Pharmaceutical compositions comprising of arsenous acid, its sodium salt and its derivatives intended for the treatment or urogenital cancer and its metastasis
a technology of arsenic acid and composition, which is applied in the direction of drug composition, biocide, extracellular fluid disorder, etc., can solve the problems of unfavorable treatment of kidney cancer, chemotherapy has not yet been shown to be helpful in treating kidney cancer, and traditional chemotherapy drugs have not been quite as useful, etc., to achieve shortening telomeres, good cytotoxic activity, and good quality of life
Inactive Publication Date: 2009-01-08
KOMINOX
View PDF3 Cites 17 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
[0051]The Inventor has explored the potency of three arsenic compounds of different valence and methylation in panels of human tumour cell lines in vitro, arsenous acid sodium salt (As3+), dimethylarsinic acid (As5+) and arsenic acid (As5+). Surprisingly arsenous acid sodium salt was the most potent and showed anti-tumour activity in a human tumour model in vivo, reason to develop arsenous acid sodium salt further as a novel arsenic compound. Arsenous acid sodium salt surprisingly was more potent in vitro and showed differential activity in leukaemia, melanoma and mammary cancer lines than As2O3. Arsenous acid sodium salt is surprisingly capable of shortening telomeres of human cancer cells, inducing cellular senescence and chromosomal abnormalities, but does not directly inhibit the telomerase activity. The effects indicate that arsenous acid sodium salt is a telomere inhibitor. Arsenous acid sodium-salt was rapidly absorbed after both i.v. and p.o. administration and remained in the plasma for prolonged periods. Surprisingly the bioavailability of oral arsenous acid sodium salt was approximately 100%. Animal toxicity studies showed that the main target organs were bone marrow and lymphoid organs. Thus arsenous acid sodium salt can be administered orally. It might be used in long-term treatment of cancer patients with solid tumours or leukaemia at dose levels below the maximum tolerated dose (MTD), alone or in combination with another treatment modality, maintaining a good quality of life.
[0052]This compound (NaAsO2) of the present invention has been developed as novel anti-cancer agent. The compound possesses good cytotoxic activity in a panel of 43 human tumour cell lines in vitro with an IC50 value of 0.6 μM. Pronounced selectivity was observed in tumour cell lines derived from leukaemia, mammary cancer and melanoma. In a head-to-head comparison arsenous acid sodium salt was surprisingly at least 15-fold more potent than the clinically used agent arsenic trioxide and had also a better differential activity. Arsenous acid sodium salt combined with 5-fluoruracil (5-FU) or vinblastine may result in additive effects. Potassium in the arsenite reduced cytotoxic activity.
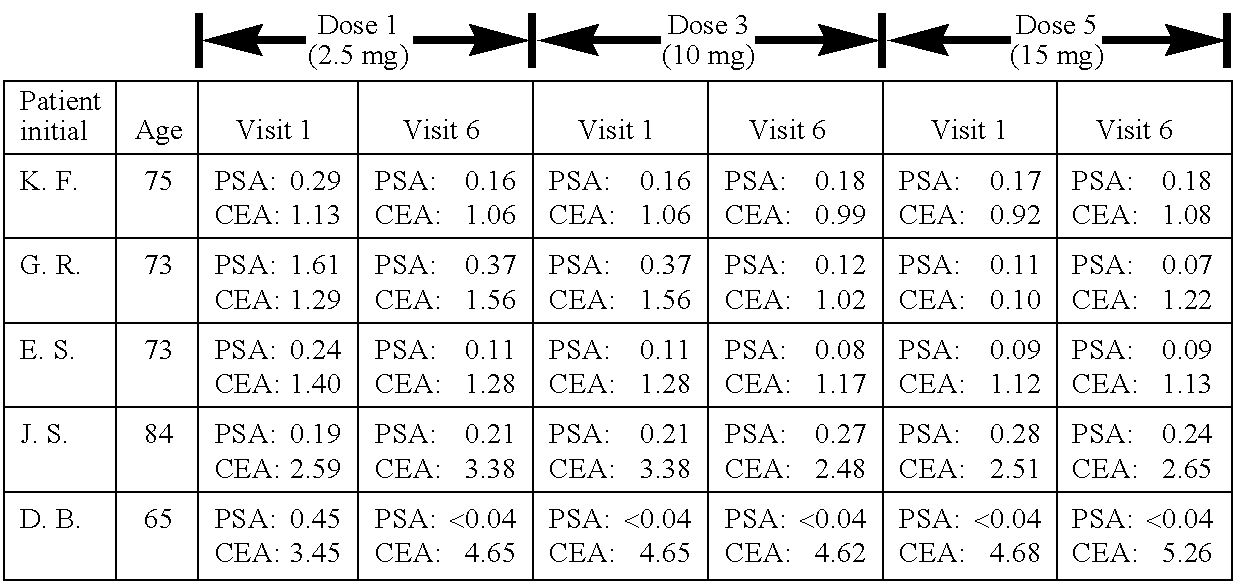
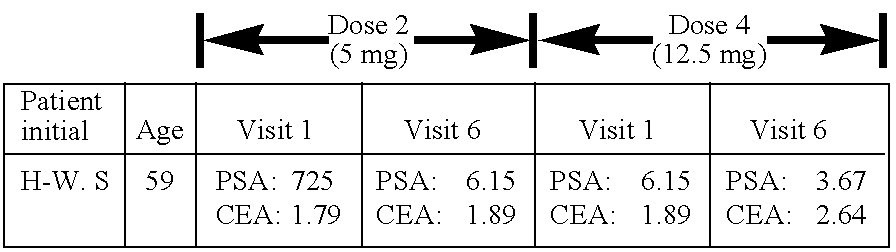
[0054]Surprisingly, oral arsenous acid sodium salt showed a high therapeutic efficacy in cancer patients suffering from urogenital cancer, mainly prostate and bone metastasis, following treatment with 2.5, 10, 12.5, 15, 17.5 and 20 mg of arsenous acid sodium salt capsules for 14 consecutive days.
[0056]Surprisingly arsenous acid sodium salt, the compound of invention, has great therapeutic and safety advantages in comparison to arsenic trioxide. Arsenic trioxide As2O3 has been shown to prolong the QT and QT interval corrected for rate (QTc), which may predispose the patient to potentially fatal aytipical ventricular tachycardia and produce complete atrioventricular block.
Problems solved by technology
Intravenous (iv) administration is a major source of discomfort and stress for cancer patients and approximately 90% of patients asked, express a preference for oral versus iv chemotherapy, predominantly because of the convenience of administration outside a clinical setting or current concerns about previous problems with intravenous access.
Chemotherapy has not yet been shown to be helpful in treating cancer of the kidney.
For this reason traditional chemotherapy drugs have not proven to be quite as useful as they have been in some of the other major cancers.
None of these agents are consistently helpful in the disease.
The bone metastases are particularly troublesome in that they can create intense pain for the patient.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
working examples
6. WORKING EXAMPLES
[0103]The following subsections describe the testing of a pharmaceutical composition comprising arsenous acid sodium salt in vivo using cancer patients. The results demonstrate that arsenous acid sodium salt administered orally is effective in the treatment of urogenital cancer.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present application relates to pharmaceutical compositions and methods for treatment of urogenital diseases and bone metastasis in a human, which pharmaceutical composition contains an effective amount of arsenous acid alkaline or earth alkaline metal salt and / or a pharmaceutically acceptable adjuvant. According to the present invention, the alkaline arsenous acid metal salt is sodium meta-arsenita (AsO2Na) or potassium meta-arsenite (AsO2K). The effective amount of arsenous acid alkaline or earth alkaline metal salt is 0.0001-1500 mg / kg, preferably 1-1000 mg / kg, more preferably 1-150 mg / kg, and most preferably 50-100 mg / kg of body weight / day. The administration form of the pharmaceutical compositions of the invention is preferably oral, such as a tablet, capsule, powder and / or solution with a pharmaceutically acceptable carrier, diluent or excipient.
Description
1. INTRODUCTION[0001]Cancer is a significant health problem in the world. Although advances have been made in cancer detection and treatment, no vaccine or other universally successful preventive or therapeutic method is currently available. Management of the disease currently relies on a combination of early diagnosis and aggressive treatment, which may include one or more of a variety of therapies such as surgery, radiotherapy, chemotherapy and hormone therapy. While such therapies provide benefit to many patients, a high mortality continues to be observed for many cancers. The development of improved anti-tumour agents would facilitate cancer prevention and treatment.[0002]Unfortunately, cancer is the leading cause of death, second only to heart disease, of both men and women. In the fight against cancer, numerous techniques have been developed and are the subject of current research directed to understanding the nature and cause of the disease and to providing methods for the co...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K33/36A61P35/00
CPCA61K33/36A61K45/06A61K2300/00A61P13/00A61P13/08A61P15/00A61P19/00A61P19/08A61P29/00A61P35/00A61P35/02A61P35/04A61P7/00A61K33/14
Inventor RADEMAKER, BERNARDUS
Owner KOMINOX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com