydroxide solution to be leached, and meanwhile the temperature of the solution is kept within the range from 50 DEG C to 100 DEG C; secondly, liquid and solid separation is carried out on the solution leached in the first step, and a leaching agent and leaching residues are obtained; the leaching residues are washed, washing water obtained after washing is completed and the leaching agent are mixed and cooled to the room temperature, and filtering is carried out to obtain sodium arsenate crystal and crystalline liquid; and the crystalline liquid is used for leaching of the lead and antimony smoke in the first step. According to the technology method, the loss of valuable metal such as lead, antimony and zinc in the arsenic leaching and removing process is reduced through the adding of sulphur, meanwhile, the arsenic removing rate is increased, and selective removing of arsenic is achieved. The leaching rate of arsenic can reach 99% or more, and the content of arsenic in the leached residues can be reduced to 0.1% or lower.
Owner:CENT SOUTH UNIV
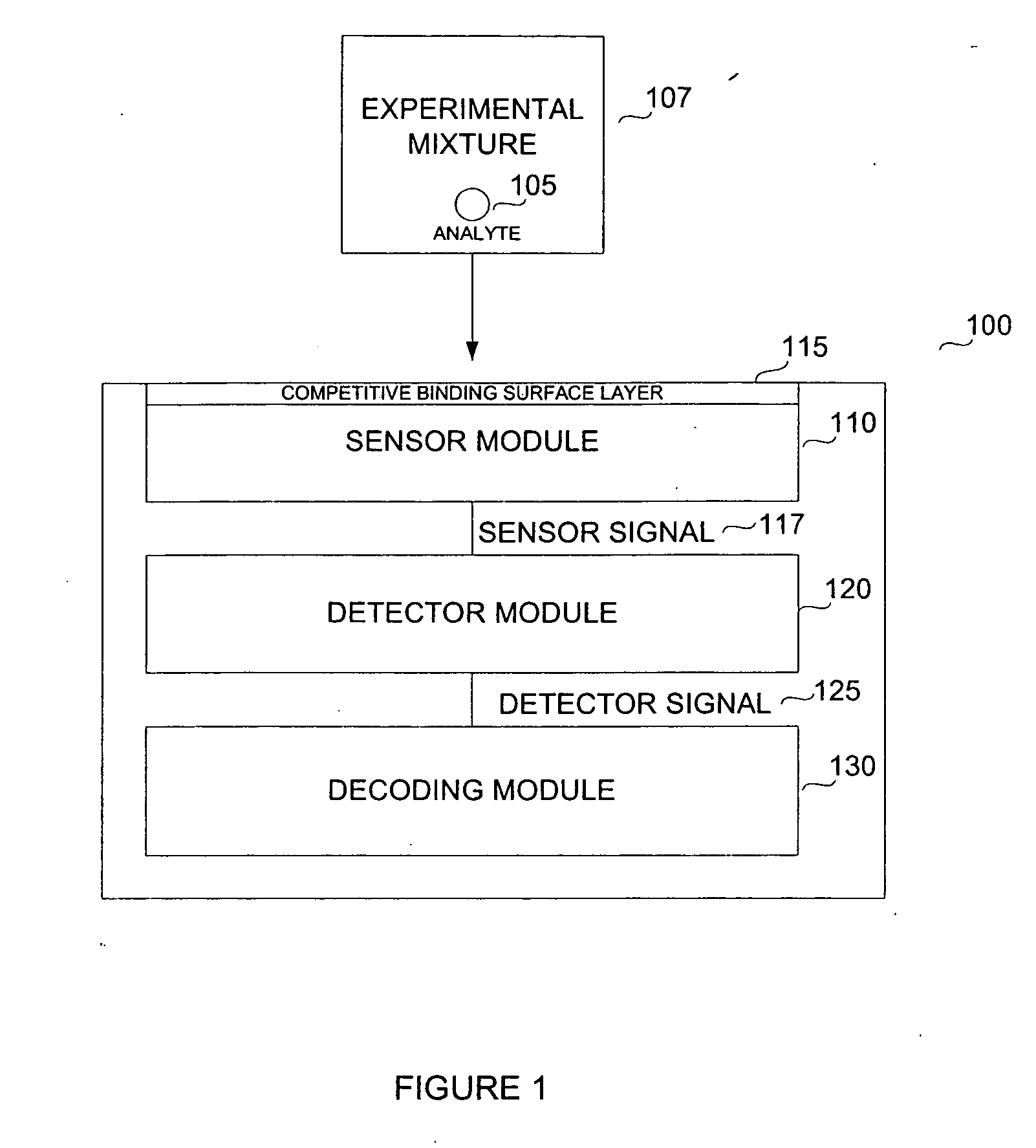
Biosensor for small molecule analytes
InactiveUS20060292581A1High sensitivityStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsEscherichia coliPhenylarsine oxide
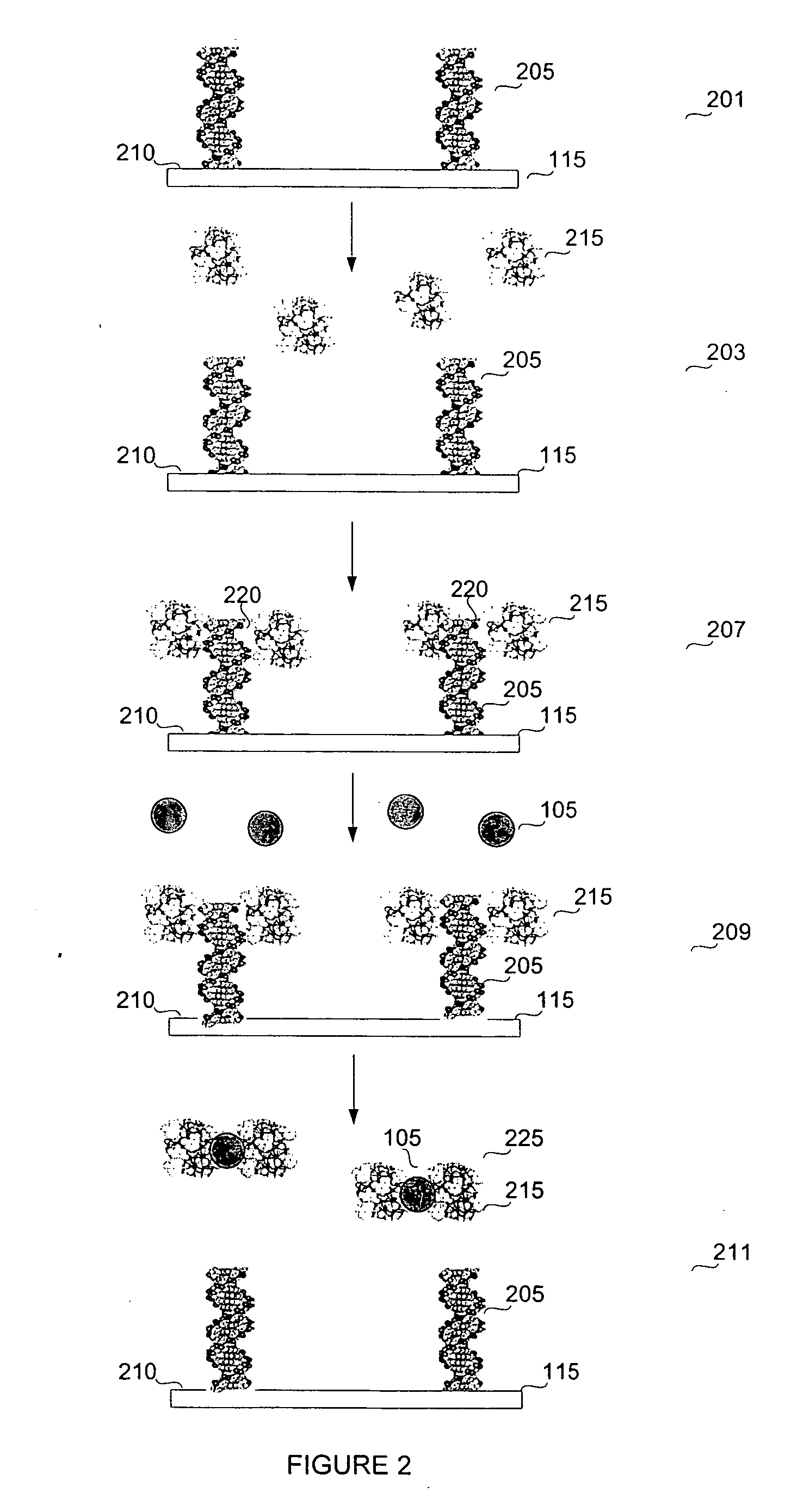
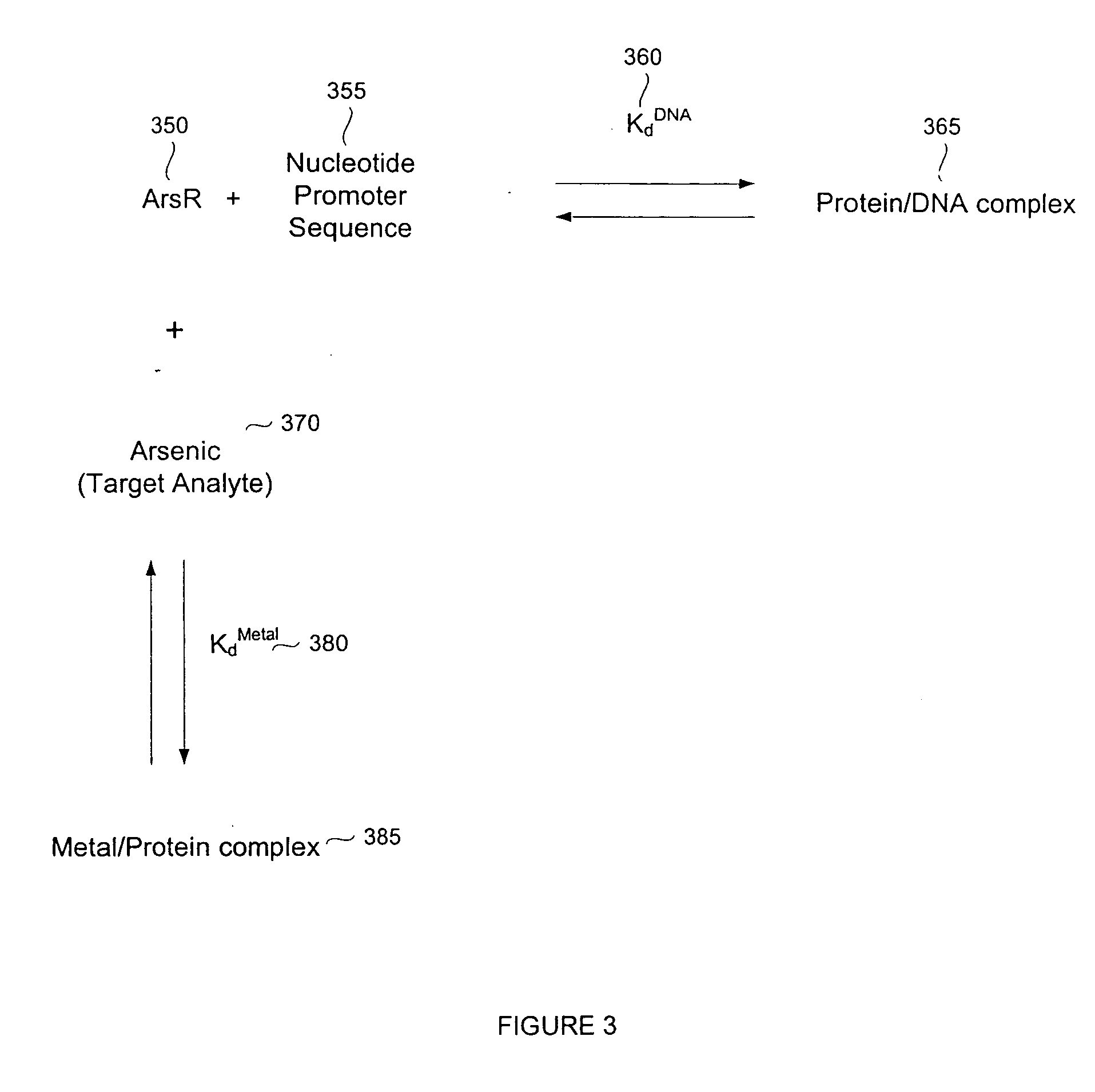
A biosensor device for detecting small molecules analytes is provided. The device employs a first class of molecules, e.g., protein that binds to both the analyte and a second class of molecules, e.g., nucleic acid. The binding of the protein to the analyte and nucleic acid can be mutually exclusive, and the presence of analyte in a sample results in a detectable displacement of protein from nucleic acid. Alternatively, binding of the protein to the nucleic acid can depend on the presence of analyte in the sample. In a specific embodiment, either the protein or nucleic acid is immobilized on a solid phase support. An arsenic detection system is exemplified. An ArsR binding sequence from the E. coli ars operon is immobilized on a gold-plated surface. ArsR protein binds to the DNA in the absence of arsenic, and is released in the presence of sodium arsenate or phenylarsine oxide. Protein release results in a change in surface plasmon resonance, and the magnitude or kinetics of the change indicate the concentration of arsenic.
Owner:LAING LANCE
Technology for treating high-arsenic copper material
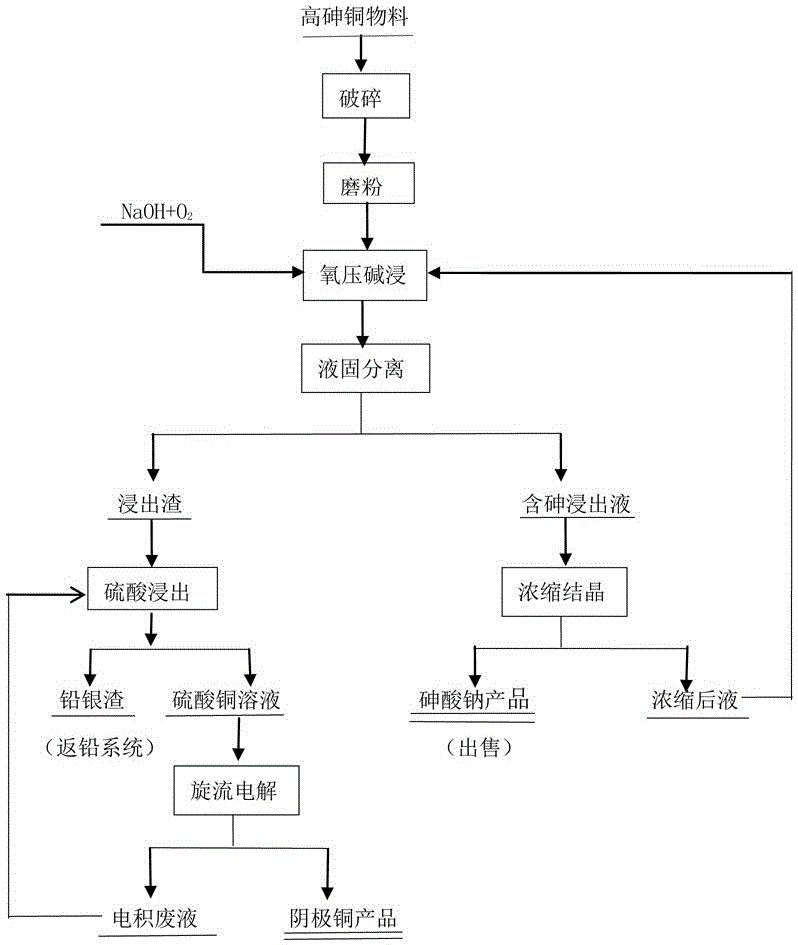
ActiveCN106555058AEnvironmental pollutionNo pollution to the environmentPhotography auxillary processesProcess efficiency improvementElectrolysisCopper sulfate
The invention discloses a technology for treating a high-arsenic copper material. The technology comprises the steps that the high-arsenic copper material is subjected to crushing, grinding, sodium hydroxide mixing and oxidizing leaching, copper in the high-arsenic copper material is oxidized and left in residues in a residual mode along with lead and noble metal of gold, silver and platinum, arsenic enters a solution in a sodium arsenate mode, leachate is subjected to concentration and crystallization, a sodium arsenate product is obtained, and a concentrated solution returns to be subject to oxygen-pressure alkali leaching; leached residues are subjected to sulfuric-acid atmospheric pressure leaching, copper enters the solution in a copper sulfate mode, acid adjustment is conducted, and vortex electrolysis is directly conducted for extracting copper; electrodeposited waste liquid is recycled; and lead and the noble metal enter lead and silver residues, and valuable elements of Pb, Ag and Au are comprehensively recycled. The technology belongs to a clean metallurgy process, is low in equipment corrosion-resistant requirement, free of pollution to environment, easy to operate and high in comprehensive recycling degree of metal and has the advantages of being higher in practicability and adaptability to raw materials and the like.
Owner:CHENZHOU CITY JINGUI SILVER IND CO LTD
Method for separating and recycling arsenic and iron from biological oxidation solution of sulfide ore
ActiveCN101586185AReduce consumptionEasy to operateIron oxides/hydroxidesCalcium/strontium/barium sulfatesTreated waterSodium hydroxide
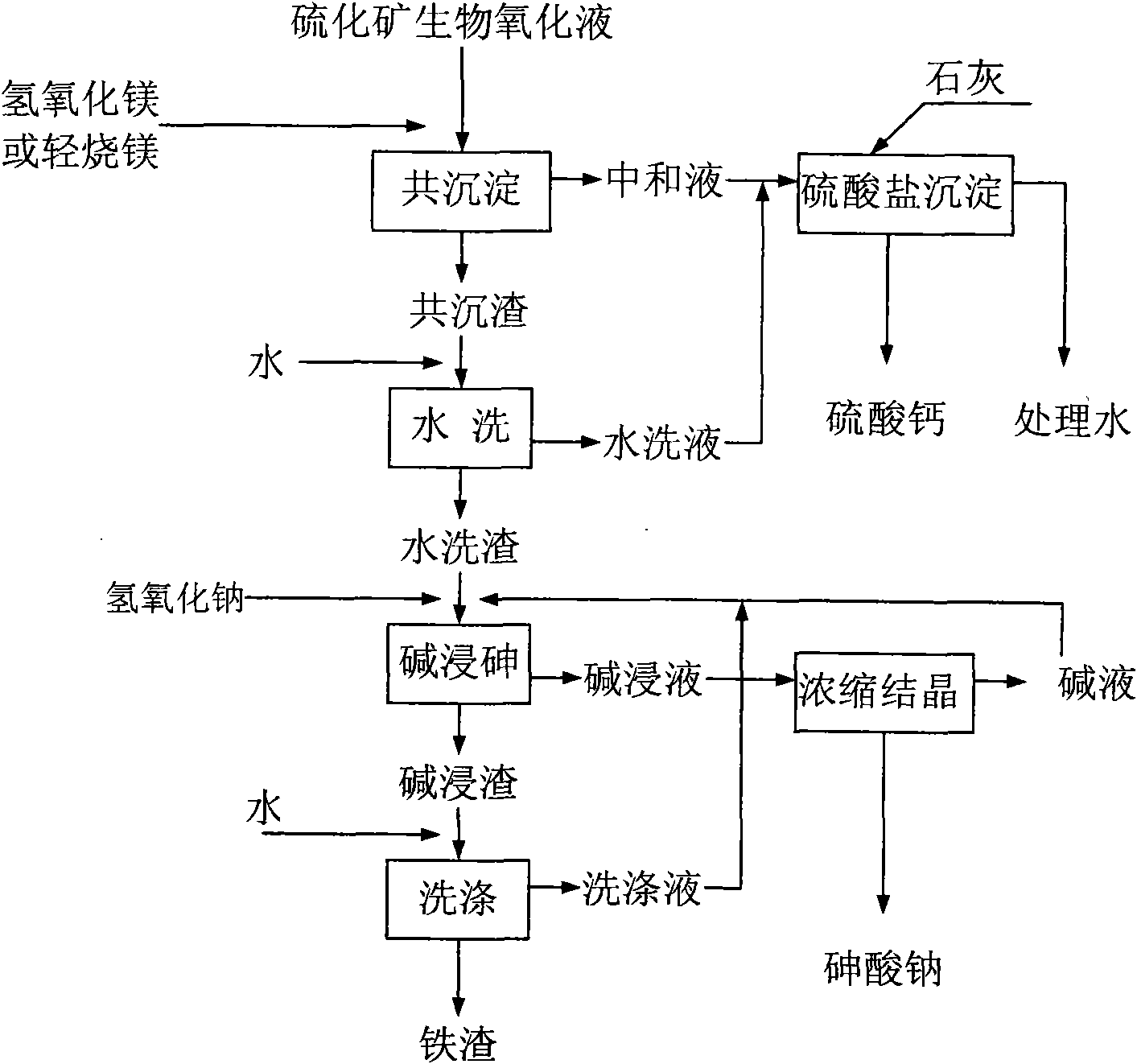
The present invention provides a method for separating and recycling arsenic and iron form the biological oxidation solution of sulfide ore and belongs to the technique. According to the method, firstly magnesium oxide is added into the biological oxidation solution of sulfide ore, then the pH value is adjusted to 5-6 so that the arsenic and iron in the biological oxidation solution generate coprecipitation, and the precipitate comprising iron and arsenic precipitate and the neutralizing agent are obtained through separating. Then the lime is added into the neutralizing solution so that the pH value obtains 8-10. The magnesium sulfate in the neutralizing agent and the lime generate calcium sulfate and magnesium hydroxide precipitate. The precipitate containing calcium sulfate and the treated water are separated. The separated water returns to the production system and the precipitate is recycled as the building material. After the coprecipitate is dried, water is added for leaching the magnesium sulfate in the coprecipitate. The precipitate after leaching and the water extract are separated. The water extract is combined with the neutralizing agent for entering the processing operation of the neutralizing agent. The arsenic in the precipitate after leaching is leached by the sodium hydroxide solution, and the arsenic leaching solution and the iron hydroxide precipitate are obtained after separation. The arsenic leaching solution is heated, evaporated and condensed to the arsenic content of 40g / L-60g / L. Then the solution is cooled for crystallizing. The sodium arsenate and the crystal mother liquor are obtained through separation. The mother liquor returns to the arsenic leaching system.
Owner:CHANGCHUN GOLD RES INST +1
Method for separating and purifying sodium salts in arsenic alkali residue
InactiveCN104818387AEfficient separation and recoveryLow As contentProcess efficiency improvementSodium saltImpurity
The invention aims at providing a method for separating and purifying sodium salts in arsenic alkali residue. The method can help separate high-purity sodium carbonate and sodium arsenate from antimony smelting arsenic alkali residue to achieve recycling of the sodium carbonate and sodium arsenate. The method comprises condensing a sodium arsenate composite salt solution at 60-70 DEG C to separate out most of Na2CO3 out of the solution, at the moment, all As is in the solution. Both magnetic fields and ultrasound can change the thermodynamic properties of chemical compounds and affect the solubility, the crystallization induction time, the width of the metastable region and the crystallization manner. A filter liquor a contains large amounts of Na+, under the coupling action of specific temperature and the magnetic field and ultrasound of the corresponding intensity, strong common-ion effects enable most of sodium arsenate to crystalize from the solution and other impurity components to stay in the solution. Meanwhile, by performing the pretreatment in the step 3) on the filter liquor a before the ultrasound and magnetic field treatment, the content of arsenic in crystalized sodium arsenate can be improved.
Owner:NANCHANG HANGKONG UNIVERSITY
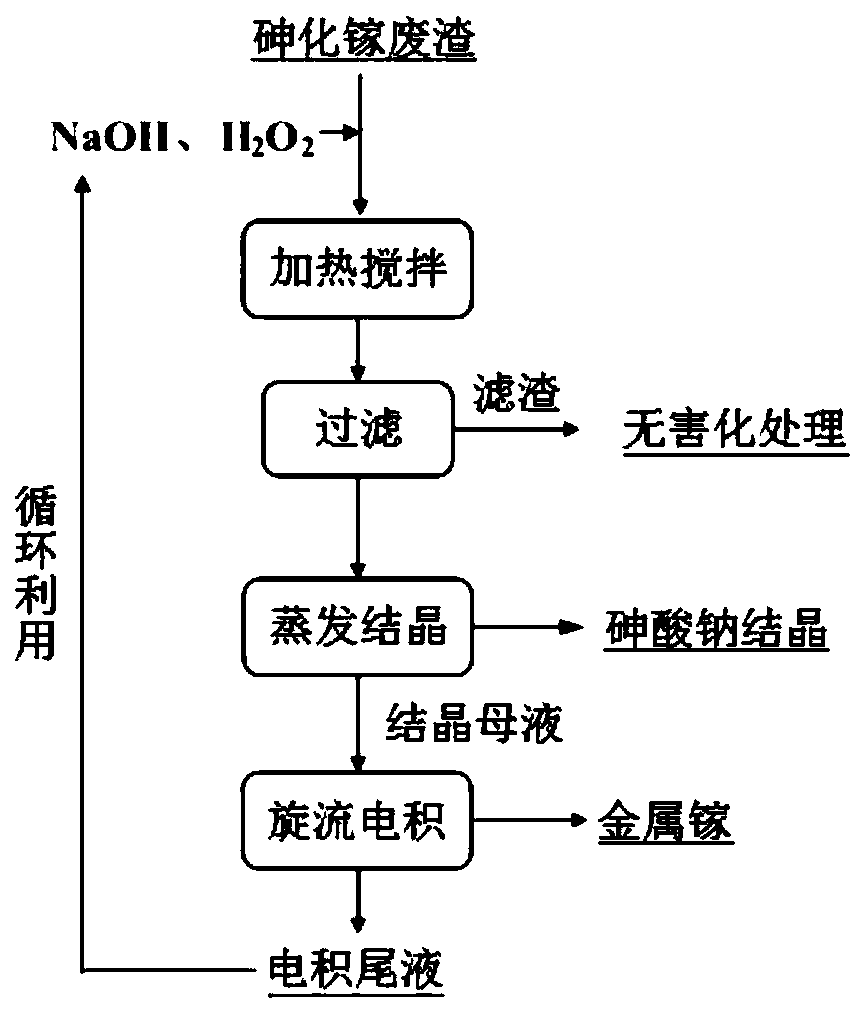
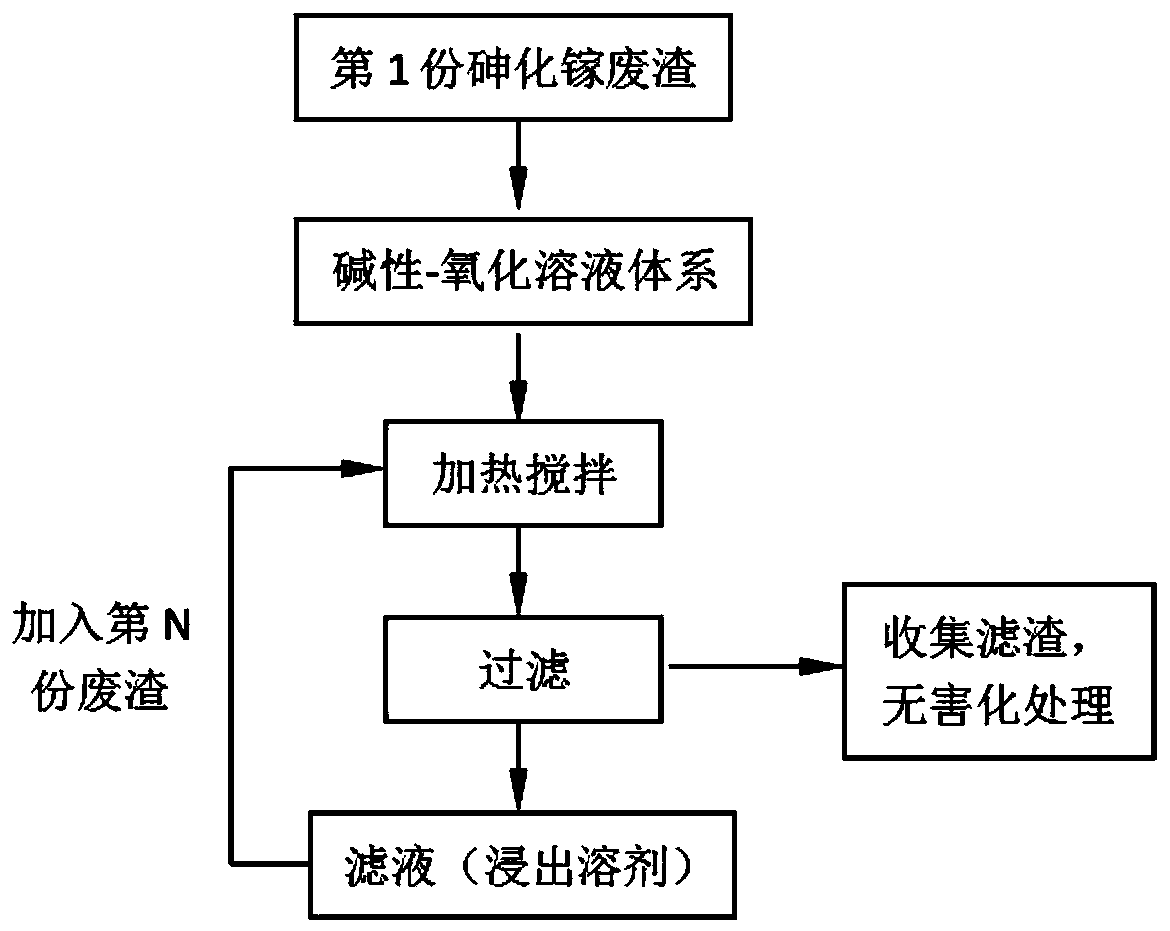
Method for recycling and preparing sodium arsenate and gallium metal from gallium arsenide waste residue
ActiveCN110938742AEasy to separateHigh purityPhotography auxillary processesArsenites/arsenatesGallium arsenateGallium
The invention relates to a method for recycling and preparing sodium arsenate and gallium metal from gallium arsenide waste residue. The method comprises the following steps of leaching the gallium arsenide waste residue in an alkaline-oxidized solution system after the gallium arsenide waste residue is dried, crushed and screened, wherein the conditions of the NaOH concentration, the oxidant concentration, the time, the temperature and the like during leaching are controlled so that arsenic and gallium are simultaneously quickly dissolved in a leaching solution to the great extent; performingevaporative crystallization to make the sodium arsenate crystallize preferentially to realize quick effective separation of the arsenic and the gallium; performing recrystallization to improve the purity of the sodium arsenate; and performing cyclone electrowinning to obtain high-purity gallium metal. The raw material gallium arsenide waste residue is from solid waste landfill and is sampled without cost; according to the method provided by the invention, the sodium arsenate and gallium metal are recycled and prepared from the waste residue; the purity of the prepared sodium arsenate is at least 95 percent; the purity of the prepared gallium metal is at least 3N; the prepared sodium arsenate and gallium metal have great utilization value; the recycled and purified sodium arsenate can serve as a chemical raw material; and the method disclosed by the invention effectively solves the problems of environment pollution and resource waste and meets the requirements of sustainable development society.
Owner:YANGZHOU NINGDA NOBLE METAL CO LTD
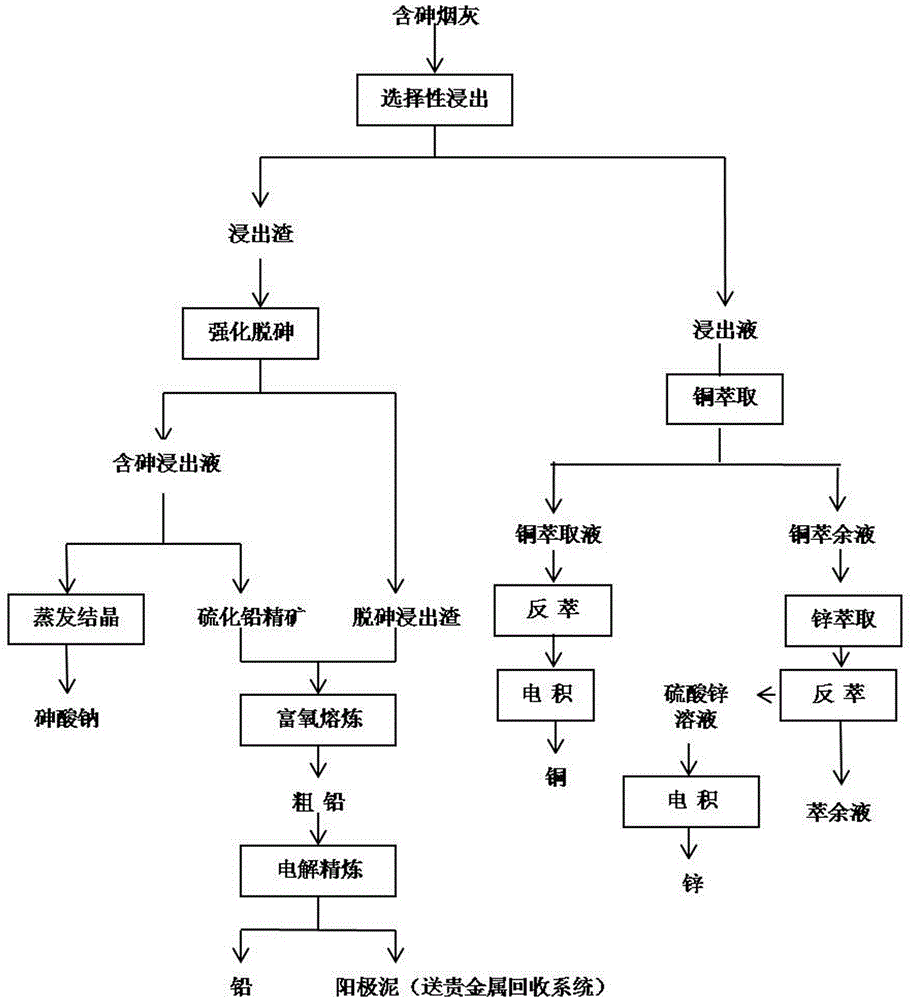
Multi-metal comprehensive recovery process for arsenic-containing smoke dust
ActiveCN103981369AEliminate pollutionEliminate the effects of pollutionProcess efficiency improvementElectrolysisHydrometallurgy
The invention provides a multi-metal comprehensive recovery process for arsenic-containing smoke dust. According to the process, leachate containing zinc and copper and leaching slag containing arsenic and lead are obtained through selective leaching, the leachate is extracted by respectively using a copper extraction agent and a zinc extraction agent, and then reverse extraction and electrodeposition are carried out so as to obtain copper and zinc; the leaching slag is leached with a mixed arsenic removing agent consisting of H2O2 and Na2S2O3 so as to obtain arsenic-containing leachate and lead-containing arsenic-removed leaching slag, the arsenic-containing leachate is concentrated and crystallized so as to obtain sodium arsenate, and the lead-containing arsenic-removed leaching slag is subjected to pyrogenic process lead extraction and electrolytic refining so as to obtain lead. According to the invention, the multi-metal comprehensive recovery process uses combined hydrometallurgy and pyrometallurgy, comprehensive recovery process and a main smelting system are effectively combined together, seamless butt joint of a part of technology and main process technology is realized, process steps are environment-friendly, metallic copper, zinc and lead are effectively recovered, and resourceful treatment of arsenic is realized.
Owner:甘肃高能中色环保科技有限公司
Absorbent material for selectively absorbing As<+5> ions and preparation method thereof
InactiveCN102172514APrecise adsorptionAccurate enrichmentOther chemical processesWater/sewage treatment by sorptionPolyethylene glycolAbsorbent material
The invention discloses a preparation method for an absorbent material capable of selectively absorbing As<+5> ions in the technical field of underground water contamination and water treatment. The method comprises: firstly, mixing chitosan dispersion with a mono-capped polyethylene glycol macromonomer, a double-capped polyethylene glycol macromonomer and a photoinitiator to prepare prepolymer solution; secondly, further adding aqueous solution of sodium arsenate to realize As<+5> ion imprinting; and finally, obtaining the absorbent material capable of selectively absorbing the As<+5> ions by acid soaking and washing neutralization. In the invention, chitosan which is a natural alkaline polysaccharide capable of forming complexes with transitional elements is dissolved in acrylic acid and methacrylic acid mono-capped and double-capped polyethylene glycol macromonomers, the As<+5> ions are used for molecular imprinting, imprinted molecules are removed by diluted hydrochloric acid after crosslinking and curing, and thus, a three-dimensional hole interpenetrating polymer network absorbent resin to be specifically bonded As<+5> ions is obtained. The absorbent material has high selectivity and high adsorbability for As<+5> ions and helps to accurate enrichment of As<+5> ions to remove and recover As<+5> ions.
Owner:SHANGHAI JIAO TONG UNIV
Process for comprehensively recovering high arsenic polymetallic material
ActiveCN102392136ALower requirementSimple operating conditionsProcess efficiency improvementArsenic pollutionPersulfate
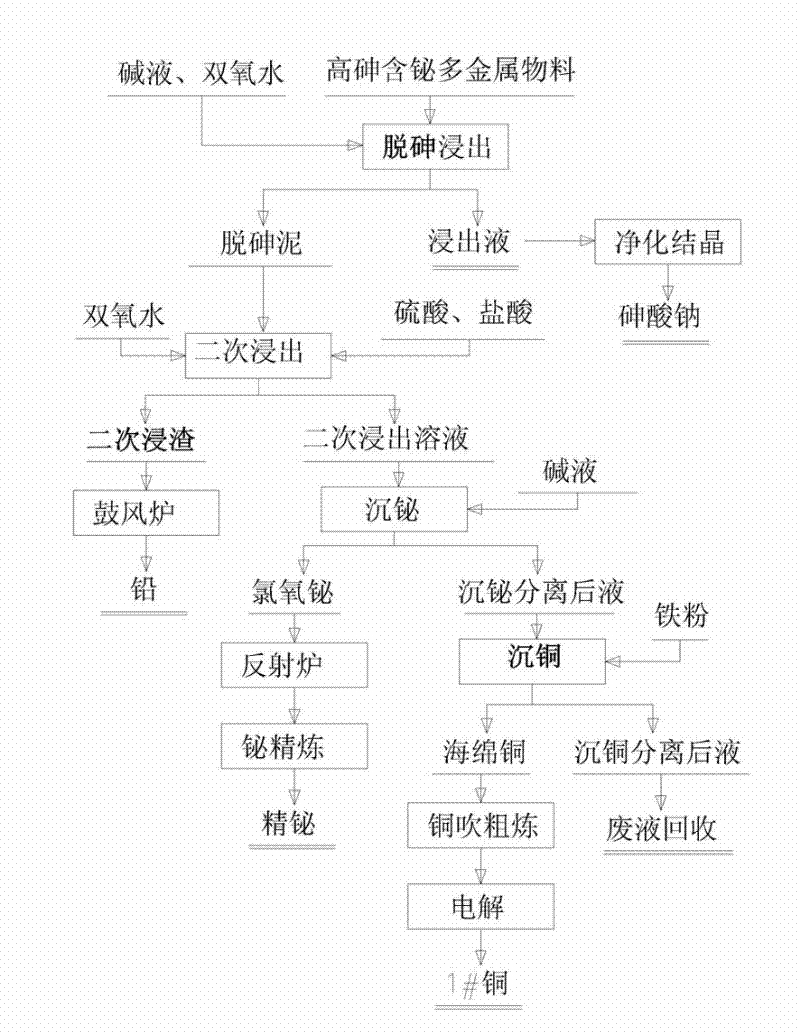
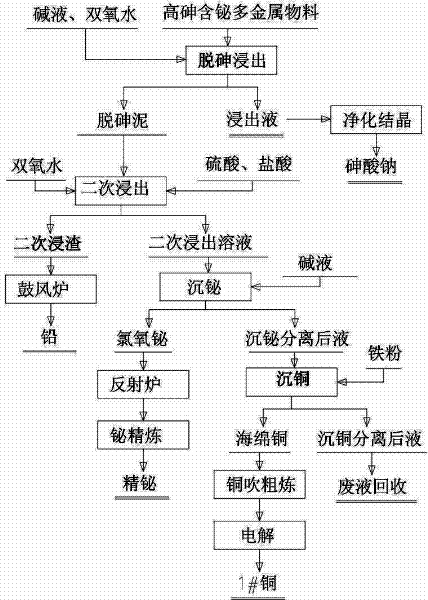
The invention relates to a process for comprehensively recovering a high arsenic polymetallic material, which belongs to the non ferrous metal comprehensive recovery field. The process comprises the following steps: leaching arsenic in the polymetallic material from the polymetallic material by sodium hydroxide, hydrogen peroxide and water, routine purifying a leachate and removing impurities, crystallizing to obtain sodium arsenate; leaching the arsenic removal mud after removing arsenic by sulfuric acid, hydrochloric acid and hydrogen peroxide twice, sending leaching residue to a blast furnace for refining lead; adding alkali loquor in a leaching solution for regulating pH value to 2.0-2.5, depositing bismuth by a bismuth chlorine oxygen form, separating and depositing bismuth chlorine oxygen and refining to obtain the refined bismuth; adding iron powder in a solution which is performed bismuth deposition and separation, electrolyzing to obtain 1#copper; sending solution which is performed copper deposition and separation to a waste liquid recovery pool for processing. The invention has the advantages of low production cost, simple operation condition and requirement, safe operation and no arsenic pollution. According to the invention, the arsenic recovery rate is greater than or equal to 96.5%, the lead recovery rate is greater than or equal to 98%, the bismuth recovery rate is greater than or equal to 95.8% and the copper recovery rate is greater than or equal to 96.5%.
Owner:郴州雄风环保科技有限公司
Method and application for removing arsenic from black copper sludge
InactiveCN108048664ALow impurity contentReduce pollutionArsenites/arsenatesProcess efficiency improvementEnvironmental resistanceSludge
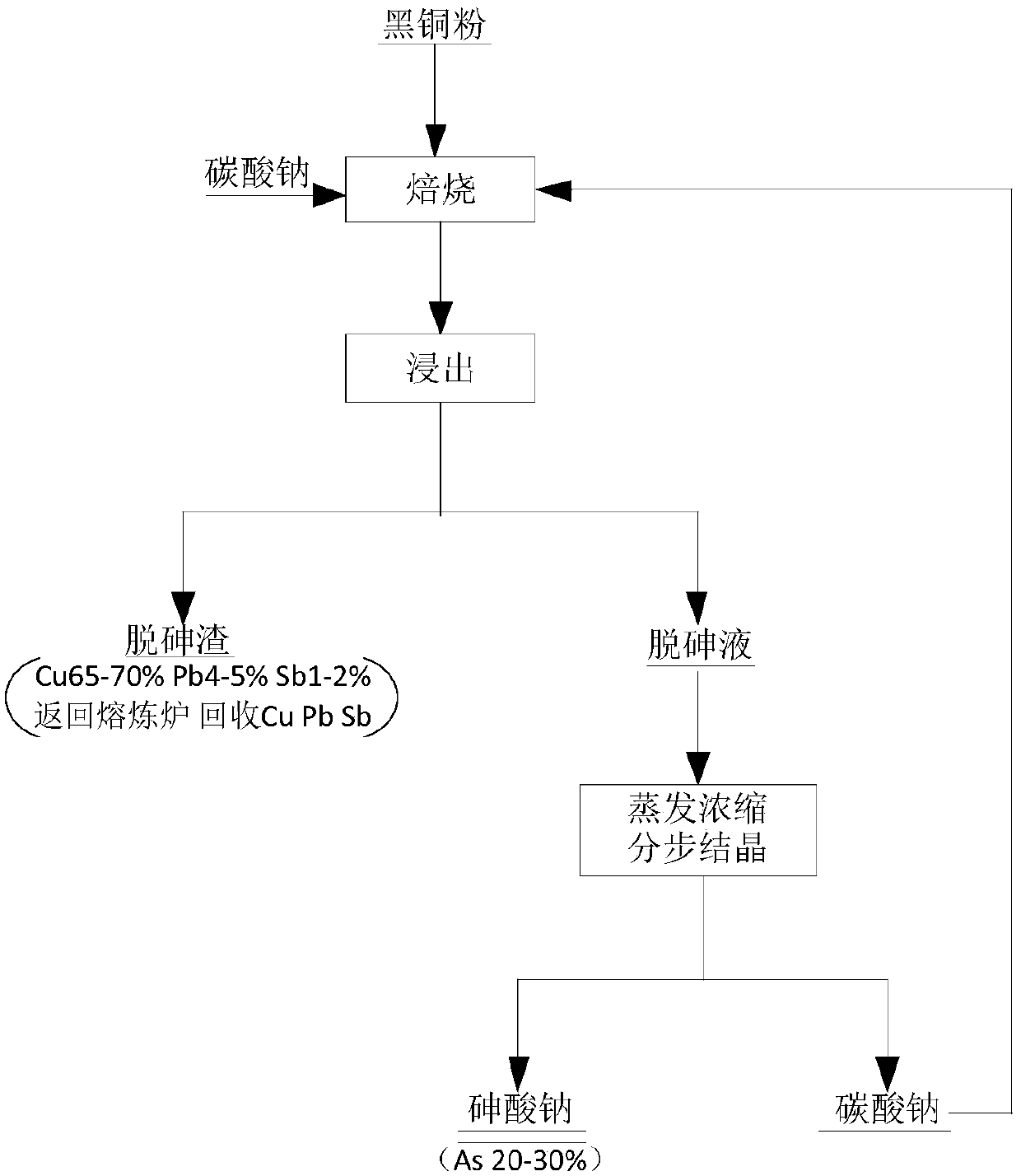
The invention provides a method and application for removing arsenic from black copper sludge, and relates to the technical field of arsenic removal. The method for removing the arsenic from the blackcopper sludge comprises the steps that the black copper sludge and alkali are mixed and roasted; and after a roasted product and water are mixed, infiltrated and filtered, arsenic removal liquid is subjected to fractional crystallization to obtain a sodium arsenate product and alkali. The method is easy and convenient to operate and high in controllability, alkali can be cyclically utilized, resource regeneration is achieved, and environment protection and high efficiency are achieved.
Owner:GUANGDONG POLYTECHNIC OF ENVIRONMENTAL PROTECTION ENG
Method for copper smelting white smoke dust arsenic removal
ActiveCN106756056ASolve the shortcomings of low arsenic dissolution rateLarge production capacityProcess efficiency improvementSlagDissolution
The invention discloses a method for copper smelting white smoke dust arsenic removal. White smoke dust collected by a copper smelting electric dust remover is used as a raw material, arsenic is converted into arsenic acid through a concentrated sulfuric acid acidification method, the arsenic acid is dissolved with water for 1-1.5 hours, and the water adding amount is 4-5 times the weight of the white smoke dust; after dissolution, solid sodium carbonate is added to adjust the pH value to 8.0-8.5, soluble metallic sulfate is converted into settlement, after being filtered to be separated from sodium arsenate salt, the settlement is washed twice with clear water, and an obtained filter cake is an arsenic-removed filter cake rich in valuable metal, wherein the arsenic content of the filter cake is lower than 0.5%; and filter liquor enters a main produced lime-molysite arsenic removal system, and newly adding of waste water treatment equipment is not needed. According to the method, the equipment is simple, heating is not needed, operation is convenient, the arsenic removal rate is high, and the arsenic content of acidized slag meets the third-level quality standard requirement of bing ore; and convenience is provided for recovery of other acid-soluble valuable metals in the white smoke dust, and the method has good application and popularization value.
Owner:KUNMING UNIV OF SCI & TECH
Method for recovering bismuth and arsenic from bismuth and arsenic-containing solution
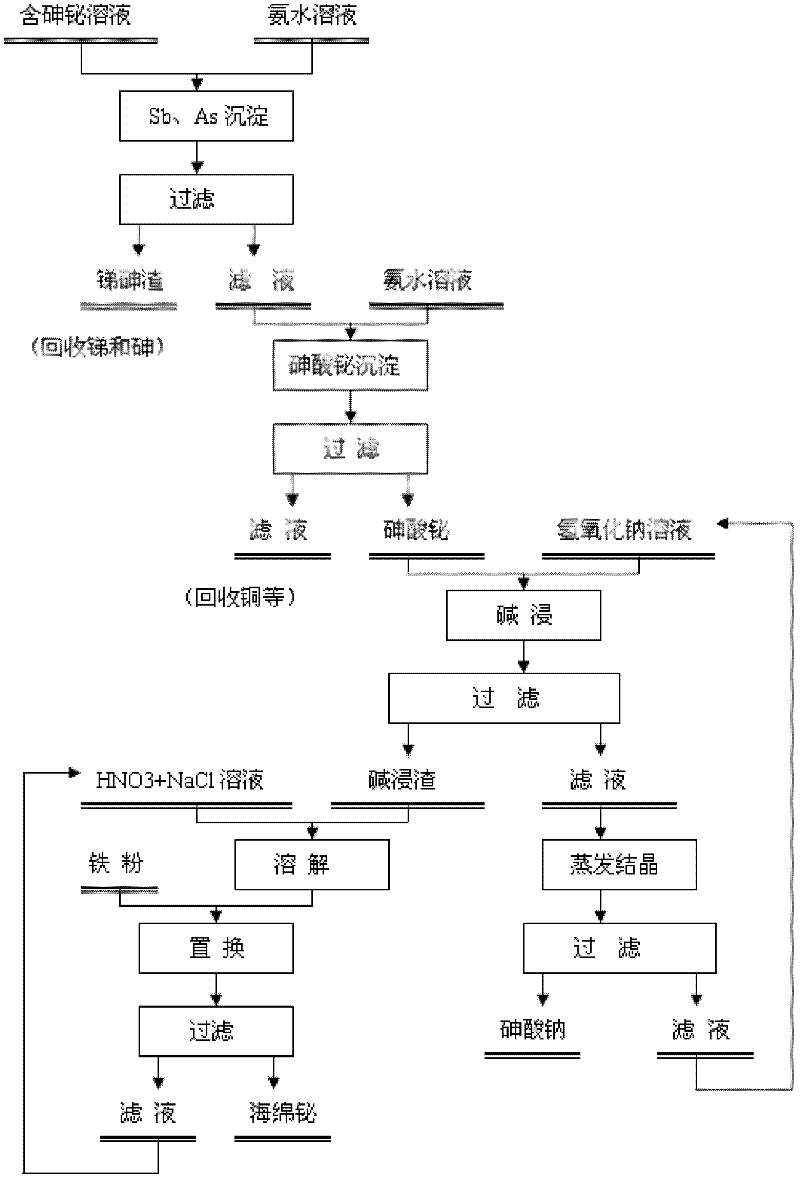
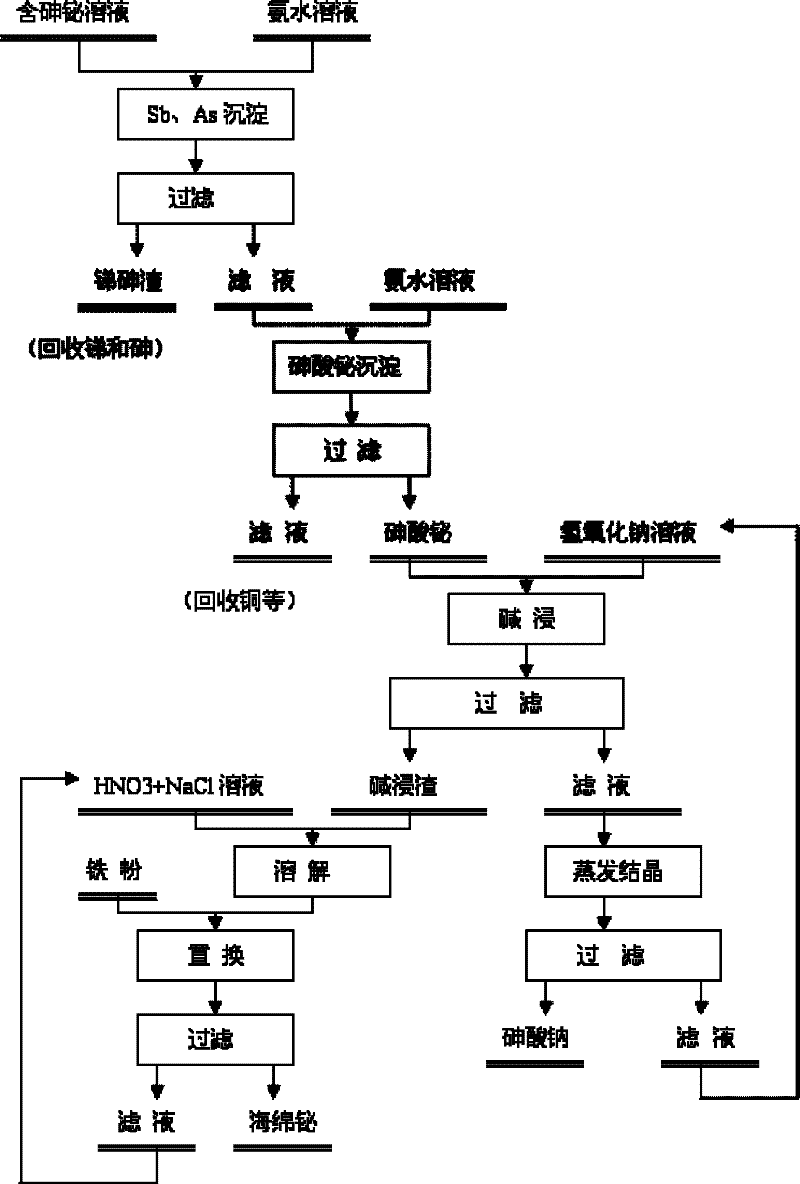
The invention discloses a method for recovering bismuth and arsenic from a bismuth and arsenic-containing solution. The method is characterized by comprising the steps as follows: adjusting the pH value of the bismuth and arsenic-containing solution to be in a certain range so as to deposit out the bismuth and the arsenic in the solution in a form of bismuth arsenate; processing the obtained bismuth arsenate deposit by a strong base solution to effectively separate the arsenic from the bismuth; and respectively recovering the bismuth and the arsenic from a basic leached residue and a basic leached solution. According to the method, the solution does not need to be subjected to arsenic removal before the bismuth deposition of the leached solution containing higher arsenic and bismuth, so that the bismuth loss in the arsenic removal process is avoided to increase the recovery rate of the bismuth in the solution; the arsenic and the bismuth in the solution are co-deposited in the form of the bismuth arsenate, and the purpose of effectively separating the arsenic from the bismuth is achieved by the basic leached bismuth arsenate deposit according to the characteristic that the arsenate deposit easily acts with strong base and is converted into soluble arsenate and insoluble metal oxide or hydroxide; the arsenic is recovered in a form of sodium arsenate while the bismuth is recovered; and due to small bismuth loss in the arsenic and bismuth co-deposition and bismuth arsenate base leaching processes, the total recovery rate of the bismuth in the whole process is guaranteed to be not less than 90%.
Owner:CENT SOUTH UNIV
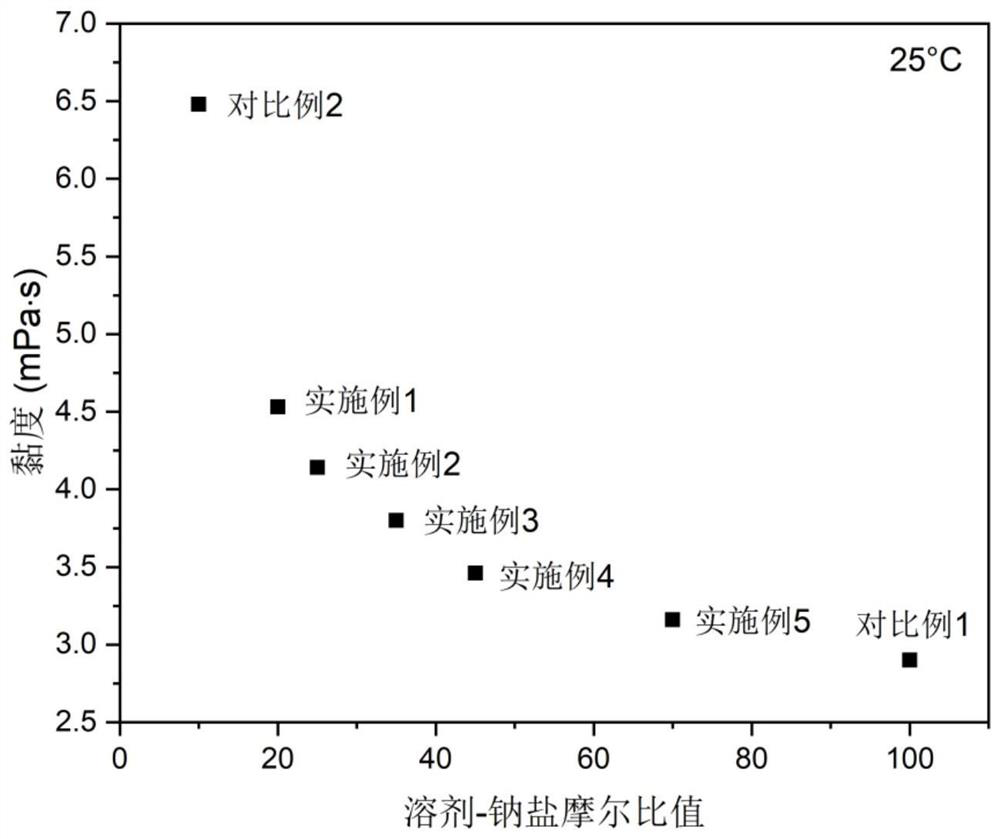
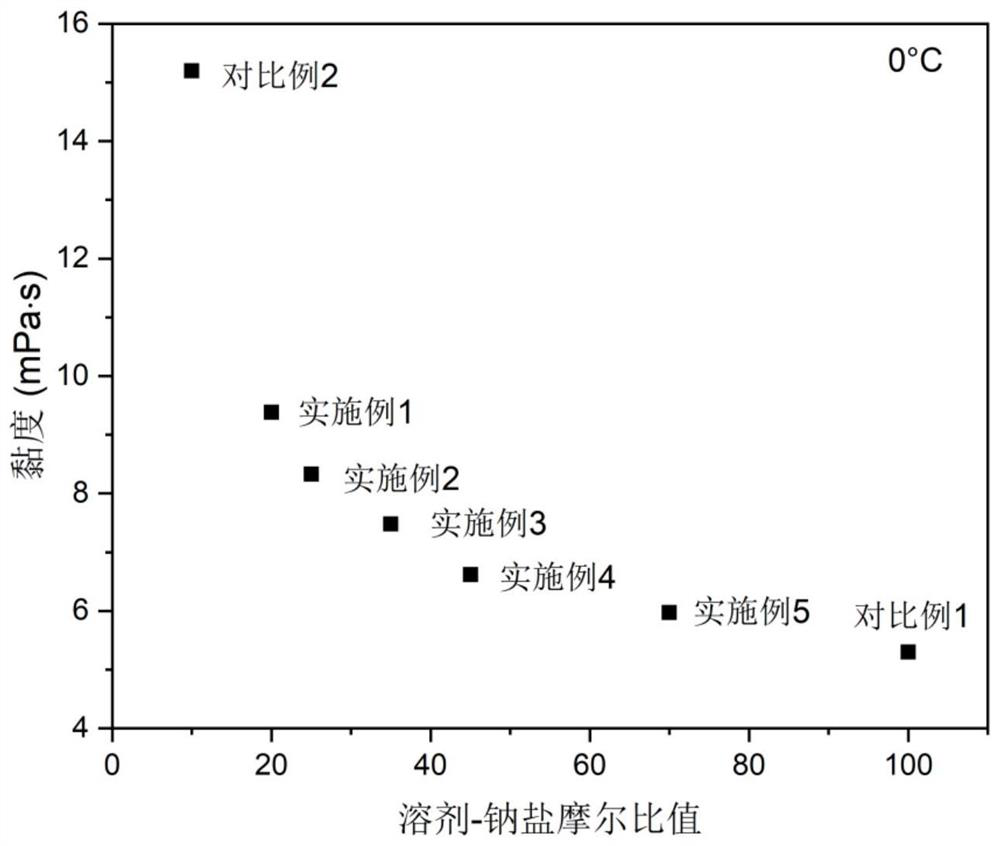
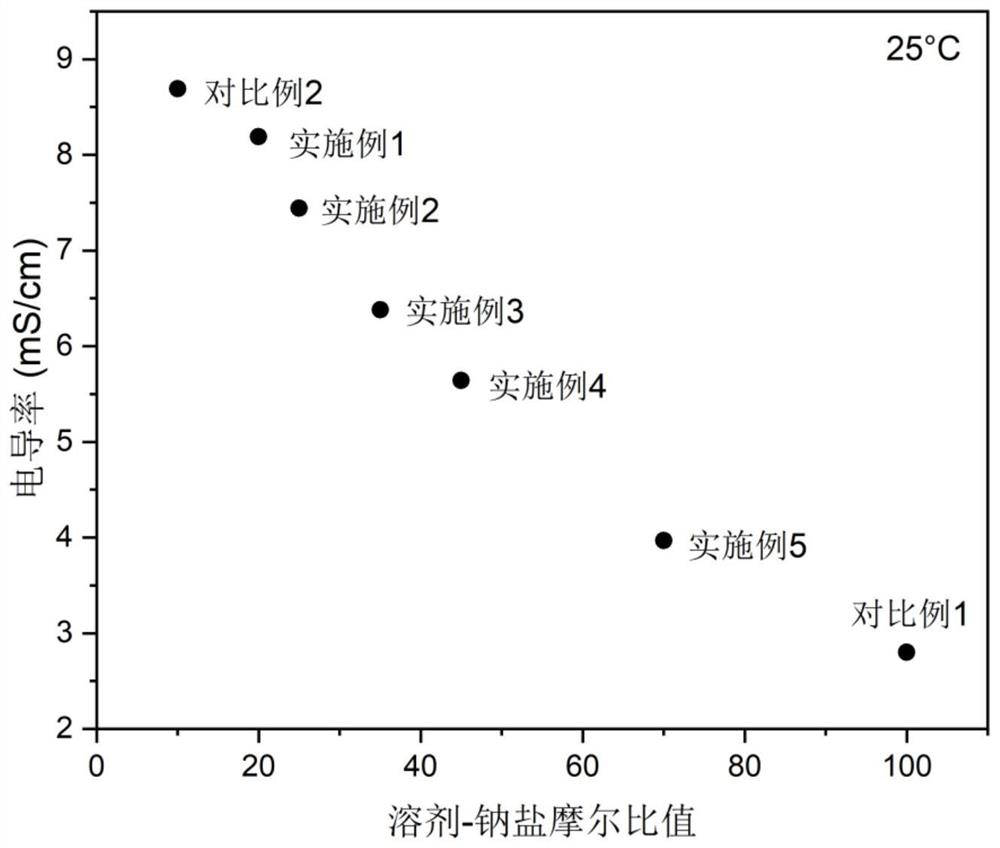
Electrolyte with high solvent-sodium salt ratio and sodium ion battery
PendingCN113299976AReduce viscosityImprove wettabilitySecondary cellsElectrolytic agentSodium tetrafluoroborate
The invention discloses an electrolyte with a high solvent-sodium salt ratio and a sodium ion battery. The electrolyte comprises sodium salt, a solvent and an additive, wherein the molar ratio of the solvent to the sodium salt is (20: 1)-(70: 1), and the addition amount of the additive accounts for 0.5%-5% of the mass of the electrolyte; the sodium salt comprises one or more of sodium perchlorate (NaClO4), sodium tetrafluoroborate (NaBF4), sodium hexafluorophosphate (NaPF6), sodium hexafluoroarsenate (NaAsF6), sodium trifluoroacetate (CF3COONa), sodium tetraphenylborate (NaB(C6H5)4), sodium trifluoromethanesulfonate (NaSO3CF3), sodium bis (fluorosulfonyl) imide (Na[(FSO2)2N]) or sodium bis (trifluoromethanesulfonyl) imide (Na[(CF3SO2)2N]).
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
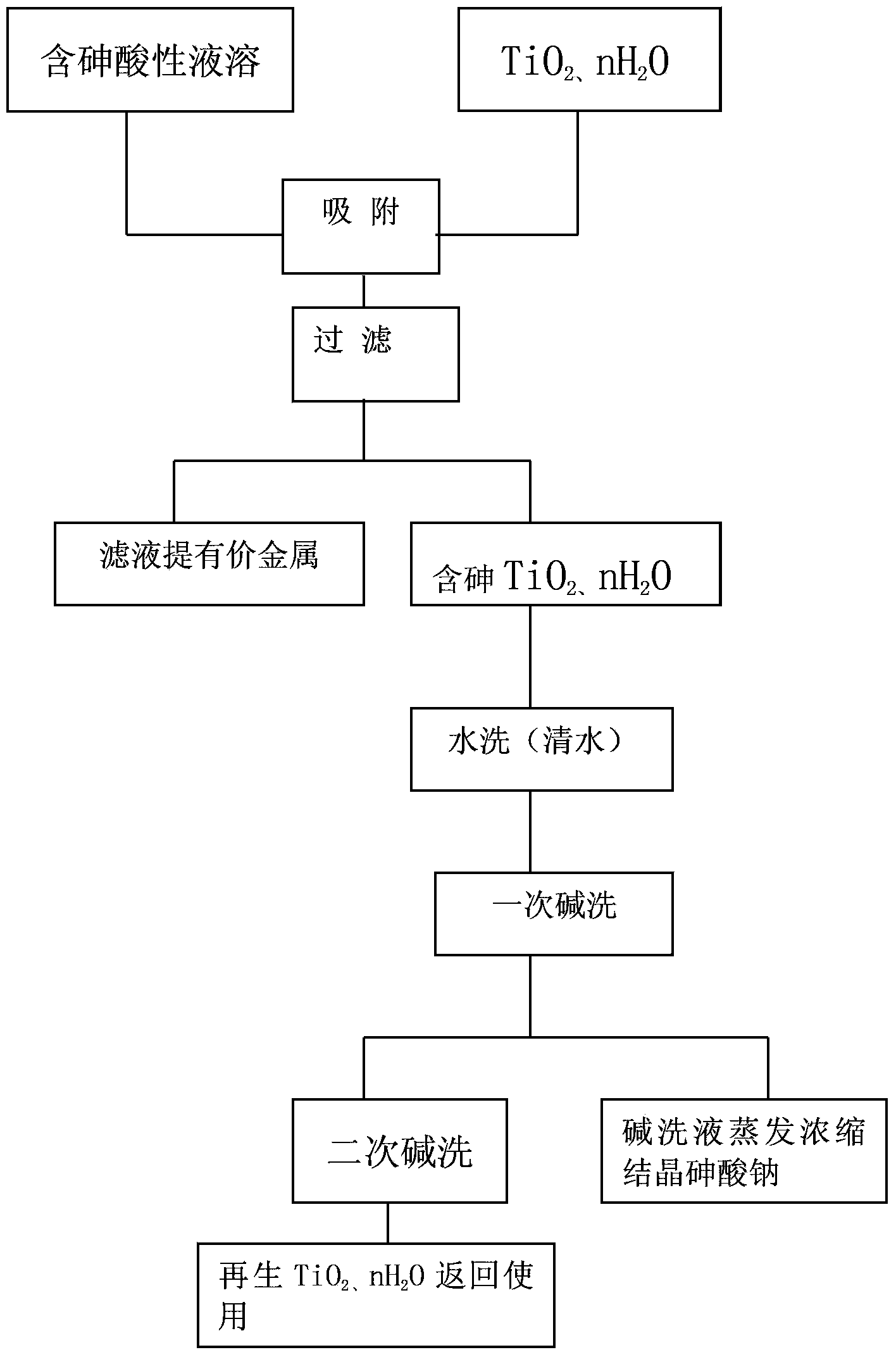
Method for removing arsenic from acid solution
InactiveCN103409625AEfficient recyclingAvoid secondary pollutionProcess efficiency improvementFiltrationIon exchange
The present invention discloses a method for removing arsenic from an acid solution, and relates to the technical field of hydrometallurgy, wherein TiO2 and nH2O can be adopted to carry out absorption removal of trivalent arsenic and pentavalent arsenic from a H2SO4 solution containing 10 mg / L-30 g / L of arsenic and a HCl solution containing 10 mg / L-30 g / L of arsenic, and acidity of an acid solution is 0.5-150 g / L. The adopted removal method can comprises: carrying out nature infiltration through an ion exchange column, carry out vacuum filtration, stirring according to a liquid-solid ratio, and carrying out pressure filtration with a pressure filter, wherein a 10-20% NaOH solution is adopted to wash and regenerate TiO2 absorbing arsenic and nH2O absorbing arsenic, an arsenic absorption effect is not reduced, the alkali washing solution is treated to obtain a sodium arsenate crystal containing more than 50% of arsenic, Zn<2+> and In<3+> are not be absorbed during arsenic removal processes of a zinc sulfate solution and an indium chloride solution, and arsenic and germanium are absorbed in a Ge-containing solution. With the method, arsenic can be effectively recovered, the problem of secondary pollution of arsenic in other methods is solved, and a certain economic value is produced.
Owner:GUIZHOU HONGDA ENVIRONMENTAL PROTECTION TECHNOLOGY CO LTD
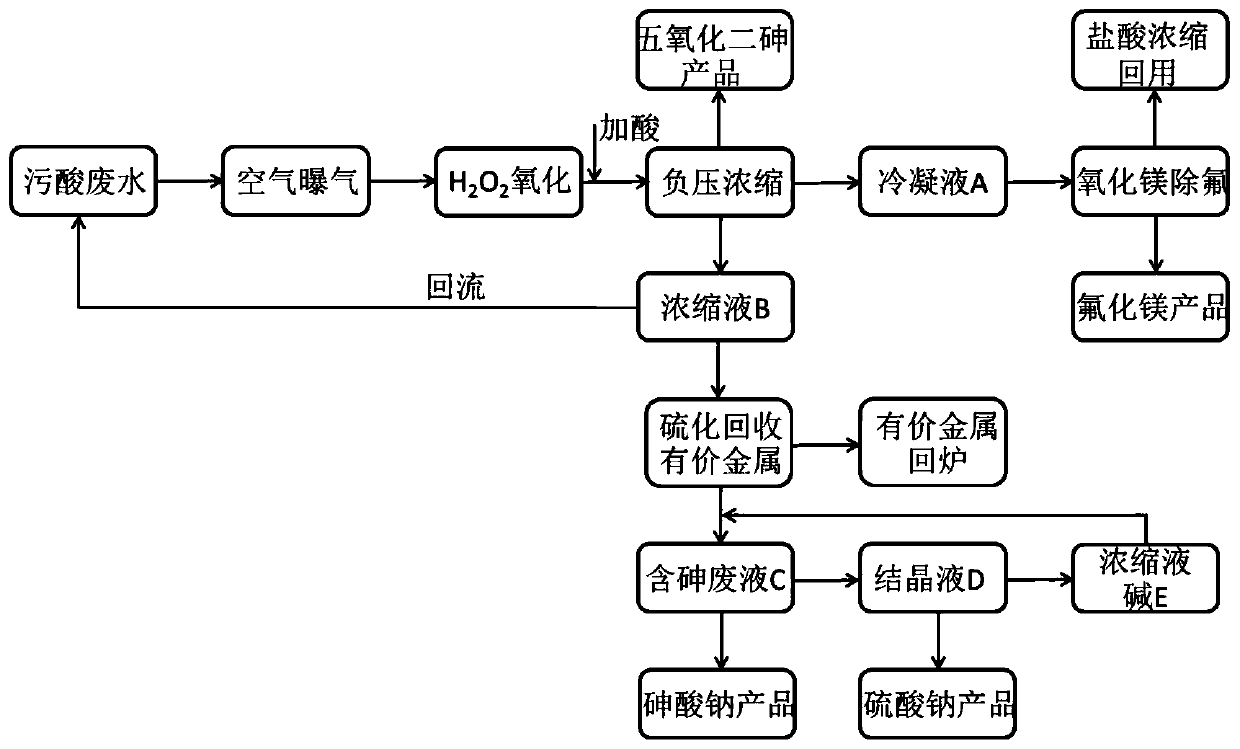
Method for resource utilization of waste sulfuric acid wastewater produced in copper smelting and acquisition of arsenic-containing products
ActiveCN111018229ANothing producedIncrease the areaMagnesium fluoridesChlorine/hydrogen-chloride purificationArsenic oxideArsenic pentoxide
The invention belongs to the technical field of industrial sewage treatment, and particularly discloses a method for resource utilization of waste sulfuric acid wastewater produced in copper smeltingand acquisition of arsenic-containing products. The method comprises the following steps: 1, oxidization of trivalent arsenic: oxidizing the waste sulfuric acid wastewater by air and hydrogen peroxidein sequence; 2, separation of arsenic from fluorine and chlorine: carrying out negative pressure evaporation after oxidation to obtain a condensate A containing fluorine and chlorine, a concentratedsolution B and arsenic pentoxide crystals; 3, separation of fluorine and chlorine: absorbing the condensate A with magnesium oxide to obtain a magnesium fluoride product and diluted hydrochloric acid;4, recovery of valuable metal: adding a sodium sulfide solution into the concentrated solution B to obtain a valuable metal precipitate and arsenic-containing waste liquid C, and returning the valuable metal precipitate to a furnace for refining; and 5, crystallization: adding caustic soda flakes or recycled concentrated alkali liquor E into the arsenic-containing waste liquor C, adjusting a pH value to 13.5-14.0 to obtain a sodium arsenate product and a crystallized filtrate D, filtering the crystallized filtrate D, and conducting concentrating to obtain a sodium sulfate product and the concentrated alkali liquor E. According to the method, waste sulfuric acid wastewater is subjected to resource recycling, and arsenic pentoxide and sodium arsenate products are obtained.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES +1
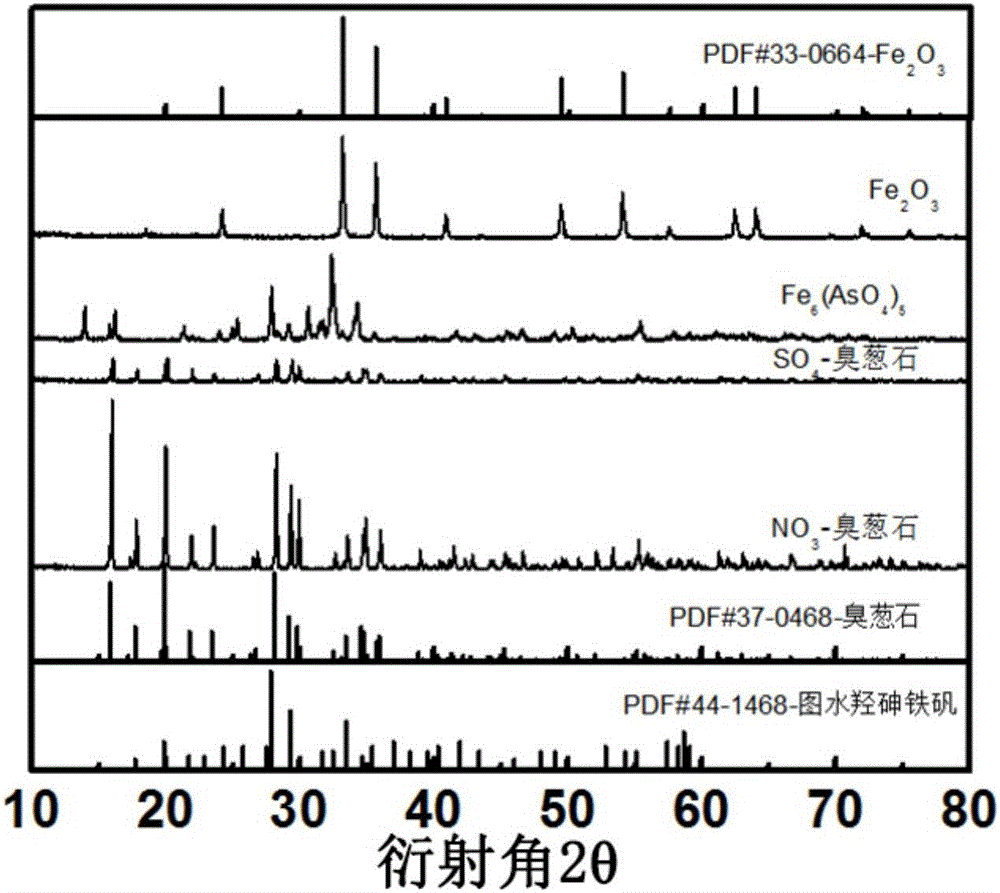
Black ferric arsenate crystal and synthesis method thereof
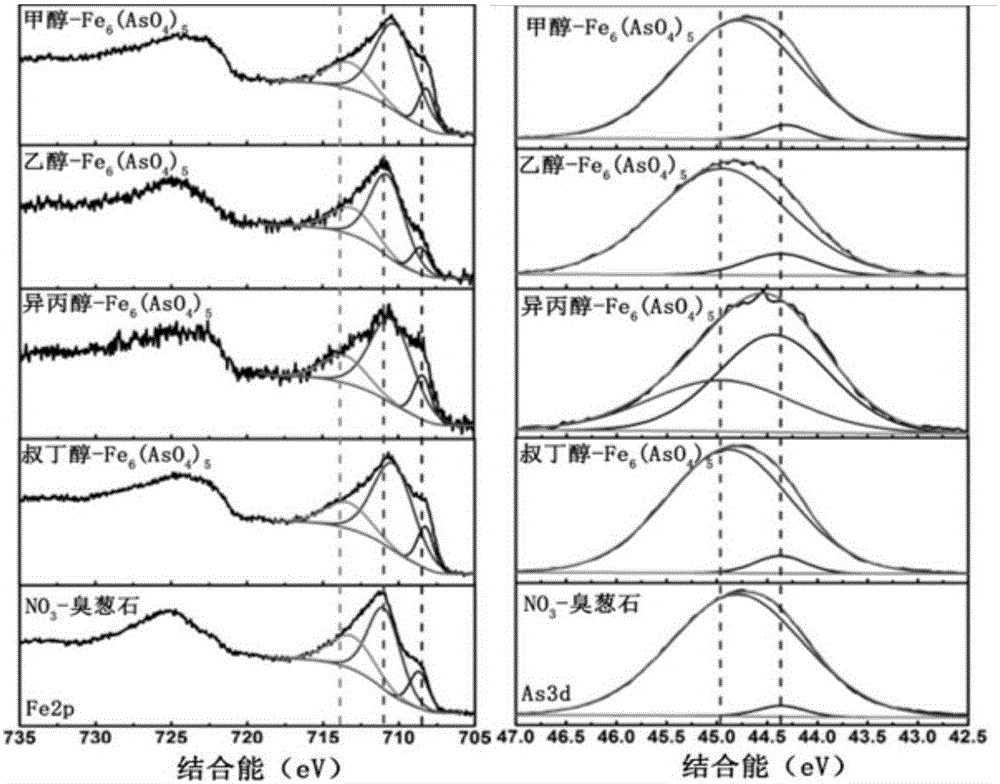
The invention discloses a black ferric arsenate crystal. The black ferric arsenate crystal is a micrometer petal-shaped particle, the particle size is 10 to 20mu m, and the appearance feature is black powder; a chemical formula of the black ferric arsenate crystal is Fe6(AsO4)5; diffraction peaks 2theta are equal to 13.9, 16.0, 21.2, 24.9, 25.2, 27.8, 28.4, 29.1, 30.5, 32.3, 34 and 34.2. The invention also discloses a synthesis method of the black ferric arsenate crystal. The synthesis method comprises the following steps of using ferric nitrate, sodium arsenate, nitric acid and deionized water to prepare a synthesis solution; mixing alcohol and the synthesis solution according to a volume ratio, so as to obtain a mixed solution; adding the mixed solution into a high-pressure reaction kettle to react for 8 to 24 hours at the temperature of 160 to 200 DEG C; after reacting, centrifuging, flushing solids by alcohol and water, and drying, so as to obtain the black ferric arsenate crystal. The synthesis method has the advantage that after the black ferric arsenate crystal is synthesized, the removal rates of Fe and As are respectively greater than 97%, so that the application potential in an As removal field is very large.
Owner:CENT SOUTH UNIV
Method for preparing high-purity rhenium powder from rhenium-containing high-arsenic copper sulfide
The invention discloses a method for high-effectively enriching rhenium from rhenium-containing high-arsenic copper sulfide. The method includes: rhenium-rich slag and a certain amount of alkali are mixed to obtain size, and pressing, heating and alkaline leaching is performed, and after filtering and washing is performed, rhenium enters extract in the form of ammonium rhenate, and a part of arsenic enters the extract in the form of sodium arsenate. Reactive metal power is heated to replace the rhenium in the extract, and after filtering and washing is performed, the rhenium oxide is enriched, and separation between the rhenium and the arsenic can be achieved. The raw material of the method is the rhenium-rich slag which is obtained by sulfide precipitation of acidic wastewater generated during a production process of copper smelting enterprises and is rich in copper and arsenic; the method can fully leach the rhenium, and the metal power is used to replace the extract to obtain a rhenium-enrichment; the process of rhenium extraction can be simplified, and the cost of rhenium extraction is reduced. The method is simple in process, is short in flow, is high in enrichment ratio, is high in material adaptability, is low in cost, is easy to industrialize, has good application value, and can increase output value and profit of the copper smelting enterprises.
Owner:KUNMING METALLURGY COLLEGE
Harmless treatment method for recycling valuable metal and arsenic resources from arsenic-containing smoke
The invention provides a harmless treatment method for recycling valuable metal and arsenic resources from arsenic-containing smoke. All valuable elements are recycled and utilized through the procedures of carrying out oxygen pressure leaching via caustic alkali; carrying out selective reduction purification on leachate; during purification, carrying out hydrogen reduction and vacuum distillation on obtained crude tellurium so as to obtain high-purity tellurium; after purification, obtaining a pure sodium arsenite solution, and using the solution for the arsenic salt purification of a zinc sulfate solution so as to remove cobalt and nickel; carrying out fluidization washing on leaching residues; after washing, solidifying arsenic in the solution, and carrying out reduction smelting on the residues; and carrying out oxidization blowing and the like. The arsenic is removed from the smoke, stibium, lead, bismuth, stannum and the like are kept in the residues from which the arsenic is removed as much as possible, and the deep separation of the arsenic and the stibium is realized. The obtained pure sodium arsenite solution is used for the arsenic salt purification of the zinc sulfate solution used for wet-process zinc smelting so as to remove the cobalt and nickel raw materials, part of sodium arsenate with high impurity content is solidified, the arsenic is precipitated, the harmless recycling of the arsenic resource is realized, and the valuable elements, such as the tellurium, the stibium, the lead and the bismuth are recycled. The comprehensive utilizing rate of the resources is high, the application range of the raw materials is wide, and a pollution problem in the extracting process of a traditional technology is solved.
Owner:CENT SOUTH UNIV
Method for separating copper and arsenic from copper-arsenic slag
ActiveCN107475519AGood for acid leachingEfficient leachingProcess efficiency improvementSlagSodium hydroxide
The invention provides a method for separating copper and arsenic from copper-arsenic slag. The method comprises the steps that the copper-arsenic slag is subjected to pressurized oxidation leaching in a sodium hydroxide system, the arsenic and a small amount of heavy metal ions enter a solution, and the remaining copper, antimony and lead enter oxidated and leached slag after being subjected to oxidation; the heavy metal ions which enter the pressurized and oxidated solution are subjected to sulfide precipitation, and a sodium arsenate solution obtained is subjected to crystallization dearsenifying and then returns to the alkaline pressurized oxidation leaching system; the copper, antimony, lead and other elements which enter the oxidated and leached slag are subjected to leaching utilizing sulfuric acid, the copper can be subjected to subsequent electrodeposition for extraction of the copper after entering the solution, and the antimony and lead are reserved in acid-leached slag and subjected to subsequent separation and recovery. By means of the pressurized oxidation leaching, the efficient leaching of the arsenic from the copper-arsenic slag can be achieved, the leaching rate of the arsenic reaches 96% or above, and furthermore, the subsequent acid leaching of the copper is facilitated through the further oxidation of the copper in the copper-arsenic slag; the precipitation rate of the heavy metal ions, including the copper, antimony and lead reaches 85% or above; and the method for separating the copper and the arsenic from the copper-arsenic slag is lower in corrosivity to equipment, friendly to environment and low in labour intensity.
Owner:CENT SOUTH UNIV
Collaborative treatment system and method for treating arsenic alkali residue grinding leaching dealkalization and cement kiln recycling
ActiveCN109776001AReduce consumptionReduce in quantityCement productionAlkali metal oxides/hydroxidesEvaporationCircular economy
The invention discloses a collaborative treatment system and method for treating arsenic alkali residue grinding leaching dealkalization and cement kiln recycling, arsenic alkali residue is sequentially subjected to crushing, grinding leaching dealkalization, alkali liquor purification and alkali liquor evaporation treatment, washing is carried out for multiple times during the grinding leaching dealkalization, the alkali content of the residue after dealkalization is greatly reduced, and sodium arsenate and sodium antimonate in the solution are converted into precipitate substances which areinsoluble in water under the action of a precipitant, so that high-temperature calcination is facilitated, arsenic and antimony in the arsenic alkali residue are removed, the alkali liquor generated in the treatment process can be evaporated and concentrated to be used as a smelting medicament or a desulfurizing agent and the like, the obtained solid can be calcined at high temperature, the calcined residue is used as a cement raw material, the production cost is saved, and the requirements of development cycle economy and sustainable development are met.
Owner:长沙中硅环保科技有限公司
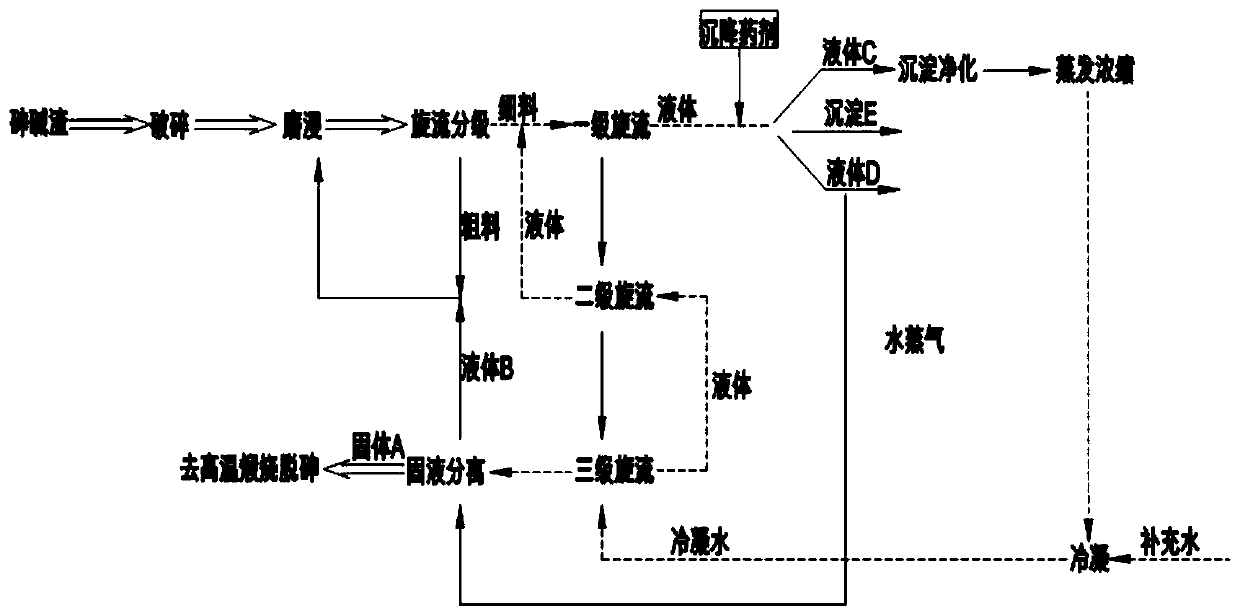
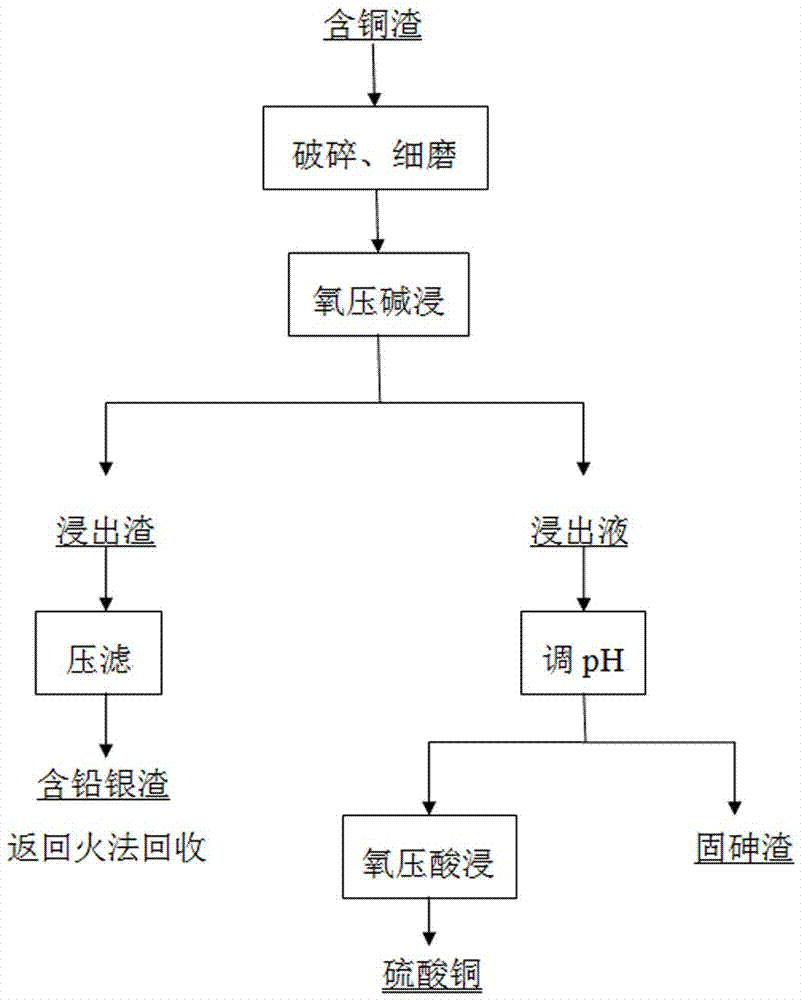
Method for efficiently cleaning and removing arsenic in copper-containing slag
InactiveCN107099669AEfficient leachingThoroughly leachedProcess efficiency improvementSlagCopper sulfate
The invention discloses a method for efficiently cleaning and removing arsenic in copper-containing slag. The method comprises steps of crushing, fine grinding, oxygen pressure alkaline leaching, pH regulation, oxygen pressure acid leaching and the like. The method sequentially comprises the following steps: efficiently cleaning and separating arsenic in the copper-containing slag as well as lead, silver and other valuable metals, and recovering copper in the copper-containing slag. The method disclosed by the invention is low in energy consumption, less in environmental pollution and high in production efficiency. The reaction process of the method is simple and rapid, and the selectivity is high. The copper in the copper-containing slag can be efficiently leached, and the metals can be completely separated. Compared with the conventional copper-containing slag treatment process technology, the arsenic is removed in a sodium arsenate precipitation form through alkaline leaching before copper leaching, so that high-efficiency cleaning and arsenic removal can be achieved, the arsenic removal effect is excellent, and the arsenic content in the leaching solution is less than 1g / L. Moreover, the copper is recovered through the oxygen pressure acid leaching, and the finally prepared copper sulfate product has the copper leaching rate of more than or equal to 96%. The arsenic in the copper-containing slag is recovered in an arsenic slag form, and secondary pollution of the arsenic is avoided. The technology has the advantages of high metal recovery rate, low production cost, high-efficiency cleaning and the like.
Owner:KUNMING METALLURGY INST
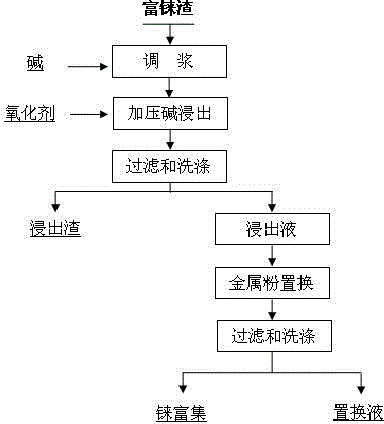
Efficient method for enriching rhenium from high-arsenic copper sulfide material containing rhenium
InactiveCN105861843ASimple processImprove leaching rateProcess efficiency improvementPregnant leach solutionSlag
The present invention discloses an efficient method for enriching rhenium from high-arsenic copper sulfide material containing rhenium. The process is as below: mixing rhenium-enriched slag with a certain amount of alkali into a slurry; pressurizing, heating and leaching alkali; filtering and washing; and sending ammonium rhenium in the form of ammonium rhenate and arsenic in the form of sodium arsenite into a leaching solution. The method uses reactive metal powder heating for replacement of rhenium in the leaching solution; and filtering and washing are carried out to obtain the enrich rhenium oxide and achieve separation of rhenium and arsenic. The raw material is rhenium-enriched slag obtained by sulfide precipitation of waste acid in produced the production of copper smelting enterprises, wherein the slag contains large amount of copper and arsenic. By the process, rhenium can be completely leached, while the metal powder substituties the leaching solution to obtain rhenium-enriched material. The method simplifies subsequent rhenium extraction procedure and reduces the cost of rhenium extraction. The invention has the advantages of simple and short process, high enrichment rate, strong adaptability of raw materials, low cost, easiness to industrialization and good application value, and can increase value and profits copper smelting enterprises.
Owner:KUNMING METALLURGY COLLEGE
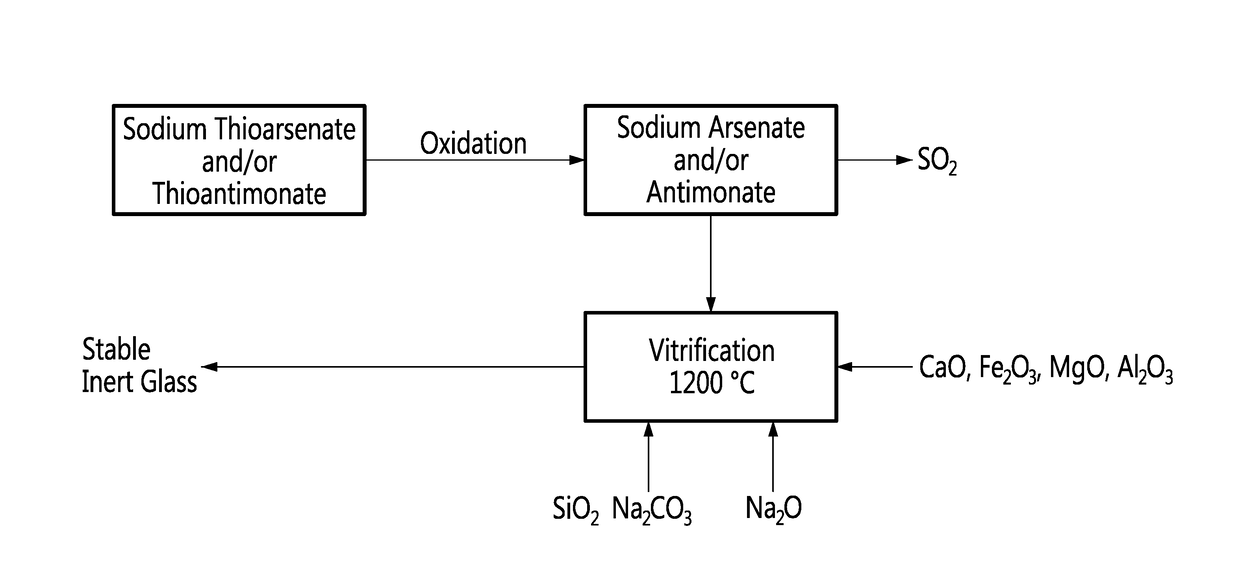
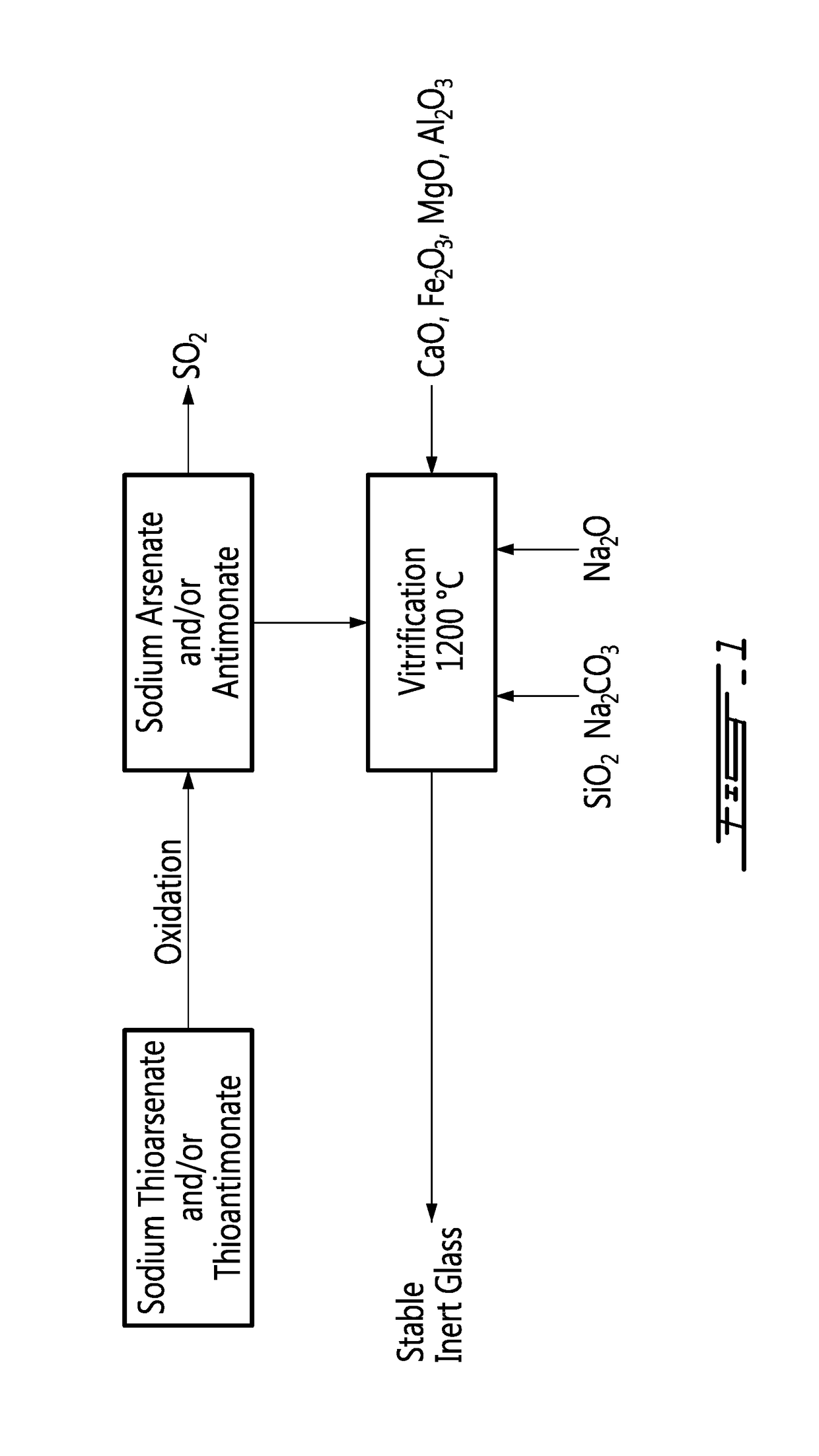
Method for vitrification of arsenic and antimony
ActiveUS20180023165A1Transportation and packagingChemistry apparatus and processesVitrificationOxygen
A method for vitrification of arsenic and antimony, comprising substituting oxygen to sulfur on thiosalts, incorporating resulting sodium arsenate and sodium antimonate into a sodium silicate glass-forming mixture and vitrifying the sodium silicate glass-forming mixture into a resulting glass sequestering the arsenic and antimony.
Owner:DUNDEE SUSTAINABLE TECH
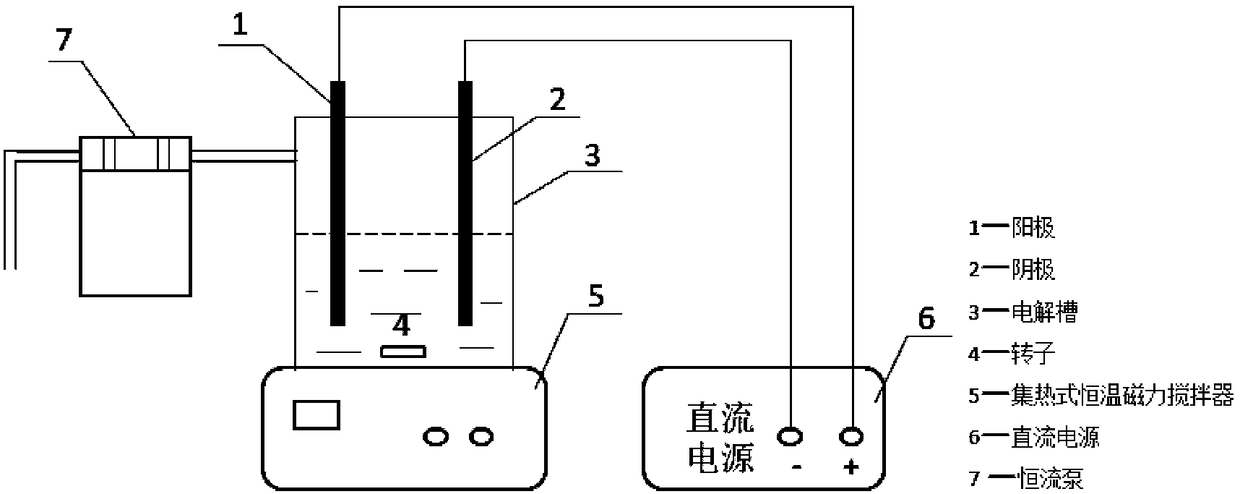
Electrochemical method for separating arsenic and alkali from arsenic-alkali residue
ActiveCN108588428AEfficient separationEasy to separateElectrolysis componentsProcess efficiency improvementElectrolysisWater immersion
The invention discloses an electrochemical method for separating arsenic and alkali from arsenic-alkali residue. The method comprises the following steps that oxidation and water immersion are carriedout on the arsenic-alkali residue so as to obtain an arsenic-alkaline residue leachate containing sodium carbonate and sodium arsenate; a sodium carbonate solution serves as an electrolyte, an iron electrode serves an anode, a carbon electrode serves as a cathode, then electrolysis is carried out, and active ferrous hydroxide is generated in the electrolyte; and the arsenic-alkali residue leachate is added into the electrolyte containing the active ferrous hydroxide, and then electrolysis is carried out to generate iron arsenate crystal precipitates. According to the method, antimony is separated from the arsenic-alkali residue by using oxidization and water immersion, and then the electrochemical method is used for converting the arsenic in the leachate into ferric arsenate particles with good crystallinity, so that efficient separation of the arsenic and the alkali is realized; the method can be used for quickly, efficiently and economically removing the arsenic from a strong alkaline solution, and the use of an oxidizing agent in the arsenic removal process is reduced; and the method is simple in process, convenient to operate and capable of meeting the industrial production.
Owner:CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com