Method for separating and recycling arsenic and iron from biological oxidation solution of sulfide ore
A technology for separation and recovery of oxidized liquid, applied in chemical instruments and methods, iron oxide/iron hydroxide, process efficiency improvement, etc., can solve the problems of high processing cost, large resource consumption, environmental pollution, etc., and achieve cost reduction, Ease of operation and reduced resource consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
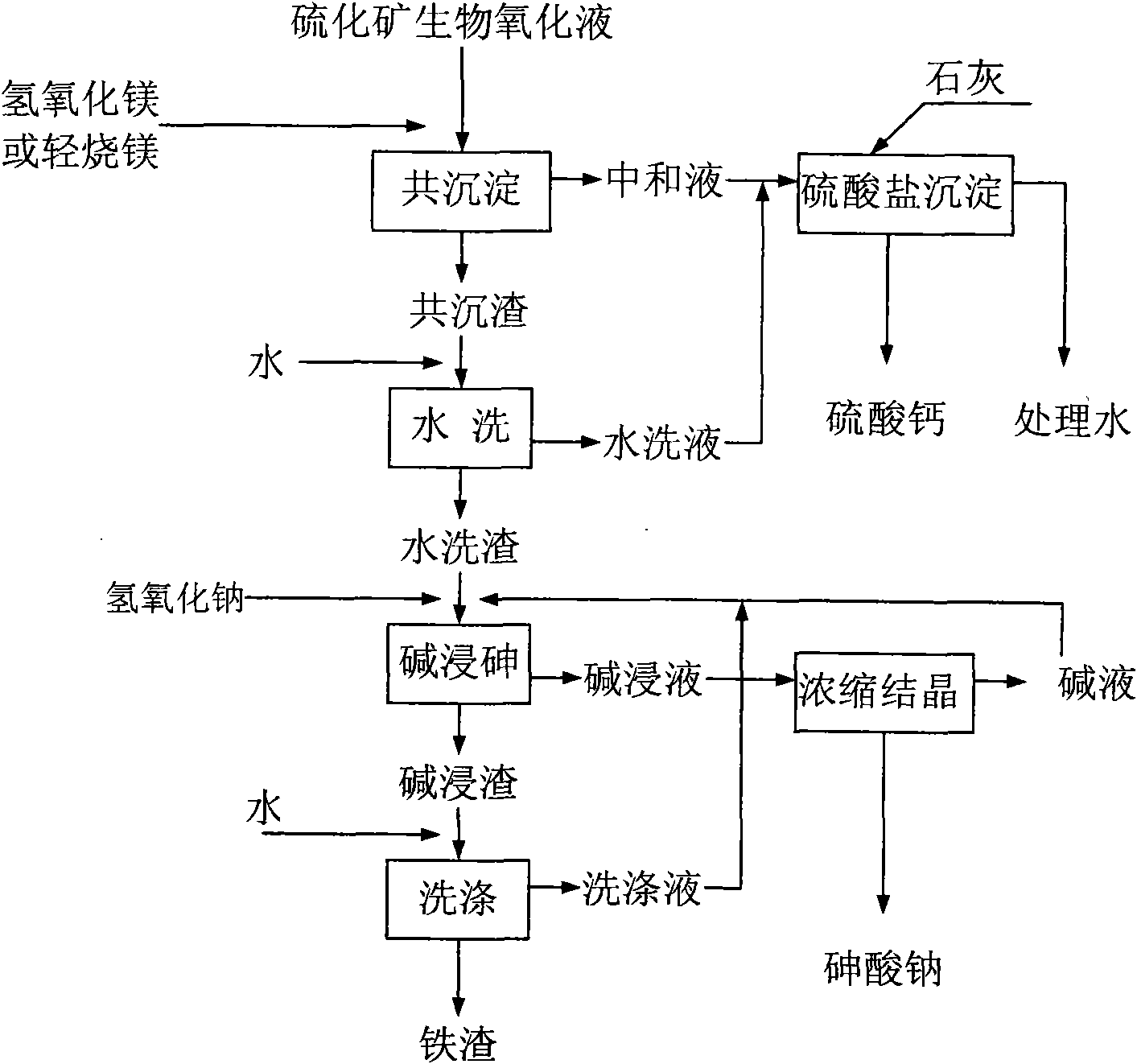
[0017] as attached figure 1 Shown, is the schematic flow sheet of the present invention:
[0018] The biological oxidation solution of sulfide ore was selected for the experiment of treating arsenic and ferric sulfate. The oxidation solution contained pentavalent arsenic 5.25g / L, ferric iron 30.4g / L, and pH=0.89;
[0019] (1) Co-precipitation operation: add light-burned magnesium that neutralizes the precipitant to the sulfide ore biological oxidation solution, and the dosage is 26kg / m 3 , adjust the pH value to 5, make co-precipitation of arsenic and iron in the sulfide ore biological oxidation solution, the precipitation rate is 99.1% and 98.2%;
[0020] (2) Treatment of neutralizing solution: add 35kg / m to the neutralizing solution produced in the co-precipitation operation 3 The lime is further processed, and the pH value is adjusted to 8, so that calcium sulfate precipitates, and the calcium sulfate precipitate and water are separated, and the water is recycled as produ...
Embodiment 2
[0026] As shown in the accompanying drawings, it is a schematic flow diagram of the present invention:
[0027] The biological oxidation solution of sulfide ore was selected for the experiment of treating arsenic and ferric sulfate. The oxidation solution contained pentavalent arsenic 8.36g / L, ferric iron 32.2g / L, and pH value 0.84;
[0028] (1) Co-precipitation operation: add neutralizing precipitant light-burned magnesium to the sulfide ore biological oxidation solution, the dosage is 27kg / m 3 , adjust the pH value to 6, make co-precipitation of arsenic and iron in the sulfide ore biological oxidation solution, the precipitation rate is 99.2% and 98.5%;
[0029] (2) Treatment of neutralizing solution: add 37kg / m to the neutralizing solution produced in the co-precipitation operation 3 The limestone is further processed, the pH value is adjusted to 9, calcium sulfate is precipitated, calcium sulfate precipitate and water are separated, and the water is recycled as production...
Embodiment 3
[0035] as attached figure 1 Shown, is the structural representation of the present invention:
[0036] The selected oxidizing solution contains 8.36 g / L of pentavalent arsenic, 32.2 g / L of ferric iron, and the pH value of 0.84 is the same as in Example 2.
[0037] (1) Co-precipitation operation; this acidic oxidation solution uses light-burned magnesium as a neutralization precipitant, and the dosage is 27kg / m 3 , adjust the pH value to 6, make co-precipitation of arsenic and iron in the sulfide ore biological oxidation solution, the precipitation rate is 99.2% and 98.5%.
[0038] (2) Treatment of neutralizing solution; add 37kg / m3 to the neutralizing solution produced in the co-precipitation operation 3 The lime is further processed, and the pH value is adjusted to 10 to precipitate calcium sulfate, and the calcium sulfate precipitate and water are separated, and the water is recycled as production water after the gas treatment, and the calcium sulfate precipitate is used as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com