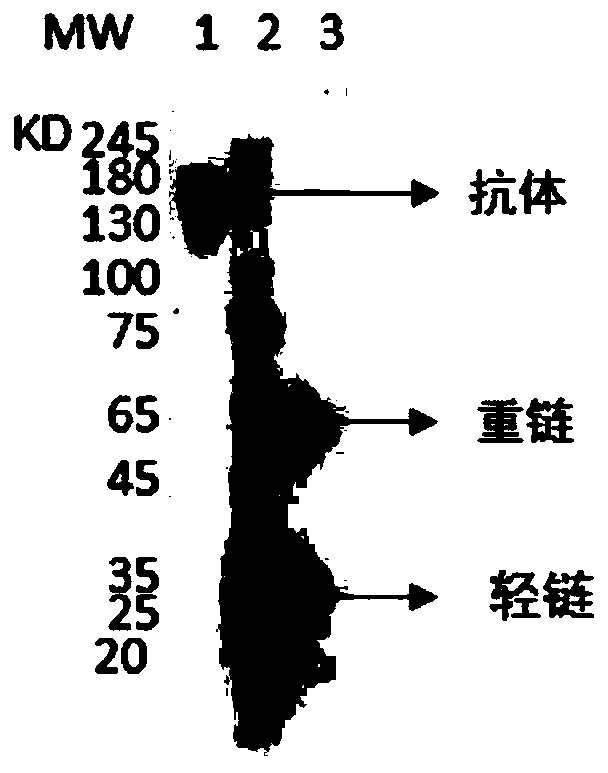
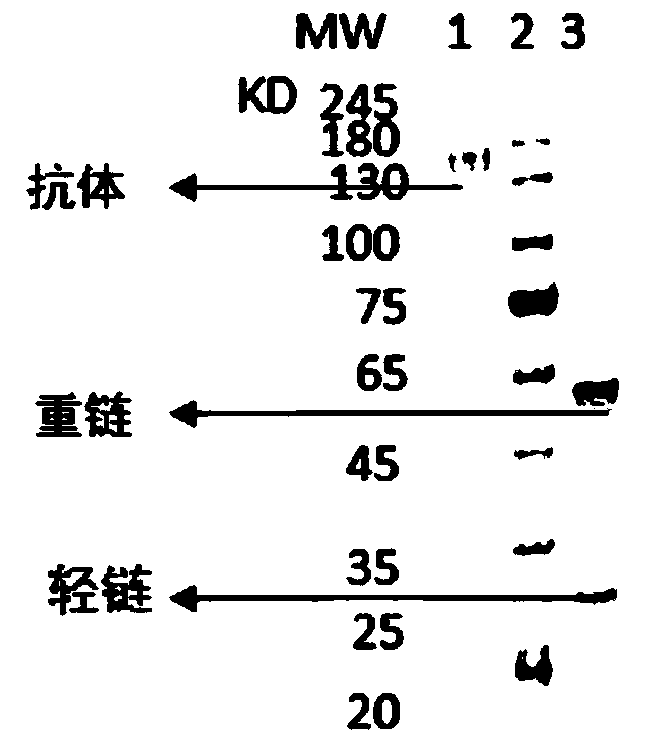
A kind of neutralizing antibody against tetanus toxin and its application
A tetanus toxin and anti-tetanus technology, applied in the field of genetic engineering, can solve the problems of exogenous virus contamination and high price of HTIG, and achieve the effects of low dosage, high affinity activity, and strong neutralizing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Sorting B cells by flow cytometry and screening and purification of anti-tetanus toxin antibodies
[0088] 1. PBMC isolation and plasma cell sorting
[0089] 15 ml of venous blood was collected from volunteers who had been vaccinated against tetanus and developed protective antibodies, and placed in anticoagulant tubes containing heparin. The 15ml blood sample was separated from PBMC (peripheral bloodmononuclear cell, peripheral blood mononuclear cell) with Ficoll (polysucrose). After PBMC counting, BD FACSria flow cytometer was used to sort single B cells from PBMCs, and the B cells with good morphology were placed in a 96-well PCR (Polymerase Chain Reaction, polymerase chain reaction) plate, so that each well contained one B cells. The 96-well PCR plate with B cells was placed in a -80°C refrigerator and stored for later use.
[0090] 2. Isolation of antibody variable region genes
[0091] Add 0.5 μM constant region primers of heavy chain and light chain...
Embodiment 2
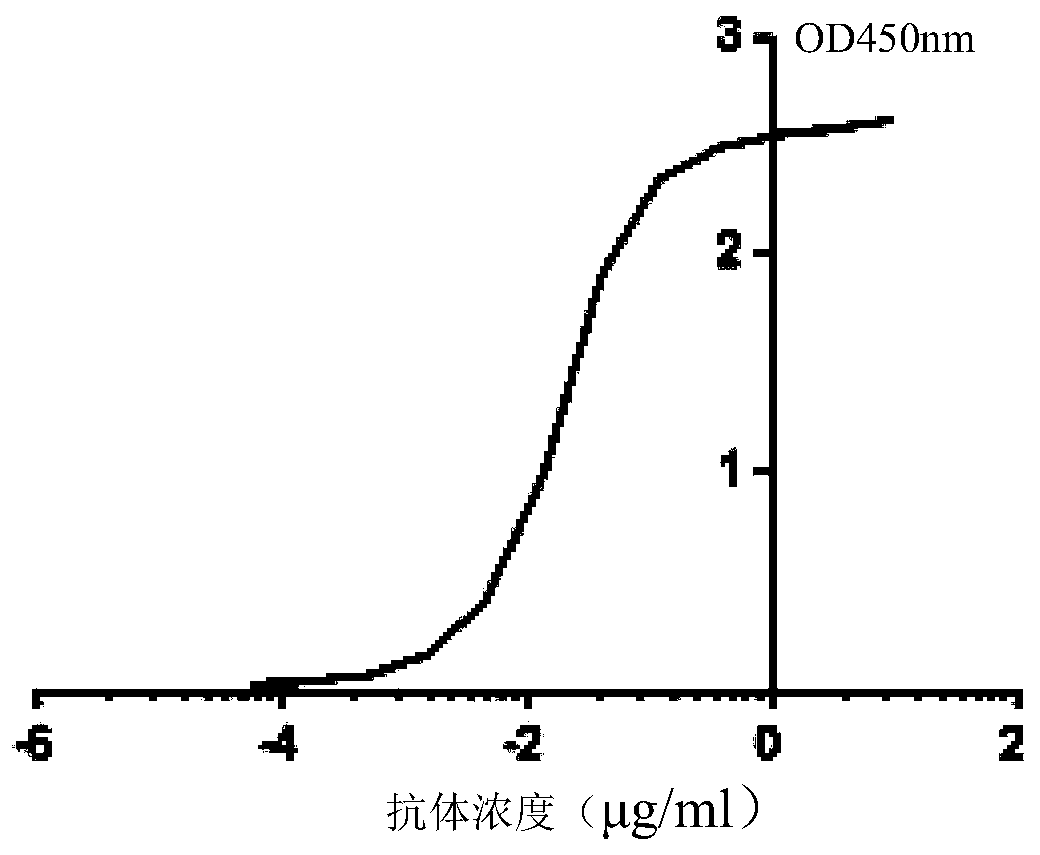
[0103] Embodiment 2 ELISA detects the neutralizing activity of antibody TRN1015
[0104] 1. Experimental process
[0105] The standard tetanus toxin was used as the antigen, and the antigen was diluted 10 times with the coating solution to coat the 96-well ELISA plate. Each well contained an overnight coating with a volume of 100 μ and the temperature was 4 ° C, and blocked with the blocking solution for 2 hours at room temperature. Serially dilute the expressed and purified antibody TRN1015 as the primary antibody and incubate at 37°C for 2h, use HRP / anti-His-tag (1:2000 dilution) as the secondary antibody and incubate at 37°C for 1h, add the substrate chromogenic solution and keep away from light at room temperature Stand for 5 minutes, stop the reaction with 2M sulfuric acid, and detect and analyze with a wavelength of 450nm.
[0106] 2. Results
[0107] The result is as image 3 As shown, after 10,000 dilutions of the expressed and purified antibody (the antibody concen...
Embodiment 3
[0108] Example 3 Kinetic Analysis of Affinity Activity of Antibody TRN1015
[0109] 1. Capture method test
[0110] The antibody affinity test adopts the capture method, the buffer is HBS-EP, and the CM5 chip is first coupled with proteinA. Then dilute the capture antibody so that its concentration is 1ug / ml, and the binding time is 50s. The analyte tetanin was sequentially passed through the chip at increasing concentrations, and the signal curves were respectively obtained. Each concentration was regarded as one cycle, and after completing one cycle, the chip was regenerated with 3M MgCl2 to return to the state of the original uncaptured antibody, and the regeneration time was 30s. The obtained signal curve was analyzed with BiaCore X-100System software to obtain the detection graph of the affinity activity between the neutralizing antibody and tetanus toxin provided in the embodiment of the present invention.
[0111] 4. Results
[0112] The result is as Figure 4 As s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com