Method for removing arsenic from acid solution
An acidic solution and removal technology, which is applied in the field of arsenic removal, can solve the problems of secondary pollution of the environment, the arsenic cannot be recycled and used, and the arsenic removal agent cannot be recycled, so as to achieve low cost of use and solve the problem of secondary pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
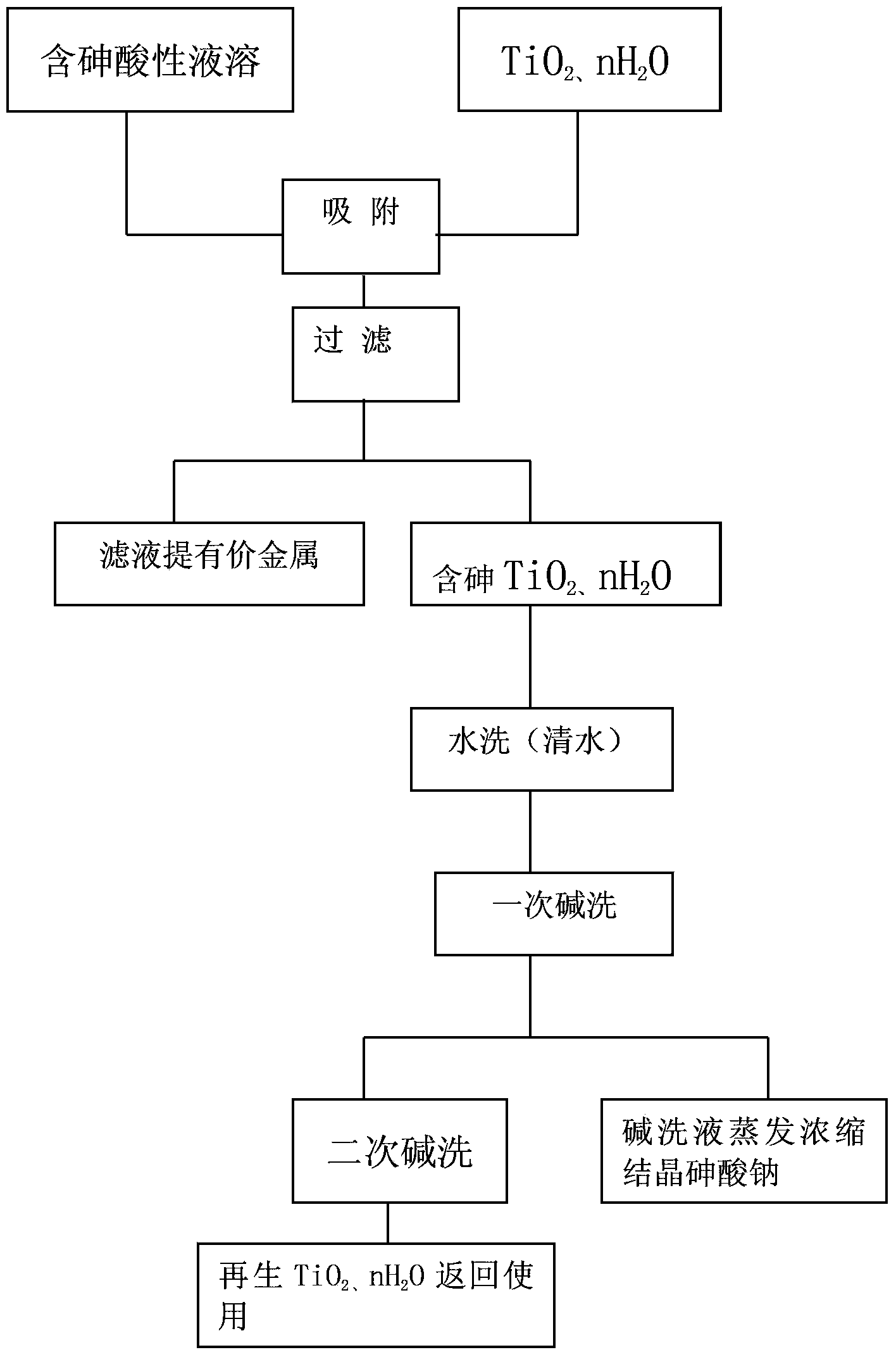
[0019] Embodiment 1: get 100 grams of TiO 2 , nH 2 O, installed in a Φ20mm glass tube, the effective column height is 100mm, and then use H containing As5, 2g / L, Zn 94g / L, Fe 0.6g / L, pH value 1.5 2 so 4 The leachate is subjected to natural osmosis filtration at a speed of 5-10ml / min, the filtrate As13.2mg / L, Zn 92.3g / LFe 0.52g / L, adsorption rate As97.6%, Zn: 2.16%Fe: 2.01%, capacity 3.6L / g TiO 2 , nH 2 The adsorption amount of O and As is 187mg / gTiO 2 , nH 2 O. The TiO that adsorbed As subsequently 2 , nH 2 O is washed once, twice with alkali, and once again with water, the total elution of As is 99.1%, one alkali wash contains As28g / L, and two alkali washes 8.3g / L.
example 2
[0020] Example 2: 200gTiO 2 , nH 2 O is put into a Φ100mm microporous ceramic funnel with an effective layer height of 20mm, and then ZnSO with a pH value of 3.2 containing As 8.7g / L and Zn106g / L 4The solution is vacuum pumped and filtered at a speed of 30-50ml / min. When the processing capacity is 40 liters of solution, the adsorption rate of As is 94.1%. After adsorption, the solution contains As 0.5g / L Zn 112g / L. / g TiO 2 , nH 2 When O, the adsorption rate drops to 68.3%, and alkali washing regeneration is carried out. Carry out vacuum pump suction filtration adsorption again. The adsorption rate of As rose to 97.8%. The first alkaline washing solution contained As 18g / L, and the second alkaline washing solution contained As9.5g / L.
example 3
[0021] Example 3: Get the In stripping liquid H containing As10% 2 o 2 The purification residue is leached with 100-120g / L HCl to obtain an HCl leaching solution containing 25g / L In and 32.8g / L As. Use 60gTiO 2 , nH 2 O is equal to 3.3 according to the liquid-solid ratio, adding HCl leaching solution, stirring adsorption and filtering for 30 minutes at room temperature. The obtained filtrate contains As24.7g / L, and the adsorption rate is 24.7%, and then regenerated TiO 2 , nH 2 060g is stirred and adsorbed, and the filtrate contains As2.46g / L, and the adsorption rate is 90%, and then 60g is used to regenerate TiO 2 , nH 2 O carries out stirring adsorption for the third time, and the obtained filtrate contains As 0.75g / L, In 22.4g / L, the adsorption rate of As is 69.5%, In 10.4%, and the total removal rate of three stirring adsorption As is 97.7%, In:10.4% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com