Hybridoma cell strain secreting tetanus exotoxin monoclonal antibody, monoclonal antibody prepared by same, Fab antibody and application
A hybridoma cell line and monoclonal antibody technology, applied in the field of medicine and biology, can solve the problems of difficulty in immunizing human plasma sources, death, and limited production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation and Identification of Monoclonal Antibody Neutralizing Tetanus Exotoxin
[0057] 1. Animal immunization
[0058] Simultaneously immunize five female Balb / c mice aged 6-8 weeks (purchased from the Experimental Animal Center of Third Military Medical University) with formaldehyde-inactivated tetanus toxoid as the antigen, inject 0.5ml each, containing 100μg of antigen, and immunize The time was 0, 3, 5, and 6 weeks, 4 times in total. The first time was added with Freund's complete adjuvant for subcutaneous multi-point injection, the second time was added with Freund's incomplete adjuvant, and the third time was intraperitoneally injected with the same dose without adjuvant After immunization, the tail blood was taken one week later and the serum titer of the immunized Balb / c mice was detected by indirect ELISA method. The splenocytes with the highest titer were used for fusion. Three days before the fusion, intraperitoneal booster immunization was ...
Embodiment 2
[0084] Example 2 Cloning of κ Light Chain and Fd Chain Genes of Tetanus Exotoxin Neutralizing Monoclonal Antibody TT5C4
[0085] 1. The cell line used is the hybridoma cell line obtained by the inventors using the above method, which can secrete high-affinity, high-specificity monoclonal antibody TT5C4 neutralizing the toxic effect of tetanus exotoxin, and the preservation number is CCTCC NO.C201257 , the subclass and subtype of antibody molecules secreted are IgG1 type.
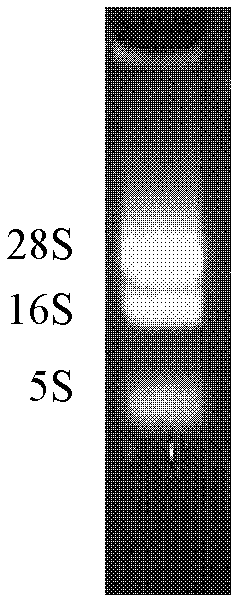
[0086] 2. Take the TT5C4 hybridoma cells in logarithmic growth phase (2×10 6 ), using TRIZOL one-step method (Invitrogen Company) to extract total RNA, and take a small amount for quantification by UV spectrophotometer and detection by 1% agarose gel electrophoresis. Then, the One step PT-PCR kit (TaKaRa Company) was used to amplify the κ light chain (VL+CL) and Fd chain (VH+CH1) genes of monoclonal antibody TT5C4. The amplified products containing the κ chain and Fd chain of TT5C4 were subjected to 1% a...
Embodiment 3
[0161] Example 3 Construction and Soluble Expression of Fab Antibody of Tetanus Exotoxin Neutralizing Monoclonal Antibody TT5C4
[0162] First, clone the correctly analyzed TT5C4κ chain and TT5C4Fd chain genes into the vector pcomb3X (purchased from Chongqing Jinmai Biotechnology Co., Ltd.) that secretes and expresses Fab antibodies, and construct the pcomb3X-TT5C4Fdκ plasmid. The coat protein III gene was self-ligated to construct the pcomb3X-TT5C4Fab expression plasmid, which was confirmed by restriction enzyme digestion and DNA sequencing analysis, and then transformed into Escherichia coli XL-blue (purchased from Shanghai Jereh Bioengineering Company) , Constructed into pcomb3X-TT5C4Fab / XL-blue recombinant, IPTG induces the expression of TT5C4Fab antibody protein. SDS-PAGE electrophoresis ELISA and Western-blot analysis showed the expression of Fab gene in E. coli. The specific steps of the operation are as follows:
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com