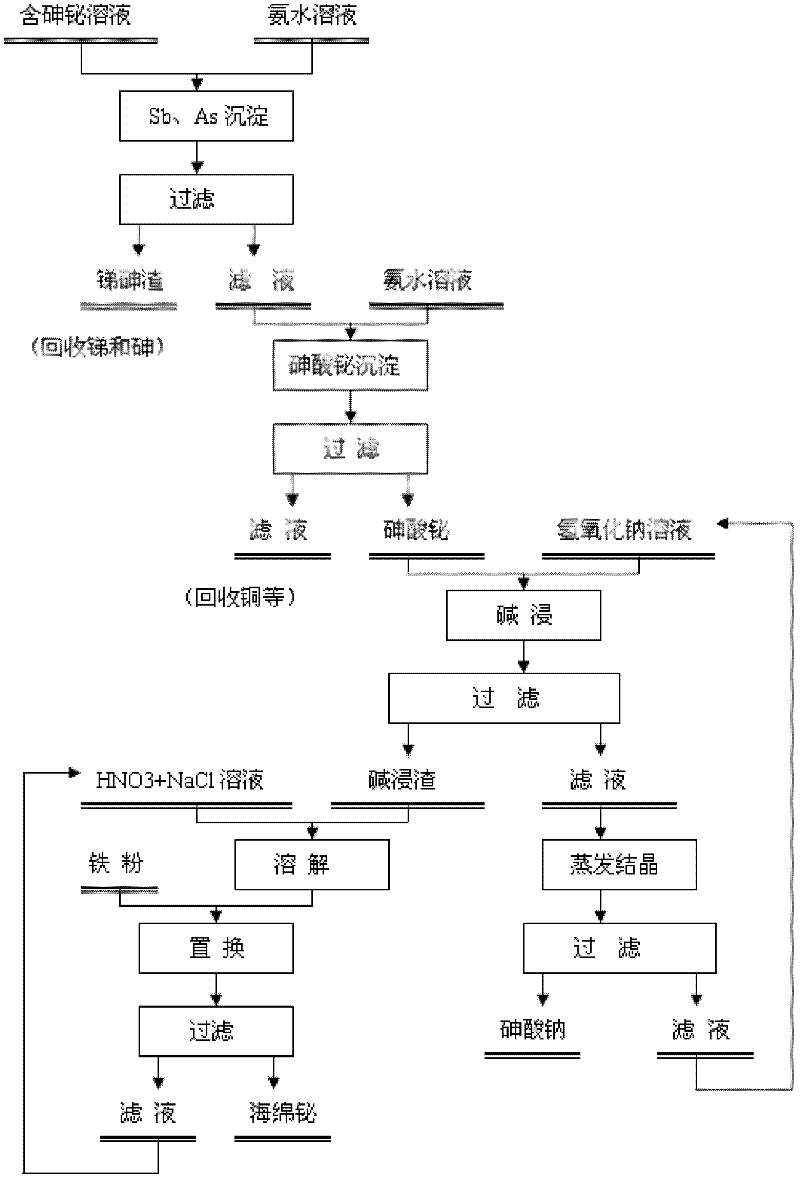
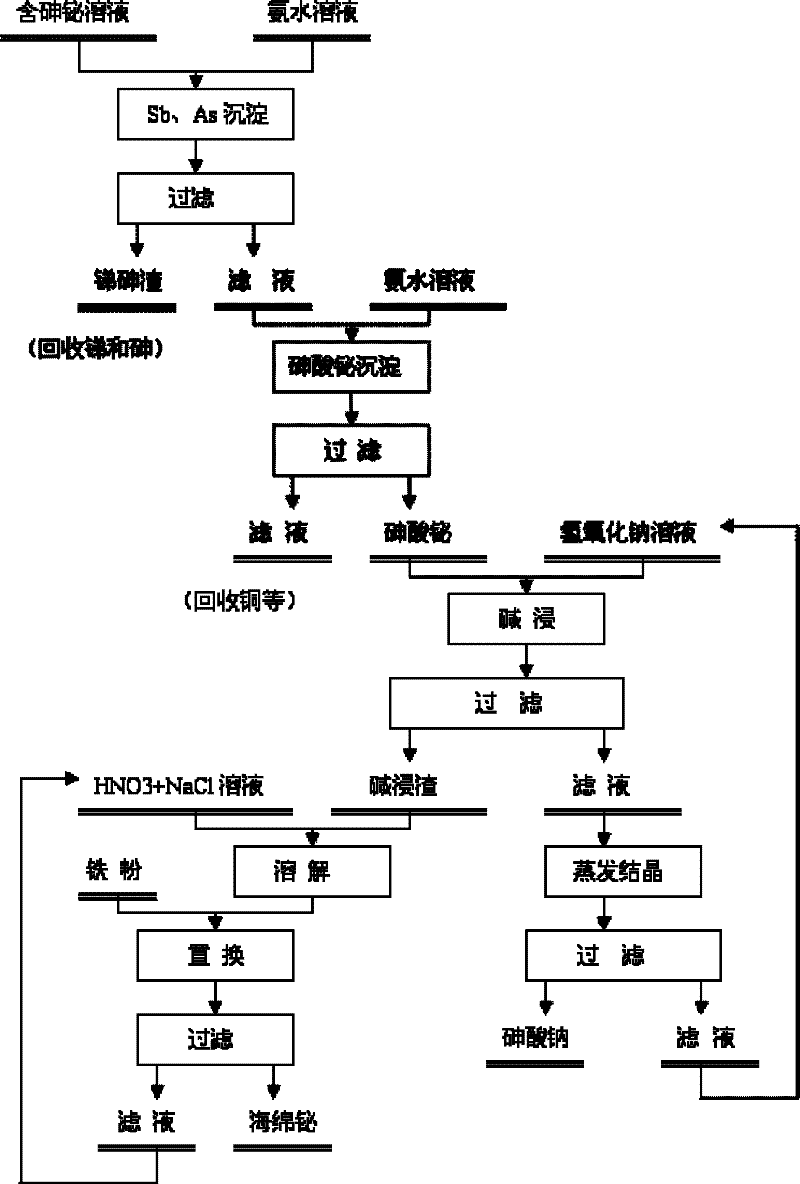
Method for recovering bismuth and arsenic from bismuth and arsenic-containing solution
A technology of solution and alkaline solution, which is applied in the field of recycling bismuth and arsenic, can solve the problems of bismuth recovery rate decline and bismuth loss, and achieve the effect of improving recovery rate and avoiding loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The leach solution obtained by leaching a metallurgical slag with sulfuric acid mainly contains As: 14.72g / L; Bi: 14.28g / L; Cu: 6.78g / L; Fe: 3.11g / L; Pb: 2.17g / L; L.
[0030] While mechanically stirring, adjust the pH value of the solution to about 0.5 with 15% (mass concentration, the same below) ammonia water, keep mechanically stirring for 30 minutes, and filter with suction to obtain a precipitate and a filtrate. The filtrate was sampled and analyzed, and the precipitation rate of each element calculated according to the filtrate composition was As 24%, Bi 3.3%, Cu 3.2%, Fe 2.8%, Pb 36%, Sb 85%.
[0031] Continue to use 15% ammonia water to adjust the pH value of the filtrate to 3.2, maintain mechanical stirring for 30 minutes, and filter with suction to obtain a precipitate and a filtrate. XRD analysis of the obtained precipitate revealed that it was mainly bismuth arsenate. According to the filtrate sampling analysis, the precipitation rates of bismuth and arsen...
Embodiment 2
[0036] The composition and operation process of the leaching solution used are the same as in Example 1, except that the pH value of the solution is adjusted to 0 with 15% ammonia water, and the precipitation rate of each element analyzed after filtration is As 16.2%, Bi 3.3%, Cu 3.2%, Fe 2.2 %, Pb 27.5%, Sb 78.9%.
[0037] Continue to use 15% ammonia water to adjust the pH value of the filtrate to 3.5, keep mechanical stirring for 30 minutes, and filter with suction to obtain a precipitate and a filtrate. XRD analysis of the obtained precipitate revealed that it was mainly bismuth arsenate. According to the filtrate sampling analysis, the precipitation rates of bismuth and arsenic in the solution are all over 98%.
[0038]Use 4mol / L sodium hydroxide solution to carry out alkali leaching treatment on the above precipitate, and stir and leaching for 1 hour under the conditions of liquid-solid ratio of 3:1 and temperature of 90°C. After liquid-solid separation, the leaching so...
Embodiment 3
[0042] The composition and operation process of the leaching solution used are the same as in Example 1, except that the pH value of the solution is adjusted to 0.8 with 15% ammonia water, and the precipitation rate of each element analyzed after filtration is As 33.2%, Bi 4.2%, Cu 4.2%, Fe 3.5 %, Pb 44.2%, Sb 88.9%.
[0043] Continue to use 15% ammonia water to adjust the pH value of the filtrate to 3.5, keep mechanical stirring for 30 minutes, and filter with suction to obtain a precipitate and a filtrate. XRD analysis of the obtained precipitate revealed that it was mainly bismuth arsenate. According to the filtrate sampling analysis, the precipitation rates of bismuth and arsenic in the solution are all over 98%.
[0044] Use 1 mol / L sodium hydroxide solution to carry out alkali leaching treatment on the above precipitate, and stir and leaching for 1 hour under the conditions of liquid-solid ratio of 6:1 and temperature of 70°C. After liquid-solid separation, the leachin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com