A binding molecule against wnv infection
A technology that combines fragments and nucleic acid molecules, applied in the field of molecular immunology, can solve the problem of no specific drugs for WNV infection, and achieve high neutralization activity, high potency, and strong affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
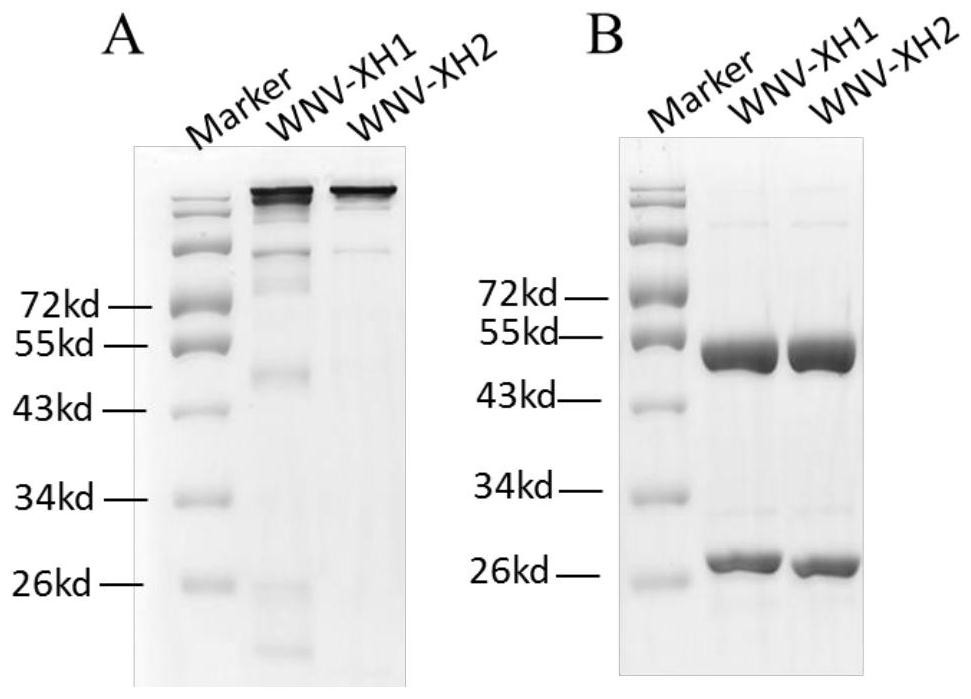
[0076] Example 1 Preparation of WN-XH2 monoclonal antibody
[0077] 1. Experimental materials
[0078] PRT / KIgG1 carrier (see patent literature: A monoclonal antibody that specifically binds to PD-1, application number: 201810273628.7)
[0079] 2. Steps
[0080] (1) Using the method of gene synthesis to synthesize the antibody heavy chain variable region sequence (sequence shown in SEQ ID NO: 4), light chain variable region sequence (sequence shown in SEQ ID NO: 8), and use them Methods of Molecular Cloning Fragments were cloned into PRT / KIgG1.
[0081] (2) Transfect the antibody expression recombinant vector constructed in step 1 into 293T cells in the logarithmic growth phase, change the fresh medium 6-8 hours after transfection, and incubate at 37°C 8% CO 2 Incubate for 96 hours in an incubator. The transfection supernatant was collected, centrifuged at 4000 rpm for 1 hour, and purified by Protein A affinity chromatography. SDS-PAGE and Western Blot experiments were us...
Embodiment 2
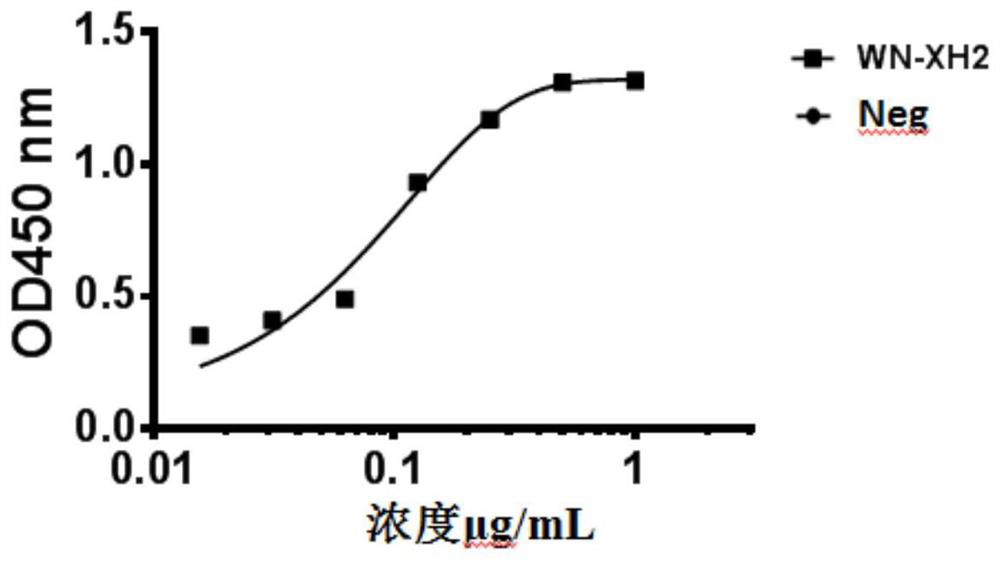
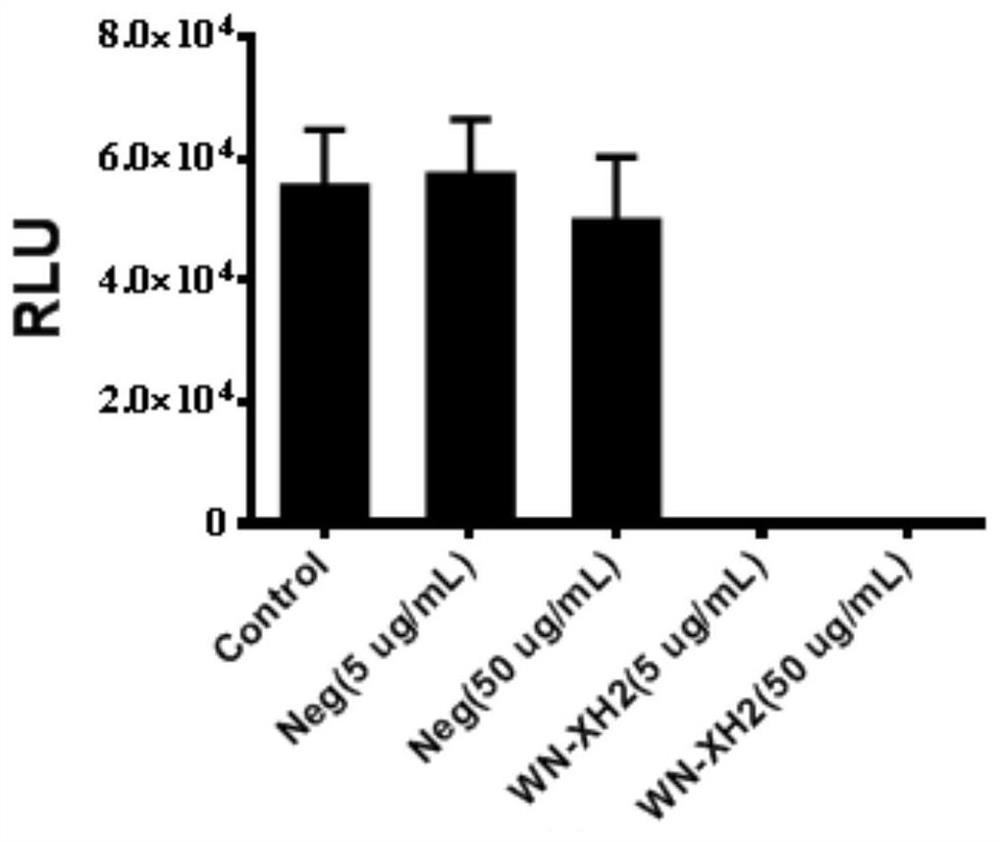
[0084] Example 2 Determination of binding activity and neutralizing activity of WN-XH2 monoclonal antibody
[0085] 1. Determination of binding activity of monoclonal antibody
[0086] Dilute the EDIII antigen of WNV to 1 μg / mL in the coating solution, add 100 μL per well to the enzyme-linked plate, and place it in a humid chamber at 4°C overnight. Wash the enzyme-linked plate 3 times with a plate washer, block with 1.5% casein, 200 μL per well, and block for 1 hour at 37°C in a humid box. Dilute the antibody to different concentrations with 1xPBS, add 100 μL per well to the enzyme-linked plate, react in a wet box at 37°C for 1 hour, wash the enzyme-linked plate 3 times, add goat anti-human (Fab') 2-HRP secondary antibody and react at room temperature for 45 minutes , wash the enzyme-linked plate 5 times and add 100 μL TMB substrate for color development, react for 3 minutes and use 100 μL 2N H 2 SO 4 The reaction was terminated, and the enzyme-linked immunosorbent assay wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com