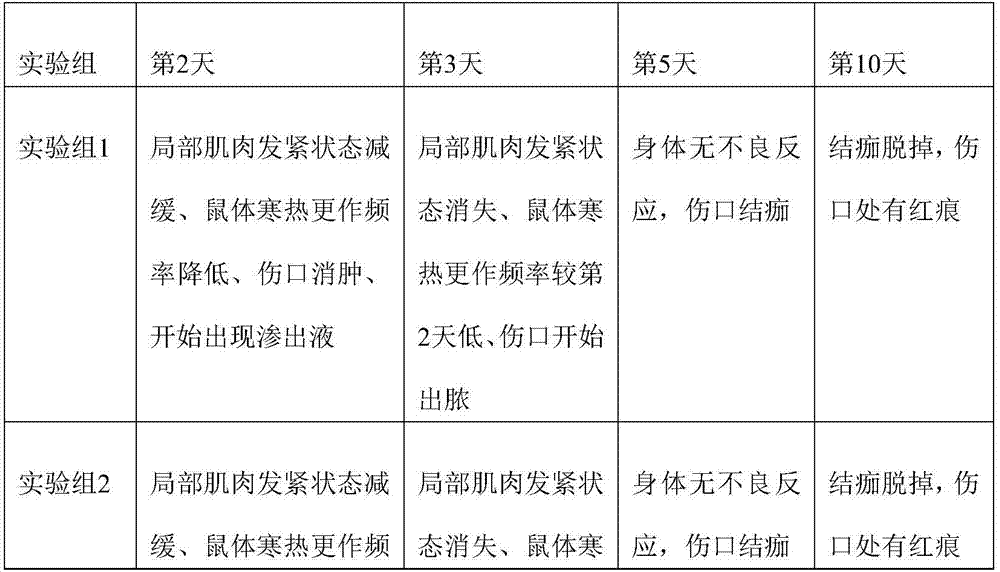
Patents
Literature
43 results about "Clostridium tetani" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clostridium tetani is a common soil bacterium and the causative agent of tetanus. When growing in soil, C. tetani is rod-shaped and up to 2.5 μm long. However, when forming spores, C. tetani becomes substantially enlarged at one end, resembling a tennis racket or drumstick. C. tetani spores are extremely hardy and can be found globally in soil or in the gastrointestinal tract of animals. If inoculated into a wound, C. tetani can grow and produce a potent toxin, tetanospasmin, which interferes with motor neurons, causing tetanus. The toxin's action can be prevented with tetanus toxoid vaccines, which are often administered to children worldwide.
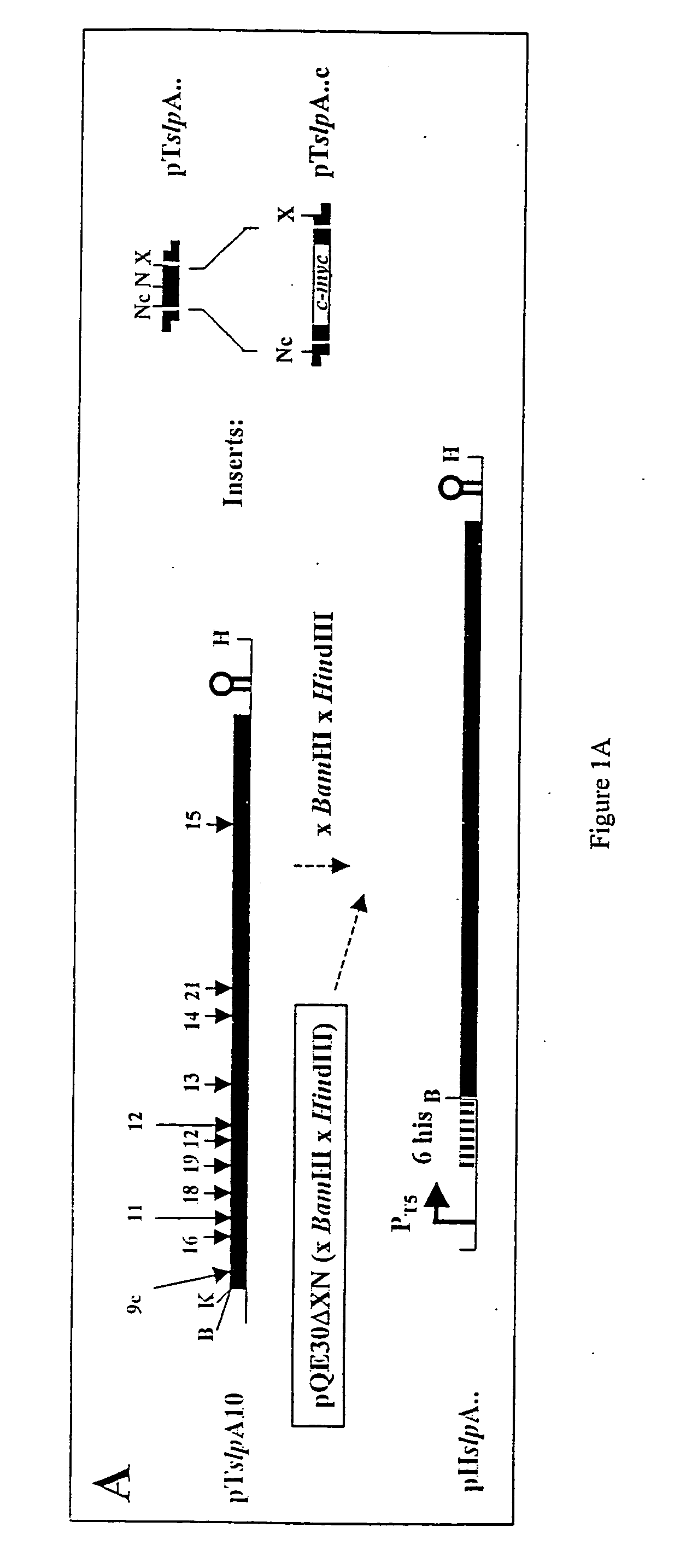
Modified bacterial surface layer proteins
InactiveUS20050233408A1Readily availableSure easyAntibacterial agentsFungiHeterologousProtein target
Modified bacterial surface layer (S-layer) proteins are disclosed where the modification is the insertion, at an internal location, of a heterologous polypeptide, or polypeptide of interest. The polypeptide is a binding or target protein, such as an antigen or antibody, or part thereof, in particular a bacterial antigen (e.g. from Clostridium tetani such as TTFC). The modified surface layer protein can then be expressed on the surface of the bacterial cell and used in a vaccine. Also disclosed are bacteria which have been modified to express a heterologous surface layer protein, but which do not as a wild-type possess an S-layer (such as L. casei), and modified bacteria which express only a modified surface layer protein (and not the wild-type S-layer protein). The wild type S-layer is completely replaced with the modified version where the polynucleotide encoding the modified version is integrated into the bacterial genome. The modified S-proteins can form crystalline arrays, sheets or layers that can be used to bind functional molecules (e.g. receptors) to solid surfaces (Au, silicon wafers) in biosensors.
Owner:LACTRYS OCTROOI
Combination vaccine
InactiveUS20090130146A1Effective seroprotectionGood immune protectionAntibacterial agentsBacterial antigen ingredientsBacteroidesDisease
The present invention relates to the combination of antigens directed against bacteria and viruses, their uses and the preparation of medicaments in order to confer protection against infectious diseases. In particular, the invention relates to a combination vaccine comprising at least one antigen of Clostridium tetani, at least one antigen from Corynebacterium diphtheriae, and at least one antigen from the TBE-flavivirus suitable to confer seroprotection against diseases and medical conditions caused by these pathogenic organisms.
Owner:NOVARTIS AG
High-level expression of tetanus toxin receptor binding domain Hc in Escherichia coli and application
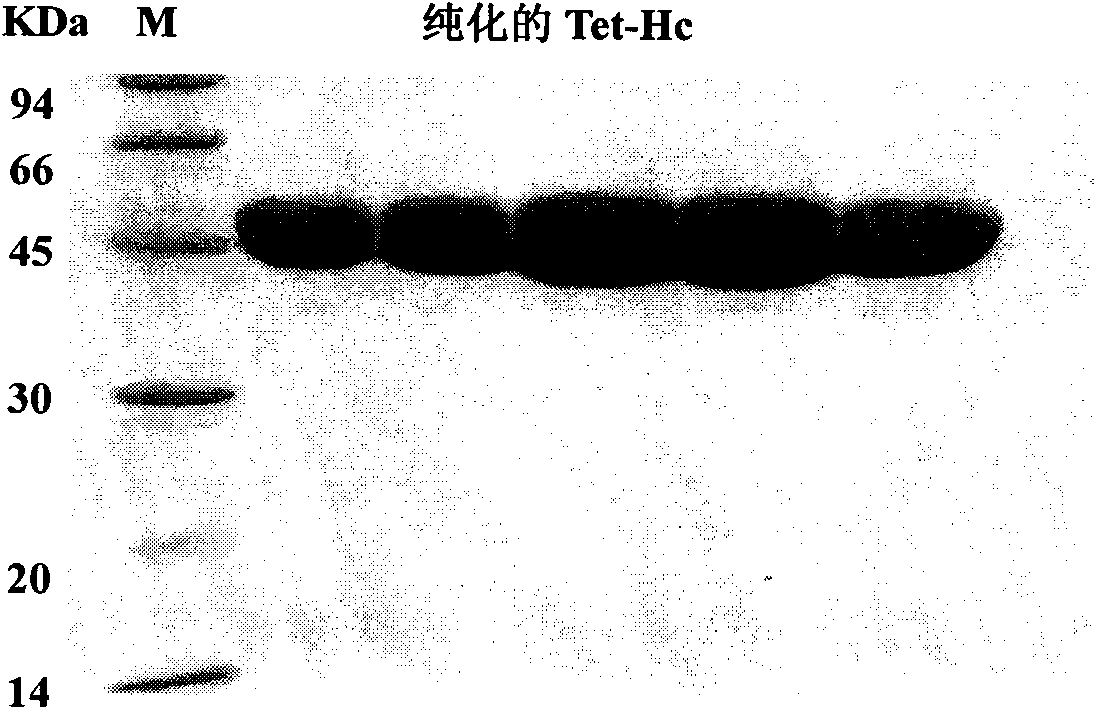
The invention relates to a method for a tetanus toxin receptor binding domain Hc to be subjected to high-level soluble expression in Escherichia coli through nucleotide sequence optimization. According to the sequencing result of a domestic C.Tetani virulent strain CMCC64008, the tetanus toxin receptor binding domain Hc sequence is analyzed and optimized, the optimized sequence is SEQ ID No.1 and the coded protein sequence is SEQ ID No.2. The synthesized Hc gene is linked into an expression vector pET32a(+) after undergoing double enzyme digestion, the recombinant Hc is subjected to high soluble expression in Escherichia coli and the target protein accounts for about 46% of the total protein in the supernatant undergoing bacteriociasis. After QFF column purification, phenyl hydrophobic column purification and SP column purification, the purity of the target protein can be more than 95% and the yield thereof is more than 300mg / L. The recombinant protein prepared by the method of the invention has good immunogenicity, can induce the mice to produce high-titre protective antibodies and can resist attack of high-dose lethal toxins. The method has extensive application prospect in large-scale high-level preparation of the tetanus toxin recombinant subunit vaccine Hc.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Method for preparing tetanus toxoid vaccine
ActiveCN102961741AReduce cloggingAvoid damageAntibacterial agentsBacterial antigen ingredientsFiltrationUltrafiltration
The invention discloses a method for preparing a tetanus toxoid vaccine. According to the process, with clostridium tetani strains as raw materials, the tetanus toxoid vaccine is prepared through the following steps of: culturing of tetanus toxoid, bacterium liquid separation, ultrafiltration and concentration, salting out, ultrafiltration desalting and the like. According to the method, firstly culture liquid is subjected to virus-free treatment and then refined, so that the porous channel plugging caused by accumulation of thalli and other impurity segments at a plate and frame membrane package during plate and frame filtering to remove thalli is reduced, and the smoothness during filtration is increased; toxoid protein and other allergens in the culture liquid are removed by changing the salting-out method; and as the desalting methods of the culture liquid after concentration and salting out adopt the tangential flow ultrafiltration method, the destruction of antigen caused by shearing of toxoid protein is reduced, and the protein precipitation is avoided. By utilizing the method, the time for preparing the tetanus toxoid vaccine is shortened, and the production efficiency is improved.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Hybridoma cell strain secreting tetanus exotoxin monoclonal antibody, monoclonal antibody prepared by same, Fab antibody and application
InactiveCN102690789AGood effectHas the effect of neutralizing tetanus exotoxinAntibacterial agentsFungiClostridium tetaniTotal rna
The invention relates to a mouse hybridoma cell strain secreting a clostridium tetani bacillus exotoxin monoclonal antibody, wherein the strain has a preservation number of CCTCC (China Center for Type Culture Collection) NO. C201257. The invention further relates to a clostridium tetani bacillus exotoxin monoclonal antibody prepared by the mouse hybridoma cell strain; the invention further provides an Fab antibody, comprising a kappa chain and an Fd chain; the kappa chain and the Fd chain are obtained by amplifying from total RNA (ribonucleic acid) of the hybridoma cell strain. The invention further relates to a medicine for preventing or treating clostridium tetani bacillus infection, comprising the clostridium tetani bacillus exotoxin monoclonal antibody and / or the Fab antibody, and a pharmaceutically acceptable carrier. According to the invention, a monoclonal antibody for effectively neutralizing tetanus exotoxin is screened with natural tetanus exotoxin, and an Fab gene engineering antibody which is produced by large scale in vitro and which has toxin neutralizing effect is prepared with a gene engineering antibody technology on the basis of the monoclonal antibody.
Owner:ARMY MEDICAL UNIV +1
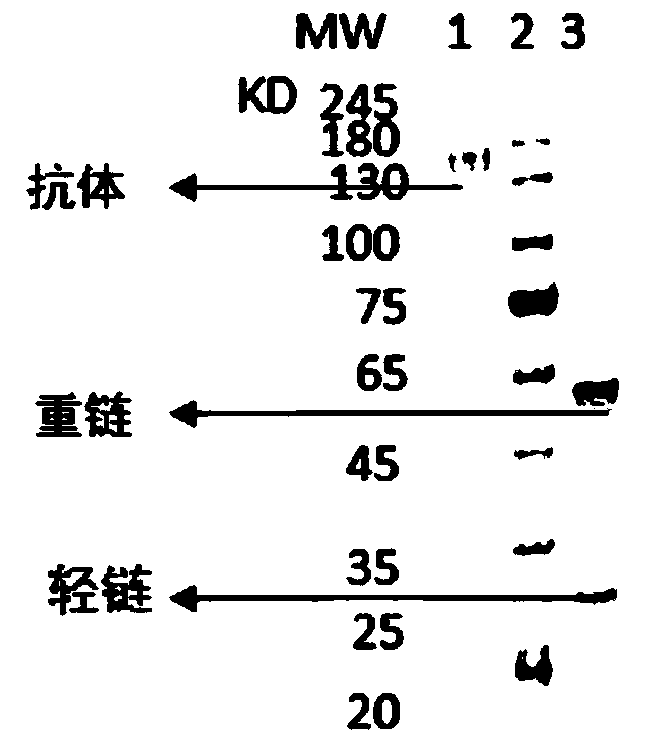
Tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof
ActiveCN108314730AAntibacterial agentsImmunoglobulins against bacteriaClostridial infectionClostridium tetani
The invention discloses a tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof. The antibody can be used for preventing, treating and diagnosing tetanus infection and / or treating one or more symptoms mediated by clostridium tetani infection. The invention further provides a method for producing an antibody capable of combining with tetanus toxin immunologically specifically.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Combination heptavalent vaccine
ActiveUS20130280293A1Reduce in quantityImprove satisfactionBacterial antigen ingredientsSsRNA viruses positive-senseClostridium tetaniImmunogenicity
The invention provides a stable immunogenic composition for prevention and prophylaxis of infections caused by rota virus, poliomyelitis virus, Haemophilius influenza, Hepatitis B, Corynebacterium diphtheriae, Clostridium tetani, Bordatella pertusis (acellular) in a single combined vaccine. The invention also provides for a bivalent immunogenic composition against rota virus and polio virus. The process of making such compositions of the multivalent antigens are also disclosed. The present invention also relates to the production and use of such vaccines for prophylaxis against the infections mentioned above.
Owner:BHARAT BIOTECH INTERNATIONAL
Modified bacterial surface layer proteins
InactiveUS20100172938A1Mitigate and avoid degradationSure easyAntibacterial agentsFungiHeterologousProtein target
Modified bacterial surface layer (S-layer) proteins are disclosed where the modification is the insertion, at an internal location, of a heterologous polypeptide, or polypeptide of interest. The polypeptide is a binding or target protein, such as an antigen or antibody, or part thereof, in particular a bacterial antigen (e.g. from Clostridium tetani such as TTFC). The modified surface layer protein can then be expressed on the surface of the bacterial cell and used in a vaccine. Also disclosed are bacteria which have been modified to express a heterologous surface layer protein, but which do not as a wild-type possess an S-layer (such as L. casei), and modified bacteria which express only a modified surface layer protein (and not the wild-type S-layer protein). The wild type S-layer is completely replaced with the modified version where the polynucleotide encoding the modified version is integrated into the bacterial genome. The modified S-proteins can form crystalline arrays, sheets or layers that can be used to bind functional molecules (e.g. receptors) to solid surfaces (Au, silicon wafers) in biosensors.
Owner:LACTRYS OCTROOI
Vaccine for inducing an improved immune reaction
ActiveUS20130273101A1Efficient preparationGood effectAntibacterial agentsSsRNA viruses negative-senseHepatitis B virusNeisseria meningitidis
The present invention relates to a pharmaceutical vaccine composition comprising: (a) a pathogen-derived antigen selected from the group consisting of Mycobacterium tuberculosis antigen, Bacillus anthracis antigen, HAV (hepatitis A virus) antigen, HBV (hepatitis B virus) antigen, HCV (hepatitis C virus) antigen, HIV (human immunodeficiency virus) antigen, influenza virus antigen, HSV (herpes simplex virus) antigen, Hib (Haemophilus influenzae type b) antigen, Neisseria meningitidis antigen, Corynebacterium diphtheriae antigen, Bordetella pertussis antigen, Clostridium tetani antigen and Varicella virus antigen; (b) a deacylated non-toxic LOS (lipooligosaccharide); and (c) a pharmaceutically acceptable carrier.
Owner:EYEGENE INC
High-level expression of tetanus toxin receptor binding domain Hc in Escherichia coli and application
The invention relates to a method for a tetanus toxin receptor binding domain Hc to be subjected to high-level soluble expression in Escherichia coli through nucleotide sequence optimization. According to the sequencing result of a domestic C.Tetani virulent strain CMCC64008, the tetanus toxin receptor binding domain Hc sequence is analyzed and optimized, the optimized sequence is SEQ ID No.1 and the coded protein sequence is SEQ ID No.2. The synthesized Hc gene is linked into an expression vector pET32a(+) after undergoing double enzyme digestion, the recombinant Hc is subjected to high soluble expression in Escherichia coli and the target protein accounts for about 46% of the total protein in the supernatant undergoing bacteriociasis. After QFF column purification, phenyl hydrophobic column purification and SP column purification, the purity of the target protein can be more than 95% and the yield thereof is more than 300mg / L. The recombinant protein prepared by the method of the invention has good immunogenicity, can induce the mice to produce high-titre protective antibodies and can resist attack of high-dose lethal toxins. The method has extensive application prospect in large-scale high-level preparation of the tetanus toxin recombinant subunit vaccine Hc.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Traditional Chinese medicine preparation for treating tetanus
InactiveCN102397479ASafe and effective treatmentEnhanced inhibitory effectAntibacterial agentsHeavy metal active ingredientsCentipedeClostridium tetani
The present invention provides a traditional Chinese medicine preparation for treating tetanus, made from cow-bezoar, periostracum cicadae, gastrodia tuber, pearl, notopterygium root, musk, uncaria, frankincense, red peony root, alum, myrrh, poria with hostwood, centipede, borneol, dahurian patrinia, pseudo-ginseng, Ligusticum wallichii, dragons blood, safflower and zhangdan in certain proportioning by weight. The product of the invention can effectively prevent and treat the tetanus, has the function of fever reduction, abirritation, convulsions stopping and convulsion and spasm relieving, has obvious inhibition effect to clostridium tetani, is low in cost, and is safe and effective on the treatment of the tetanus with 95% of effective rate and 89% of curative ratio.
Owner:高英豪
An improved process of conjugation and novel synthetic oligosaccharide- protein conjugates obtained thereof
InactiveUS20170246313A1High yieldHigh purityAntibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiTetanus toxoids
The present invention relates to an improved process of conjugation to obtain synthetic oligosaccharide-protein (OS-PR) conjugates. The process of synthetic OS-PR conjugation is a rapid process providing oligosaccharide-protein conjugates which are highly immunogenic and elicit specific and homogenous immune responses. The synthetic oligosaccharide comprising of four to eight repeating units of respective monomers and at least one in-built terminal amino linker, said synthetic polysaccharide mimics natural polysaccharide obtained from gram negative bacteria such as Neisseria meningitidis serogroups A, C, Y, W, X and Haemophilus influenzae and carrier protein is obtained from gram positive bacteria such as Clostridium tetani (tetanus toxoid) or Corynebacterium diphtheriae (CRM197) or their recombinant versions. The conjugation chemistry of the said oligosaccharide-protein conjugate of the present invention is thio-ether linkage. The present invention takes complete process time in the range of 14-22 hours. The said oligosaccharide-protein conjugates are useful in production of monovalent vaccine or multivalent combination vaccines and as diagnostic tool.
Owner:MSD WELLCOME TRUST HILLEMAN LAB PVT LTD
Technology for preparing antibacterial peptide
InactiveCN103695505AShorten the production cycleReduce use costMicroorganism based processesFermentationSynechococcusBacillus licheniformis
The invention provides a technology for preparing an antibacterial peptide. The technology comprises the following steps of mixing 2-4 parts by weight of bacillus licheniformis, 0.5-1.5 parts by weight of bacillus cereus and 1-2 parts by weight of bacillus natto to obtain a uniform mixture, carrying out mixture fermentation at a temperature of 36-39 DEG C for 6-10h to obtain storage strains, carrying out storage strain fermentation in a fermentation tank at a temperature of 36-39 DEG C for 27-37h, carrying out plate-frame pressure filtration on the fermented storage strains, and carrying out spray drying on the filtered storage strains to obtain the antibacterial peptide. The triple strain fermentation product obtained by the technology has effects on hemolytic streptococcocci, pneumococcocci, enterococcus, corynebacterium diphtheria, bacillus anthracis, gram-positive anoxic bacillus, achalme bacillus, clostridium tetani, clostridium difficile, eubacterium and lactic acid bacillus. The technology can shorten a production period by 1 / 3 and reduce a use cost by 60%.
Owner:潍坊市东方海洋生物科技研发中心
Specific protein composition and application method thereof
PendingCN105770890AGood control effectNearbyMuscular disorderImmunoglobulins against virusesRabiesBite wounds
The invention provides a specific protein composition and an application method thereof targeted to passive immunity and clostridium tetani infection prevention of a patient who is bitten or scratched by a mad dog or other mad animals.The specific protein composition and the application method thereof are mainly characterized in that the specific protein composition is formed by mixing human rabies immune globulin and human tetanus immune globulin, wherein the valence of antibodies of the human rabies immune globulin is not lower than 20 IU / ml, the valence of antibodies of the human tetanus immune globulin is not lower than 8 IU / ml, and the value ratio of the valence of antibodies of the human rabies immune globulin to the valence of antibodies of the human tetanus immune globulin is 2.5: 1.The application method comprises the steps of conducting thorough debridement in time; firstly, taking a half of the specific protein composition for conducting subcutaneous infiltration injection on the periphery of the injured part of the patient, and conducting intramuscular injection with the remaining half.The specific protein composition is mainly used for preventing the patient who is bitten or scratched by a mad dog or other mad animals from suffering from rabies and tetanus; the injection amount of the human rabies immune globulin is 10-40 IU / kg, and the injection amount of the human tetanus immune globulin is 4-40 IU / kg.
Owner:苏文全
Traditional Chinese medicine formula for treating tetanus
InactiveCN107375392APromote growthSimple componentsAntibacterial agentsAnthropod material medical ingredientsClostridium tetaniPilea notata
The invention belongs to the technical field of Chinese herbal medicines and particularly relates to a traditional Chinese medicine formula for treating tetanus. The formula comprises components as follows: all grass of pilea notata, herba sambuci chinensis, cacumen tamaricis, kelp and cockroaches. The traditional Chinese medicine composition for treating tetanus adopts a simple formula and is lower in cost, higher in clostridium tetani inhibition activity and remarkable in treatment effect and promotes wound growth, a use method is simple and convenient, the cure rate is high, and the composition is a good medicine for treating the tetanus and suitable for clinical use and popularization.
Owner:NANNING UNIV
Hybridoma cell strain secreting tetanus exotoxin monoclonal antibody, monoclonal antibody prepared by same, Fab antibody and application
InactiveCN102690789BGood effectHas the effect of neutralizing tetanus exotoxinAntibacterial agentsFungiGenetic engineeringBacillus infections
The invention relates to a mouse hybridoma cell strain secreting a clostridium tetani bacillus exotoxin monoclonal antibody, wherein the strain has a preservation number of CCTCC (China Center for Type Culture Collection) NO. C201257. The invention further relates to a clostridium tetani bacillus exotoxin monoclonal antibody prepared by the mouse hybridoma cell strain; the invention further provides an Fab antibody, comprising a kappa chain and an Fd chain; the kappa chain and the Fd chain are obtained by amplifying from total RNA (ribonucleic acid) of the hybridoma cell strain. The invention further relates to a medicine for preventing or treating clostridium tetani bacillus infection, comprising the clostridium tetani bacillus exotoxin monoclonal antibody and / or the Fab antibody, and a pharmaceutically acceptable carrier. According to the invention, a monoclonal antibody for effectively neutralizing tetanus exotoxin is screened with natural tetanus exotoxin, and an Fab gene engineering antibody which is produced by large scale in vitro and which has toxin neutralizing effect is prepared with a gene engineering antibody technology on the basis of the monoclonal antibody.
Owner:ARMY MEDICAL UNIV +1
Non-toxic tetanus toxin and clostridium novyi alpha toxine recombinant fusion protein
ActiveCN110041437AImprove stabilityReduce Biosecurity RisksAntibacterial agentsBacterial antigen ingredientsVaccine antigenToxin protein
The invention relates to non-toxic tetanus toxin and clostridium novyi alpha toxin recombinant fusion protein. The prepared recombinant fusion protein is produced in the manner that The prepared recombinant fusion protein is produced in the manner that through codon optimization, a tetanus toxin C fragment, C terminal of clostridium novyi alpha toxin, and N-end non-toxic epitope of clostridium novyi alpha toxin are subjected to fusing expression, so that the immunogenicity of two kinds of toxin protein can be reserved to the maximum extent, and biology potential safety hazard of natural toxincan be avoided. The recombinant fusion protein can be used for preparing clostridium tetani and clostridium novyi subunit vaccines. Compared with the clostridium tetani and clostridium novyi subunit vaccines commercialized in China at present, the non-toxic tetanus toxin and clostridium novyi alpha toxin recombinant fusion protein has the advantages of being simpler in preparation technology, lower in immunizing dosage, better in vaccine effects and the like, the biology security risk in the production process of the vaccine is greatly reduced, and the non-toxic tetanus toxin and clostridium novyi alpha toxin recombinant fusion protein is an ideal candidate vaccine antigen for upgrading and regenerating two clostridial toxin vaccines. When the non-toxic tetanus toxin and clostridium novyialpha toxin recombinant fusion protein and other antigens are in jointed preparation of a combined vaccine, the using dosage of the combined vaccine does not need to be increased, and the combined vaccine can be prepared.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Process of conjugation and novel synthetic oligosaccharide-protein conjugates obtained thereof
InactiveUS10105438B2High yieldHigh purityAntibacterial agentsBacterial antigen ingredientsThio-Tetanus toxoids
The present invention relates to an improved process of conjugation to obtain synthetic oligosaccharide-protein (OS-PR) conjugates. The process of synthetic OS-PR conjugation is a rapid process providing oligosaccharide-protein conjugates which are highly immunogenic and elicit specific and homogenous immune responses. The synthetic oligosaccharide comprising of four to eight repeating units of respective monomers and at least one in-built terminal amino linker, said synthetic polysaccharide mimics natural polysaccharide obtained from gram negative bacteria such as Neisseria meningitidis serogroups A, C, Y, W, X and Haemophilus influenzae and carrier protein is obtained from gram positive bacteria such as Clostridium tetani (tetanus toxoid) or Corynebacterium diphtheriae (CRM197) or their recombinant versions. The conjugation chemistry of the said oligosaccharide-protein conjugate of the present invention is thio-ether linkage. The present invention takes complete process time in the range of 14-22 hours. The said oligosaccharide-protein conjugates are useful in production of monovalent vaccine or multivalent combination vaccines and as diagnostic tool.
Owner:MSD WELLCOME TRUST HILLEMAN LAB PVT LTD
A fully native human neutralizing monoclonal antibody against tetanus toxin and its applications
ActiveUS20210002356A1Improve the shortageHigh affinityAntibacterial agentsImmunoglobulins against bacteriaNatural antibodyAntiendomysial antibodies
The invention describes fully native human neutralizing monoclonal antibodies against tetanus toxin. The invention developed fully native human neutralizing monoclonal antibodies against tetanus toxin through a systematic high through-put platform that is specialized for identifying and developing human native antibody. The neutralizing monoclonal antibodies described in the invention can be used in the prevention, treatment and detection of Clostridium tetani infection. The fully human neutralizing monoclonal antibodies developed in the invention have a high affinity toward tetanus toxin, as well as possessing high neutralizing activities against the toxin, safe of use with high disease prevention effectiveness, free of exogenous virus contamination, and are widely applicable to various human groups with strong industrial applications.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Vaccine for inducing an improved immune reaction
InactiveCN103384532AExcellent immune boosting effectImprove stabilityAntibacterial agentsSsRNA viruses negative-senseHepatitis B virusHuman immunodeficiency virus (HIV)
The present invention relates to a pharmaceutical vaccine composition comprising: (a) a pathogen-derived antigen selected from the group consisting of Mycobacterium tuberculosis antigen, Bacillus anthracis antigen, HAV (hepatitis A virus) antigen, HBV (hepatitis B virus) antigen, HCV (hepatitis C virus) antigen, HIV (human immunodeficiency virus) antigen, influenza virus antigen, HSV (herpes simplex virus) antigen, Hib (Haemophilus influenzae type b) antigen, Neisseria meningitidis antigen, Corynebacterium diphtheriae antigen, Bordetella pertussis antigen, Clostridium tetani antigen and Varicella virus antigen; (b) a deacylated non-toxic LOS (lipooligosaccharide); and (c) a pharmaceutically acceptable carrier.
Owner:EYEGENE INC
Method and kit for detecting clostridium tetani by using loop-mediated isothermal amplification method
InactiveCN101948923AGuaranteed reliabilityMeet specific requirementsMicrobiological testing/measurementClostridium tetaniLoop-mediated isothermal amplification
The invention relates to the field of molecular biology, and particularly discloses a method and a kit thereof for detecting clostridium tetani by using a loop-mediated isothermal amplification method. The method comprises the following steps of: amplifying clostridium tetani spasm toxin genes in a sample DNA by using loop-mediated isothermal amplification technology and using an upstream inner primer, a downstream inner primer, an upstream outer primer and a downstream outer primer, wherein the nucleotide sequences of the primers are expressed as SEQ ID No: 1-4; and detecting the amplification results. The kit comprises the upstream inner primer, the downstream inner primer, the upstream outer primer, the downstream outer primer, DNA quick extraction liquid and isothermal amplification liquid. The method and the kit thereof for detecting the clostridium tetani by using the loop-mediated isothermal amplification method have the advantages of high clostridium tetani specificity, high sensitivity and simple and quick detection process.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Medium for promoting toxin-producing capability of clostridium tetani
InactiveCN105483055AImprove toxin production capacityHigh yieldBacteriaMicroorganism based processesSodium acetateMonopotassium phosphate
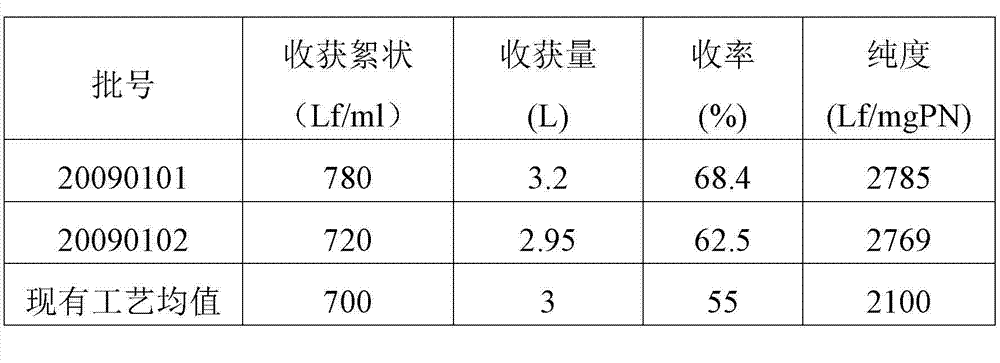
The invention discloses a medium for promoting toxin-producing capability of clostridium tetani. One liter of the medium is prepared from 1.00+ / -0.05 g of beef gastric enzyme digestive juice total nitrogen, 1.00+ / -0.05 g of cheese digestive juice total nitrogen, 5.0+ / -0.1 g of sodium acetate, 1.00+ / -0.05 g of disodium hydrogen phosphate, 1.00+ / -0.05 g monopotassium phosphate, 60+ / -2 ml of glycerinum, 2.5+ / -0.1 g / of glucose, 5.0+ / -0.1 ml of a factor I#, 2.00+ / -0.05 ml of a factor II#, 5.0+ / -0.1 g of yeast extract powder, and 0.0025+ / -0.001g of FeCl3.6H2O. The toxin-producing capability is improved by 38-48 Lf / ml, and the protein yield is increased by 36%.
Owner:YUXI WALVAX BIOTECH CO LTD
Broad-spectrum anti-tumor compound preparation
InactiveCN106667907ANo deathSignificant antitumor effectBacteria material medical ingredientsSolution deliveryBacteroidesSerratia
The invention relates to a broad-spectrum anti-tumor compound preparation, and belongs to the technical field of medical preparations. The broad-spectrum anti-tumor compound preparation is prepared by compounding a bacterial suspension formed by a plurality of bacteria and an auxiliary material, wherein the bacterial suspension comprises any one or more of bordetella pertussis, diphtheria bacillus, clostridium tetani, streptococcus, typhoid bacillus, bacillus paratyphosus A, bacillus paratyphosus B, salmonella enteritidis, rubella bacteria, measles bacteria, dothienesis bacterial suspension, staphylococcus aureus and serratia marcescens; or the bacterial suspension is a combination of one or more of bordetella pertussis bacterial suspension, a corynebacterium diphtheria bacterial suspension, a clostridium tetani bacterial suspension, a streptococcus bacterial suspension, a typhoid bacillus bacterial suspension, a bacillus paratyphosus A bacterial suspension, a bacillus paratyphosus B bacterial suspension, a salmonella enteritidis bacterial suspension, a rubella bacteria bacterial suspension, a measles bacteria bacterial suspension, a dothienesis bacterial suspension bacterial suspension, a staphylococcus aureus bacterial suspension and a serratia marcescens bacterial suspension. The broad-spectrum anti-tumor compound preparation has the advantages of spectrum property, good tumor killing effect and the like when being applied to anti-tumor treatment.
Owner:孙仁娥
A Recombinant Fusion Protein of Avirulent Tetanus Toxin and Clostridium perfringens β Toxin
ActiveCN109942718BImprove stabilityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsVaccine PotencyClostridium perfringens beta toxin
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method and kit for detecting clostridium tetani by using loop-mediated isothermal amplification method
InactiveCN101948923BStrong specificityHigh sensitivityMicrobiological testing/measurementClostridium tetaniNucleotide
The invention relates to the field of molecular biology, and particularly discloses a method and a kit thereof for detecting clostridium tetani by using a loop-mediated isothermal amplification method. The method comprises the following steps of: amplifying clostridium tetani spasm toxin genes in a sample DNA by using loop-mediated isothermal amplification technology and using an upstream inner primer, a downstream inner primer, an upstream outer primer and a downstream outer primer, wherein the nucleotide sequences of the primers are expressed as SEQ ID No: 1-4; and detecting the amplification results. The kit comprises the upstream inner primer, the downstream inner primer, the upstream outer primer, the downstream outer primer, DNA quick extraction liquid and isothermal amplification liquid. The method and the kit thereof for detecting the clostridium tetani by using the loop-mediated isothermal amplification method have the advantages of high clostridium tetani specificity, high sensitivity and simple and quick detection process.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Composite nanometer antibacterial agent having bacterium specific recognition capacity and application of composite nanometer antibacterial agent
ActiveCN107441490ATo achieve the purpose of antibacterialAntibacterial agentsOrganic active ingredientsEscherichia coliSide effect
The invention discloses a composite nanometer antibacterial agent having a bacterium specific recognition capacity and an application of the composite nanometer antibacterial agent, and belongs to the technical field of high-molecular polymers. The composite nanometer antibacterial agent consists of silver nanoparticles, boron fluoride dipyrrole molecules and polygalactose, wherein the silver nanoparticles constitute silver nanospheres; the polygalactose is represented in the form of polygalactose chains; the silver nanospheres are connected to one end of each of the plurality of polygalactose chains; one of the boron fluoride dipyrrole molecules is connected to the other end of each polygalactose chain; and all boron fluoride dipyrrole molecules surround the silver nanospheres, so that a sphere-like structure is also formed. The composite nanometer antibacterial agent particle prepared by the invention has a selective recognition capacity on pseudomonas aeruginosa, escherichia coli, clostridium tetani and staphylococcus aureus, and the particle is not or is least adsorbed by normal human cells; therefore, on the basis of causing relatively low toxic and side effects to human body, an antibacterial purpose can be selectively achieved.
Owner:WUHAN TEXTILE UNIV
Method for preparing tetanus toxoid vaccine
ActiveCN102961741BReduce cloggingAvoid damageAntibacterial agentsBacterial antigen ingredientsCulture fluidFiltration
The invention discloses a method for preparing a tetanus toxoid vaccine. According to the process, with clostridium tetani strains as raw materials, the tetanus toxoid vaccine is prepared through the following steps of: culturing of tetanus toxoid, bacterium liquid separation, ultrafiltration and concentration, salting out, ultrafiltration desalting and the like. According to the method, firstly culture liquid is subjected to virus-free treatment and then refined, so that the porous channel plugging caused by accumulation of thalli and other impurity segments at a plate and frame membrane package during plate and frame filtering to remove thalli is reduced, and the smoothness during filtration is increased; toxoid protein and other allergens in the culture liquid are removed by changing the salting-out method; and as the desalting methods of the culture liquid after concentration and salting out adopt the tangential flow ultrafiltration method, the destruction of antigen caused by shearing of toxoid protein is reduced, and the protein precipitation is avoided. By utilizing the method, the time for preparing the tetanus toxoid vaccine is shortened, and the production efficiency is improved.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
A kind of neutralizing antibody against tetanus toxin and its application
ActiveCN108623681BStrong neutralizing activityHigh affinity activityAntibacterial agentsImmunoglobulins against bacteriaDiseaseClostridium tetani
Owner:CHANGCHUN BCHT BIOTECH
Traditional Chinese medicine preparation for treating tetanus
InactiveCN104906521AGood inhibitory effectReduce the burden onAnthropod material medical ingredientsMuscular disorderBasella rubraBiology
The invention discloses a traditional Chinese medicine preparation for treating tetanus. The traditional Chinese medicine preparation is prepared from the following raw materials in parts by weight: 11-16 parts of boenninghausenia albiflora, 9-13 parts of bamboo juice, 10-15 parts of drumfish bladder, 7-10 parts of periostracum cicada, 9-12 parts of rhizoma arisaematis, 10-15 parts of typhonium giganteum, 3-6 parts of gecko, 12-16 parts of radix curcumae, 10-17 parts of common calanthe herb, 8-11 parts of rhizoma typhonii, 9-14 parts of wadalee-gum-tree, 6-14 parts of ginseng fruits, 13-20 parts of radix pseudostellariae, 11-15 parts of centella and 9-16 parts of basella alba. The traditional Chinese medicine preparation has the efficacies of dispelling wind heat, relieving spasm, tranquilizing mind and allaying excitement, is capable of effectively preventing and treating tetanus, and has an obvious inhibiting effect on clostridium tetani; the traditional Chinese medicine preparation disclosed by the invention is low in loss of effective components, high in curative effect, free of side effects and low in price; and the burden of a patient can be relieved.
Owner:钊桂英
Culture medium for clostridium tetani
InactiveCN106834153AIncrease proliferation rateGood training effectBacteriaMicroorganism based processesYeastClostridium tetani
The invention provides a culture medium for Clostridium tetani, which comprises the following ingredients in parts by weight: 8 parts of maltose, 6 parts of yeast powder, 2 parts of cucumber juice, 1 part of Folium Isatidis, 1 part of serine, 1 part of realgar, 1 part of black rice; on this basis, a specific amount of ferrous chloride, mulberry bark or distiller's grains can be added. On the basis of the common materials used in the culture medium, certain Chinese herbal medicines are added to the formula, which unexpectedly achieves an outstanding culture effect. It is especially suitable for the culture of Clostridium tetani, and has a good application prospect.
Owner:ZHISHENG TIANJIN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com