Manufacture of copper arsenite and application of the same
A copper arsenite, reaction technology, applied in the direction of arsenate/arsenite, process efficiency improvement, photography process, etc., can solve the problem of high solubility of extraction agent, cumbersome treatment process, long follow-up process of chemical precipitation method, etc. problem, to achieve the effect of improving physical quality and chemical quality, improving physical and chemical quality, and remarkable removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
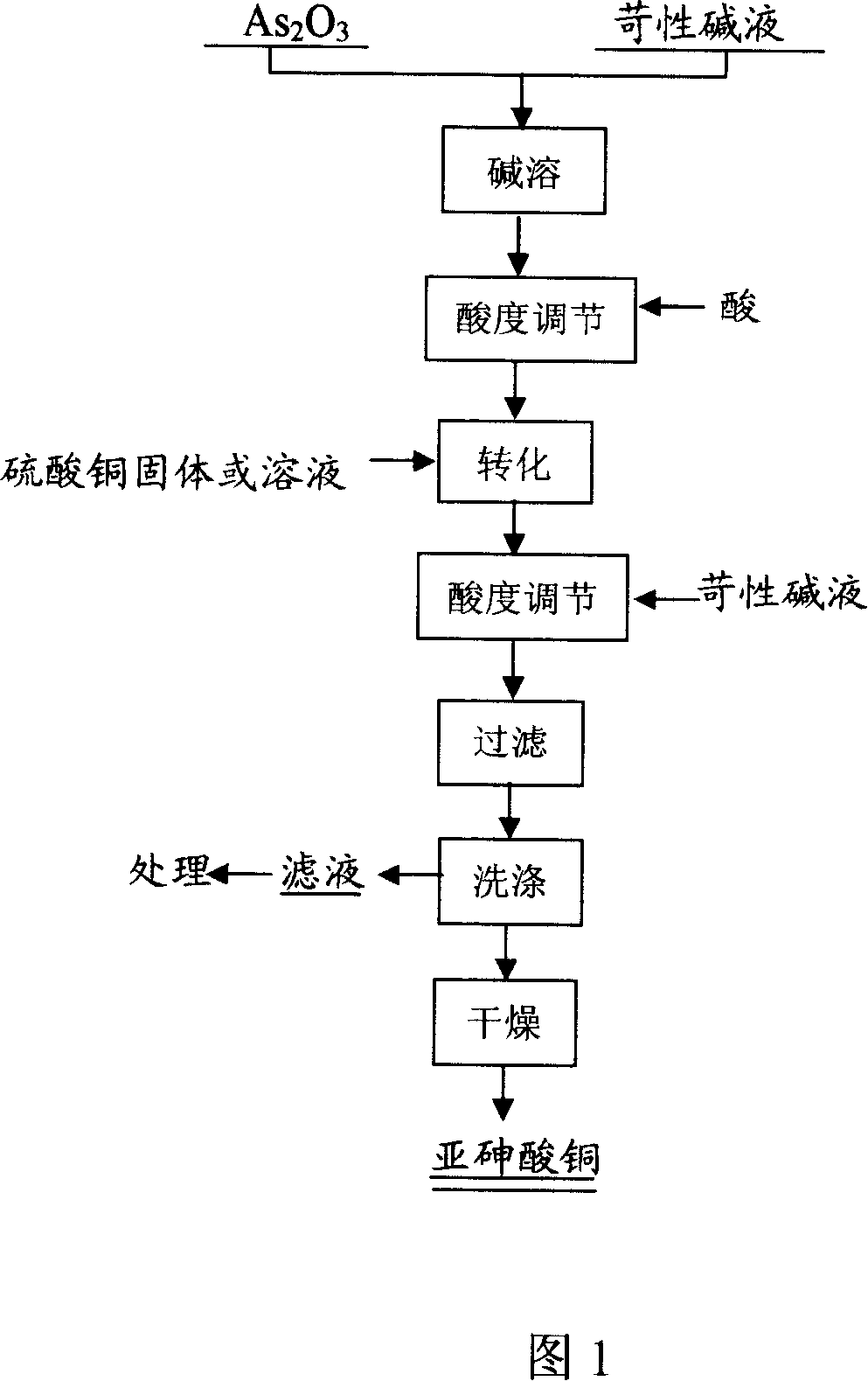
[0023] Example 1: Weigh 40g of arsenic trioxide and add it to 800mL of sodium hydroxide solution with a concentration of 1mol / L. After stirring for 1 hour, add concentrated sulfuric acid to adjust the pH value of the solution to 8.0, add 150g of copper sulfate pentahydrate, stir to dissolve, and add 6mol / L sodium hydroxide solution to adjust the pH value of the solution to 6.0, stir and react at this acidity for 1 hour and then filter to obtain green copper arsenite, bake it at 110°C for 8 hours to obtain dry copper arsenite, its chemical formula is Cu 5 As 4 o 12 h 2 .
[0024] Take 20g of copper arsenite and add it into 1L of copper electrolyte, stir and react at 65°C for 8h, then filter.
[0025] The comparison of electrolyte composition before and after purification is shown in Table 1.
[0026] Table 1 Composition / g·L of electrolyte before and after purification -1
[0027] the element
[0028] It can be seen from Table 1 that the removal rates of Sb and...
example 2
[0029] Example 2: Weigh 200kg of arsenic trioxide and add to 4m 3 Concentration is in the sodium hydroxide solution of 1mol / L, after stirring for 2 hours, add concentrated sulfuric acid to adjust the pH value of the solution to 7.0, add 800kg copper sulfate pentahydrate, stir and dissolve, add 6mol / L sodium hydroxide solution to adjust the pH value of the solution 6.8, stirred and reacted at this acidity for 2 hours, then settled and washed 4 times, then filtered with a belt filter, dried and packaged.
[0030] Take 2100kg of copper arsenite prepared by the above method and add it to the electrolytic cell in batches, and inject it into the 40m 3 In the copper electrolyte, the dissolved electrolyte flows into the low-level tank, and is pumped into the high-level tank by the circulation pump and into the filter by the circulation pump, and the circulation is repeated for 16 hours. The filter capacity is to filter once every 4 hours. The circulation temperature is 63~65℃, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com