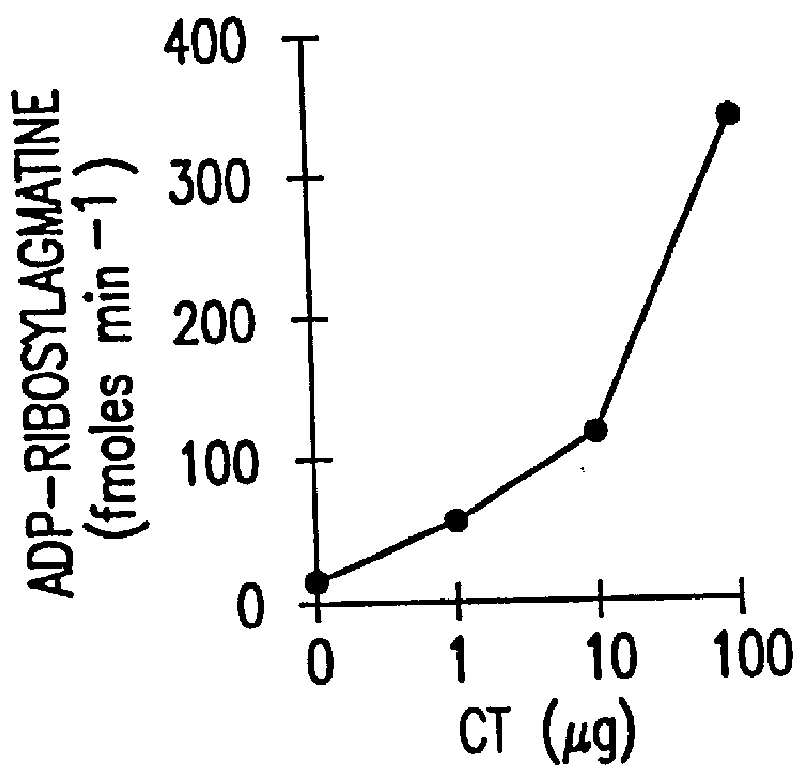
Patents
Literature
106 results about "Toxiferine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Toxiferine (C-toxiferine I) is a curare toxin. It is a bisindole alkaloid derived from Strychnos toxifera and a nicotinic acetylcholine receptor antagonist. This alkaloid is the main toxic component of Calabash curare, and one of the most toxic plant alkaloids known to man. The lethal dose (LD50) for mice has been determined as 10 - 60 µg/kg by intravenous administration. It is a muscle relaxant that causes paralysis of skeletal muscle, which takes approximately 2 hours to recovery for a moderate dose, and 8 hours of total paralysis with a 20-fold paralytic dose. The paralysis can be antagonized by Neostigmine...
Toxin peptide therapeutic agents
ActiveUS20070071764A1Avoid it happening againRelieve symptomsNervous disorderAntipyreticHalf-lifeSjögren syndrome
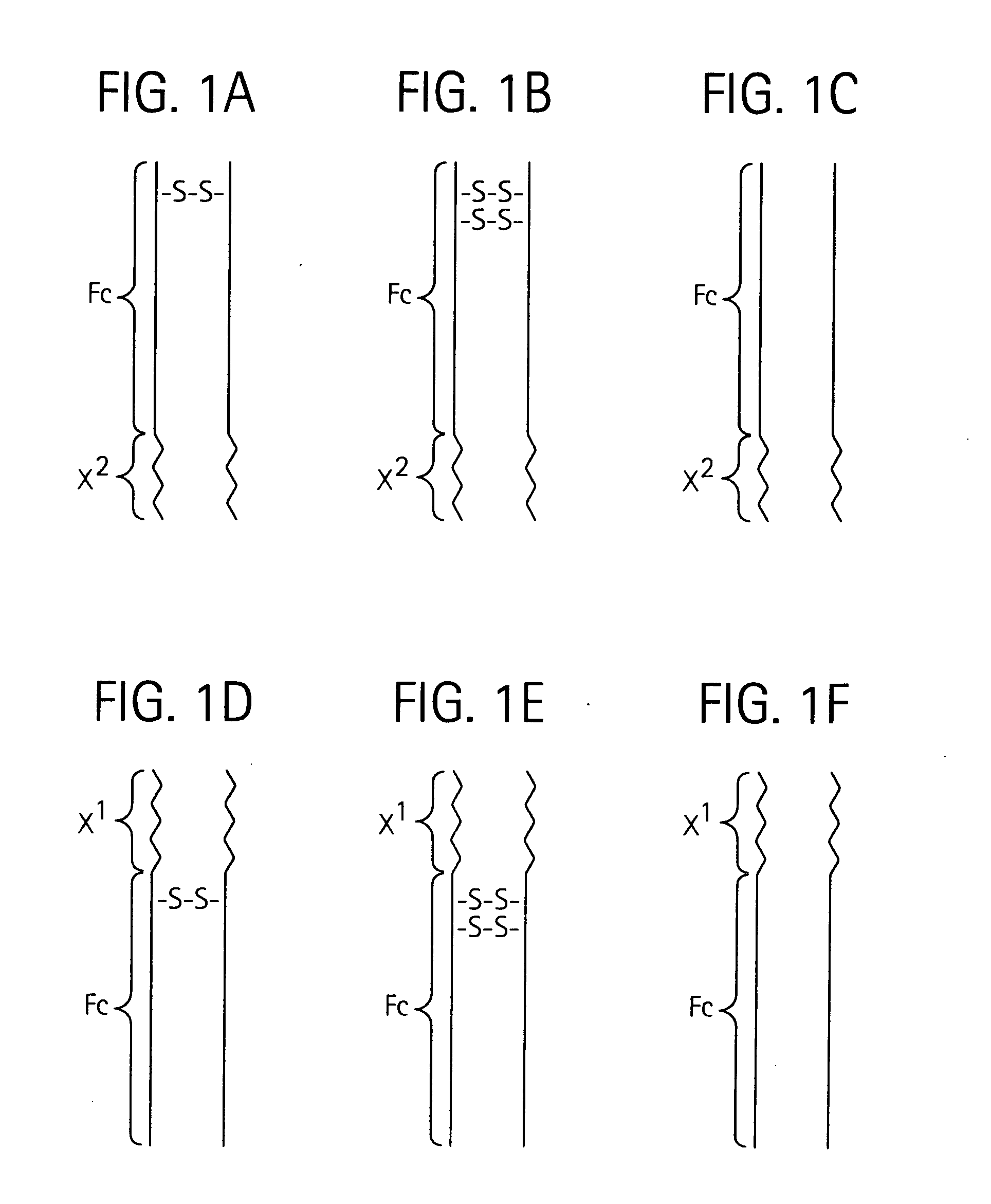
Disclosed is a composition of matter of the formula (X1)a—(F1)d—(X2)b—(F2)e—(X3)c (I) and multimers thereof, in which F1 and F2 are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X1, X2, and X3 are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
Mutant enterotoxin effective as a non-toxic oral adjuvant
InactiveUS6019982AEnhance immune responseLack of activityAntibacterial agentsPeptide/protein ingredientsAntigenAdjuvant
Methods and compositions are provided herein for the use of a novel mutant form of E. coli heat-labile enterotoxin which has lost its toxicity but has retained its immunologic activity. This enterotoxin is used in combination with an unrelated antigen to achieve an increased immune response to said antigen when administered as part of an oral vaccine preparation.
Owner:TULANE EDUCATIONAL FUND
Modified clostridial neurotoxins with altered biological persistence
InactiveUS20020127247A1Increase and decrease persistenceIncrease or decrease stabilityNervous disorderPeptide/protein ingredientsDiseaseToxin
The present invention discloses modified neurotoxins with altered biological persistence. In one embodiment, the modified neurotoxins are derived from Clostridial botulinum toxins. Such modified neurotoxins may be employed in treating various conditions, including but not limited to muscular disorders, hyperhidrosis, and pain.
Owner:ALLERGAN INC
Immunogenic peptide composition comprising measles virus Fprotein Thelper cell epitope (MUFThl-16) and N-terminus of β-amyloid peptide
InactiveUS6906169B2Increase the gapHigh cross reactivityNervous disorderPeptide/protein ingredientsFibrilDisease patient
The present invention relates to a composition comprsing a peptide immunogen useful for the prevention and treatment of Alzheimer's Disease. More particularly, the peptide immunogen comprises a main functional / regulatory site, an N-terminal fragment of Amyloid β (Aβ) peptide linked to a helper T cell epitope (Th) having multiple class II MHC binding motifs. The peptide immunogen elicit a site-directed immune response against the main functional / regulatory site of the Aβ peptide and generate antibodies, which are highly cross-reactive to the soluble Aβ1-42 peptide and the amyloid plaques formed in the brain of Alzheimer's Disease patients. The antibodies elicited being cross reactive to the soluble Aβ1-42 peptide, promote fibril disaggregation and inhibit fibrillar aggregation leading to immunoneutralization of the “soluble Aβ-derived toxins”; and being cross-reactive to the amyloid plaques, accelerate the clearance of these plaques from the brain. Thus, the composition of the invention comprising the peptide immunogen is useful for the prevention and treatment of Alzheimer's Disease.
Owner:UNITED NEUROSCIENCE LIMITED
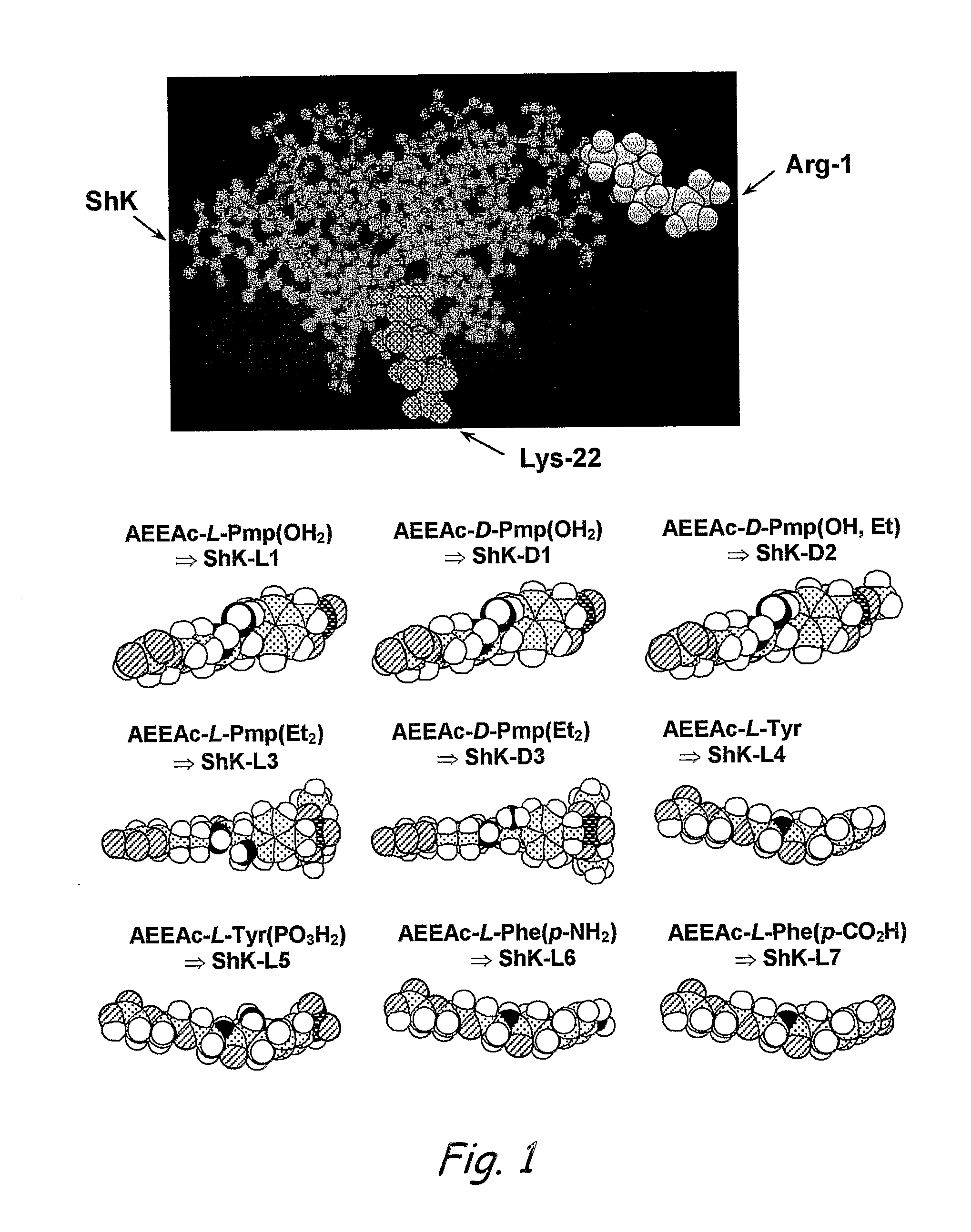
Analogs of Shk Toxin and Their Uses in Selective Inhibittion of Kv1. 3 Potassium Channels
Analogs of ShK toxin and methods for using such ShK analogs. The ShK analogs generally comprise ShK toxin attached to a chemical entity (e.g. an atom, molecule, group, residue, compound, moiety, etc.) that has an anionic charge. In some embodiments the chemical entity attached to the ShK toxin may comprise an amino acid residue. The ShK analogs may be administered to human or non-human animal subjects to cause inhibition of potassium channels or to otherwise treat diseases or disorders. In some embodiments, the chemical entity to which the ShK toxin is attached may be chosen to provide selective inhibition of certain potassium channels (e.g., Kv1.3 channels) over other potassium channels (e.g., Kv1.1 channels). In come embodiments, the chemical entity to which the ShK toxin is attached may include a fluorophore, thereby providing a fluorophore tagged ShK analog. Such fluorophore tagged ShK analogs may be used in flow cytometry alone, or in conjunction with class II tetramers that can detect autoreactive cells.
Owner:BACHEM AMERICAS +1
Amino acid sequences for therapeutic and prophylactic use against diseases due to clostridium difficile toxins
InactiveUS7151159B2Antibacterial agentsFungiClostridium difficile toxin AClostridium difficile (bacteria)
The invention relates to monoclonal antibodies capable of recognizing and neutralizing epitopes from the ligand domain, the translocation domain or the catalytic domain of the enterotoxin (toxin A) and cytotoxin (toxin B) from Clostridium difficile, as well as their production and therapeutic and prophylactic applications to diseases caused by said toxins.
Owner:VON EICHEL STREIBER CHRISTOPH
Low Dosage Serotonin 5-HT2A Receptor Agonist To Suppress Inflammation
ActiveUS20100016280A1Inhibit inflammationInhibition effectBiocideNervous disorderSerotonin 2A ReceptorsBiological activation
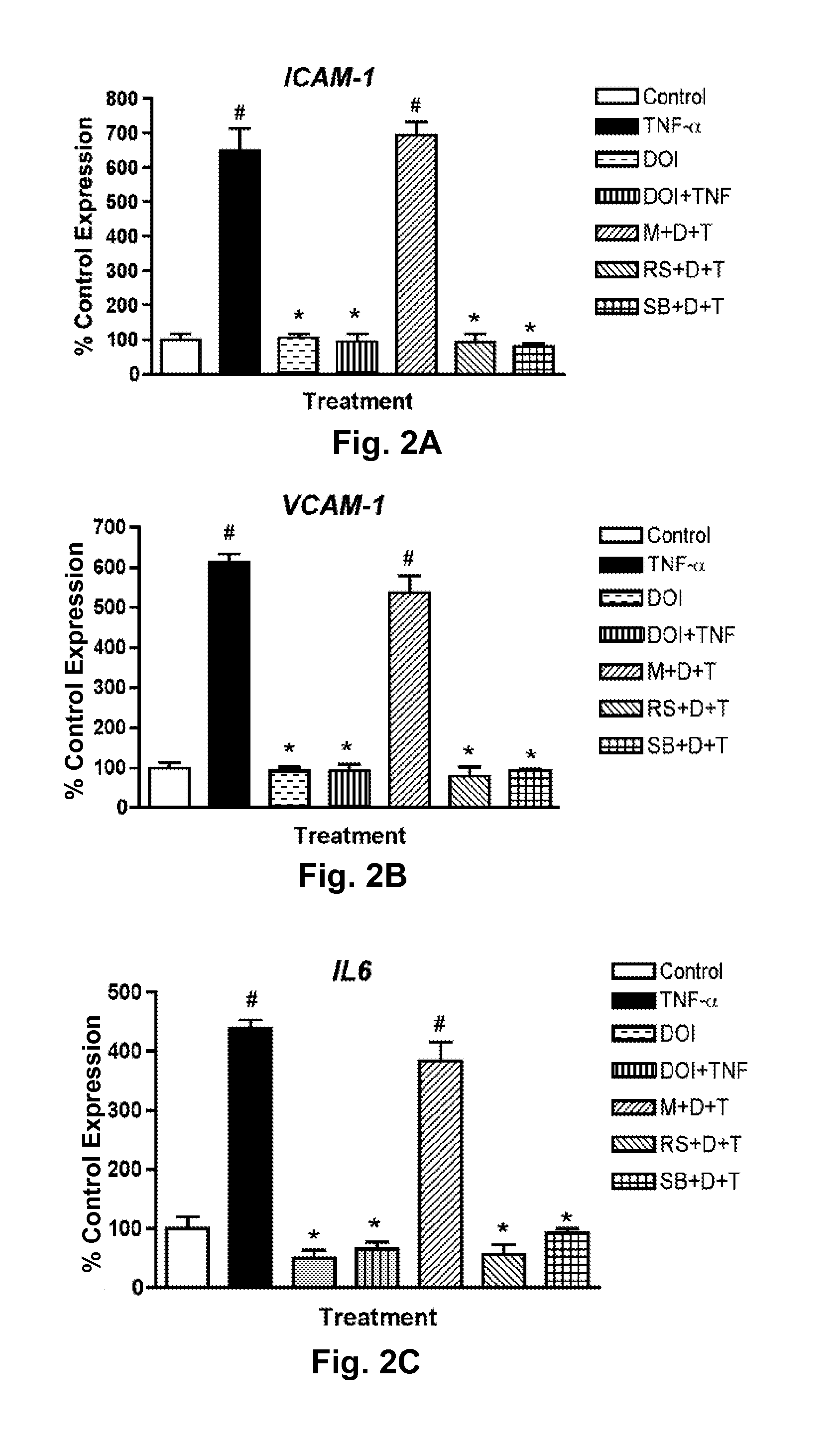
Activation of 5-HT2A receptors using agonists at surprisingly low concentrations was shown to potently inhibit TNF-α-induced inflammation in multiple cell types. Significantly, proinflammatory markers were also inhibited by the agonist, (R)-DOI, even many hours after treatment with TNF-α. With the exception of a few natural toxins, no current drugs or small molecule therapeutics demonstrate a comparable potency for any physiological effect. TNF-α and TNF-α receptor mediated inflammatory pathways have been strongly implicated in a number of diseases, including atherosclerosis, asthma, rheumatoid arthritis, psoriasis, type II diabetes, depression, schizophrenia, and Alzheimer's disease. Importantly, because (R)-DOI can significantly inhibit the effects of TNF-α many hours after the administration of TNF-α, potential therapies could be aimed not only at preventing inflammation, but also treating inflammatory injury that has already occurred or is ongoing.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Remedies for dissease with hypermyotonia
InactiveUS20060153877A1Highly preventive effectLow efficacyBacterial antigen ingredientsNervous disorderWrinkle skinDisease
An M toxin of type A botulinum toxin (HA-negative substance) and a mixture of L toxin and LL toxin (HA-positive substance) are compared and examined in inhibitory action for neuromuscular transmission and therapeutic index. As a result, it is found that M toxin of type A botulinum toxin has characteristics of: 1) having an excellent inhibitory action for neuromuscular transmission; 2)showing a high therapeutic index; 3) showing a low antigenicity and 4) suffering from little reduction in efficacy even after repeatedly administered, compared with the mixture of L toxin and LL toxin. Owing to these characterics, the M toxin of type A botulinum toxin is particularly useful as a therapeutic agent for diseases caused by hypermyotonia such as strabismus, blepharospasm, facial spasms, spasmodic torticollis, paralysis after cerebral apoplexy, infantile cerebral paralysis, spasmodic phonopathy, headache such as migraine, chronic pain such as lumbago, stiff shoulder, muscular relaxation disorder accompanied with onset of Parkinson's disease or multiple sclerosis, myofascial pain syndrome, masticatory spasm, chronic anal fissure, urinary inconsistency, grinding of teeth, facial myokymia, tic, topical dystonia and wrinkles.
Owner:SANTEN PHARMA CO LTD
Biotoxin sorbent and method for preparing the same
InactiveUS20100189871A1Good compatibilityImprove efficiencyOther chemical processesAnimal feeding stuffDiseaseBiotechnology
The present invention provides a combination used for absorbing toxins in feed, comprising 1-10% by weight of clay and 90-99% by weight of yeast manna oligosaccharide, wherein the yeast manna oligosaccharide is extract of yeast cell wall which is separated from Saccharomyces cerevisiae. The biotoxin sorbent product can be used to feed any animals, including livestock, fowls, marine lives and ruminants. When mixing with feed, owing to its strong toxin adsorption capability, this absorbent may reduce the amount of toxins entering bodies of animals, thereby improving productivity and health of animals, reducing diseases caused by toxins.
Owner:ANGELYEAST CO LTD
Biologically active, hemagglutinin from type A Clostridium botulinum and methods of use
InactiveUS6994859B1Alleviate abnormal firingReduce releaseAntibacterial agentsNervous disorderHemagglutininBotulinum toxin type C
An isolated, biologically active 33 kDa hemagglutinin purified from the type A Clostridium botulinum neurotoxin complex and its uses are described.
Owner:SINGH BAL RAM
Application of N-acetly glucosamine in the preparation of medicine for treating local injury and full body syndrome due to virus or bacterial infestation
InactiveCN1533776AMaintain normal physiological functionAvoid complicationsAntibacterial agentsOrganic active ingredientsWhole bodyCurative effect
An application of N-acetylaminoglucose in preparing the medicines for treating the local injury and general sympton caused by virus or bacterium infection is disclosed. It has high curative effect up to 90%.
Owner:ARMY MEDICAL UNIV +2
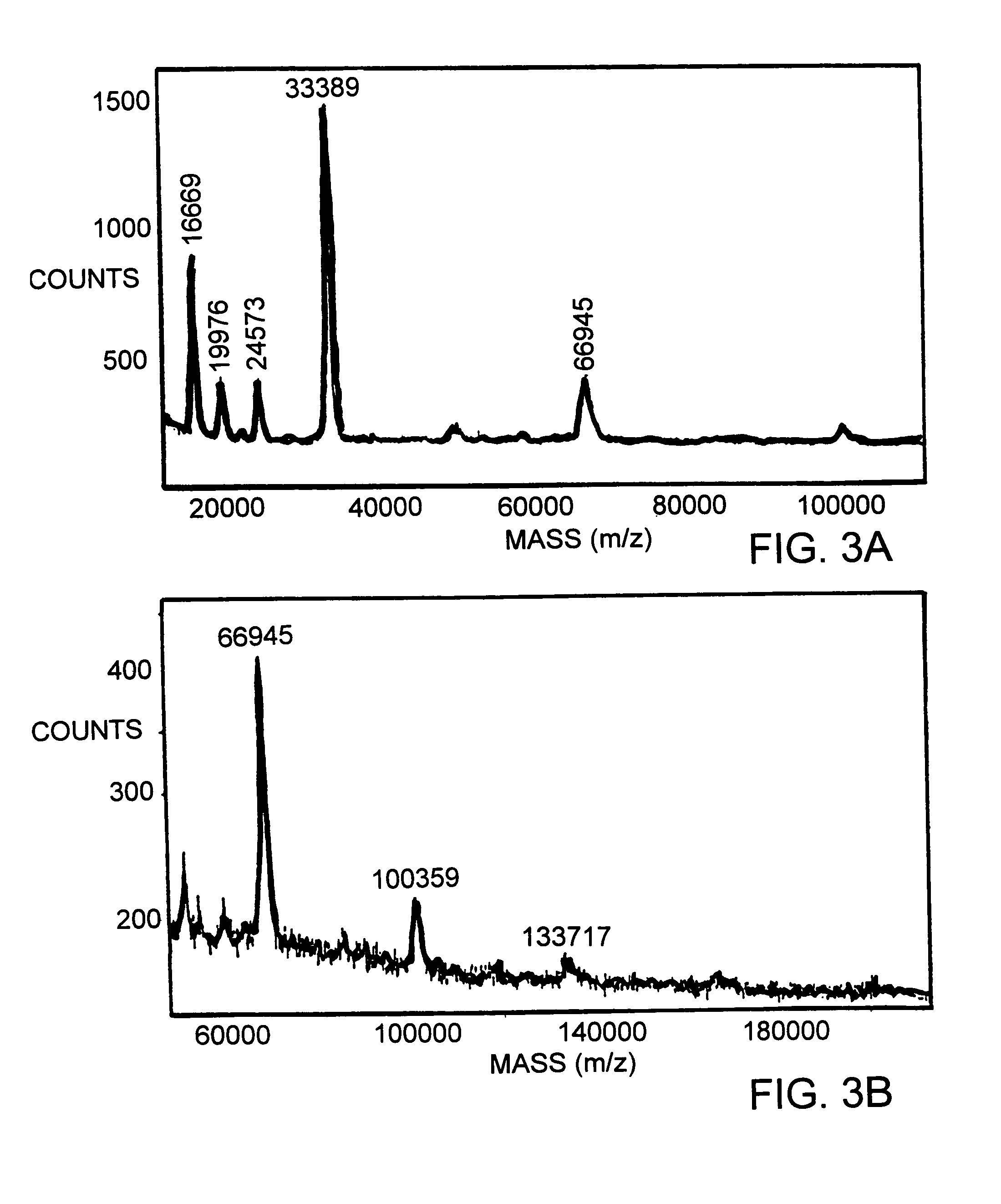
Recombinant proteins containing shiga-like toxin and vascular endothelial growth factor fragments
The present invention is directed to an isolated polypeptide including: (1) the A subunit of Shiga-like bacterial toxin, wherein said subunit has the nucleic acid sequence of SEQ ID NO:9; and (2) human vascular endothelial growth factor wherein the growth factor has the nucleic acid sequence of SEQ ID NO:10; wherein the isolated polypeptide possesses ribosome inactivating activity. The present invention is also directed to compositions for inhibiting endothelial cell growth in a patient.
Owner:SIBTECH
Nucleic acids encoding recombinant proteins containing Shiga-like toxin and vascular endothelial growth factor
InactiveUS7267973B2Good curative effectReduce supplySenses disorderFungiDiseaseRibosome Inactivation
The present invention is directed to an isolated nucleic acid encoding a fusion protein comprising (1) the A subunit of Shiga-like bacterial toxin, or a truncated or mutated version thereof; and (2) human vascular endothelial growth factor, or a truncated or mutated version thereof; wherein the fusion protein possesses ribosome inactivating activity and ability to bind to cellular VEGF receptors. The present invention is also directed to polypeptides the above combination of toxin and growth factor, as well as expression vectors and transformed cells incorporating the above nucleic acid. The invention is also directed to pharmaceutical compositions and methods for treating patients suffering from diseases relating to angiogenesis.
Owner:SIBTECH
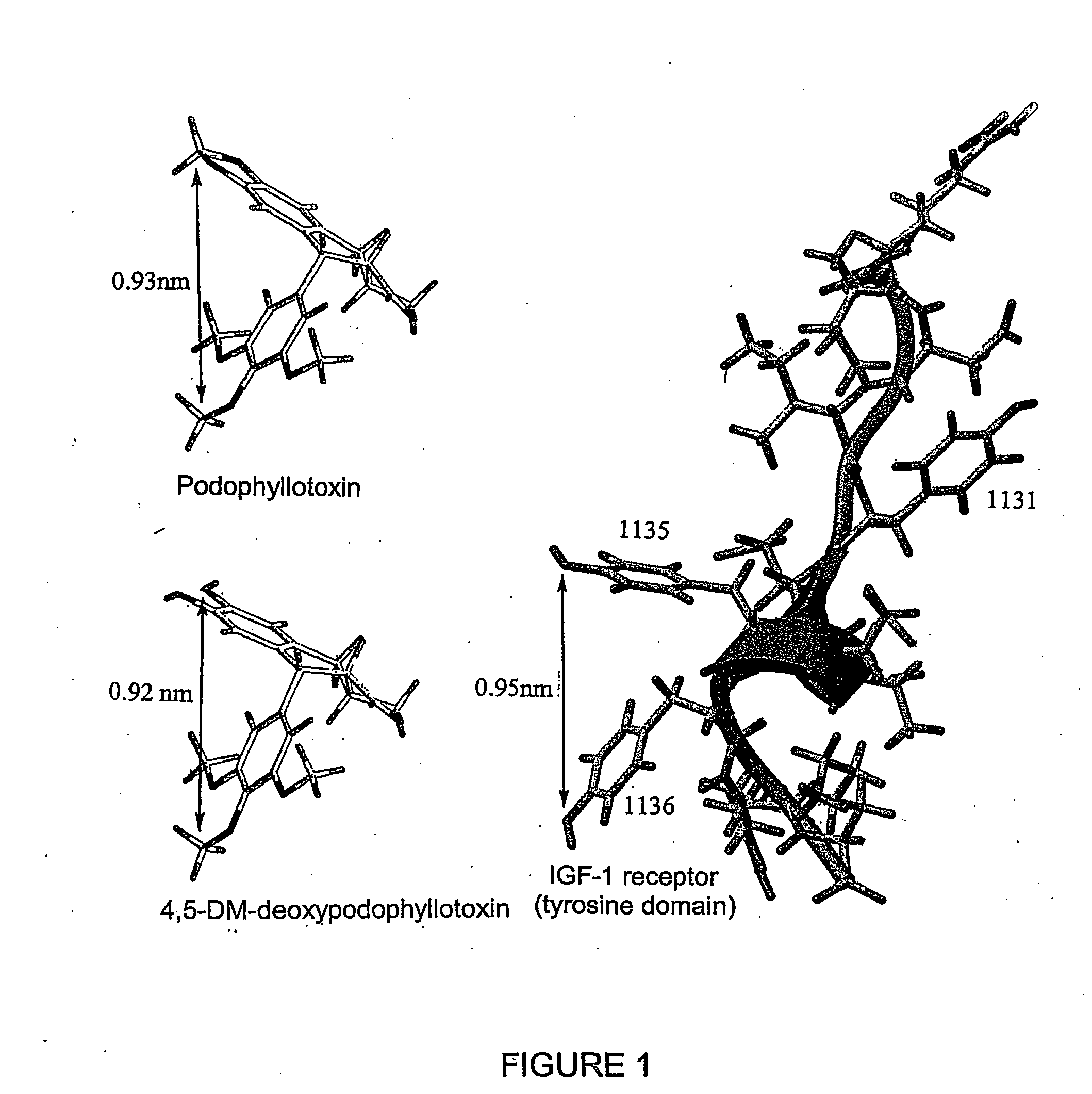
Podophyllotoxin derivatives as igf-1r inhibitors
The invention refers to new compounds, e.g. podophyllotoxin derivatives, as well as to the use thereof and of known compounds as specific inhibitors of the insulin-like growth factor-1 receptor (IGF-1R). Said compounds can be used for treatment of IGF-1 / IGF-1R dependent diseases, such as cancer, psoriasis, arteriosclerosis, certain endocrine and metabolic disorders etc.
Owner:AXELAR
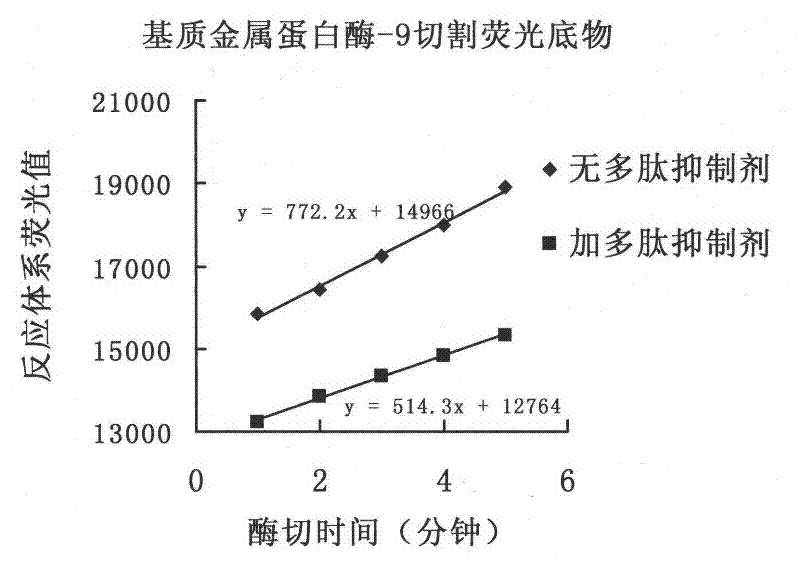
Matrix metalloproteinase-9 polypeptide inhibitor 4 and its application
ActiveCN102268069AImprove survival rateAvoid damagePeptide/protein ingredientsAntipyreticFactor iiWilms' tumor
The invention relates to the field of medicaments, in particular to a polypeptide which has the effects of inhibiting substrate metal prolease-9 and tumor necrosis factor liberase and relieving the damage of an acute inflammatory reaction on an organism. The sequence of the polypeptide is Pro-(D-Pyr)-(D-Cys)-Bip-Arg-Gly-Glu which is a brand new sequence (D-Pyr is D-type pyridine alanine, D-Cys isD-type cysteine, and Bip is diphenyl alanine). The polypeptide can be used for inhibiting the activities of the substrate metal prolease-9 and the tumor necrosis factor liberase on 1 micromole level in vitro and increasing the survival rate of an endotoxic shock mouse in an in-vivo test, and has a potential new medicament development value.
Owner:CHINA PHARM UNIV
Purification method, extract and preparation of cobra venom neurotoxin
InactiveCN102351951AEasy to separateShorten the timeNervous disorderPeptide/protein ingredientsCobra venomPurification methods
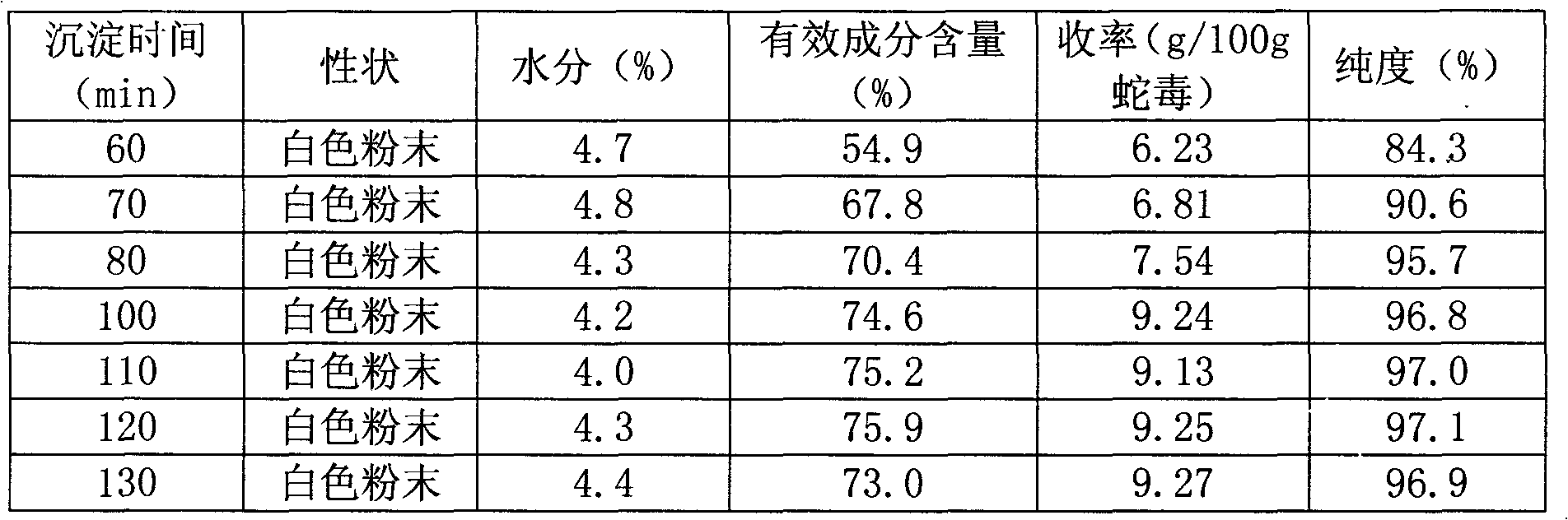
The invention discloses a purification method, an extract and a preparation of cobra venom neurotoxin. The extract of the cobra venom neurotoxin is prepared from cobra crude venom through extraction and purification. A filter membrane filtering method is firstly adopted for removing germs, particles and macromolecular sensitizers in solution, the time and the cost are saved for the subsequent processes, and the safety of products is enhanced; an ion exchange method is adopted for eluting and separating the neurotoxin, and the separation of neurotoxin protein is improved; and finally, acetone is adopted for directly precipitating the cobra venom neurotoxin, and impurities in final products are removed. Compared with the prior art, the extract and the preparation obtained in the invention have the advantages that the product yield, the content and the purity are improved, the adverse reaction of the medicine is effectively reduced, the curative effect is improved, and the quality of products is ensured.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Centipede toxin anti-tumour active polypeptide
InactiveCN101899095AHas antitumor activityGrowth inhibitionPeptide/protein ingredientsPeptidesReversed-Phase Liquid ChromatographyAmino acid composition
The invention discloses polypeptide separated from centipede toxin, and determination on anti-tumour activity thereof is carried out. Ultrafilteration and reversed-phase chromatography separation methods are adopted to separate and purify one polypeptide composed of twelve amino acids from centipede toxin, the sequence thereof detected by applying edman degradation method is Phe-Thr-Gly-Gly-Asp-Glu-Ser-Arg-Ile-Gln-Glu-Gly, and the molecular weight thereof measured by applying mass-spectrometric method is 1296.05Da. The anticancer cell viability of the polypeptide is determined by applying MTT method. The polypeptide in the invention has anti-tumour activity, has inhibition action on propagation and growth of human umbilical vein endothelial cell, is in dose-response relationship and can be applied to treatment and auxiliary treatment of various cancers.
Owner:CHINA PHARM UNIV
Application of parthenolide as platelet-activating factor (PAF) antagonist
InactiveCN102579426AReduce contentAntibacterial agentsOrganic active ingredientsThrombusHepatic fibrosis
The invention discloses application of parthenolide as a platelet-activating factor (PAF) inhibitor. The parthenolide can be used as a safe PAF antagonist to be applied to treatment, auxiliary treatment and prevention of diseases such as thrombus, atherosclerosis, ischemic cardio-vascular disease, cerebral ischemia, acute pancreatitis, endotoxic shock, asthma, hepatic fibrosis and hepatocirrhosis, injury of nerve, gastrointestinal ulcer and necrosis, psoriasis, systemic lupus erythematosus and acquired immune deficiency syndrome related to platelet-activating factor.
Owner:韩颖
Nutritional formula for managing chronic obstructive pulmonary disease (COPD) and lung health
The present invention is a novel nutritional product composition formulated to be used as medical foods for managing COPD. The formula comprises clary sage root extract, L-Leucine, L-Isoleucine, L-Valine and L-Glutamine. The most active chemicals that import antioxidants proprieties are Tanshinones. The composition enhances the activity of Phase 2 enzymes that deactivate and react to oxygen species and many toxins present in tobacco smoke. Preparation of highly enriched Tanshinones improved the lung function in patients with chronic asthmatic bronchitis.
Owner:SUBBIAH VEN
Injectable combination of adrenergic receptor agonists with fillers, for decreasing skin reactions due to injection
ActiveUS9050246B2Reduce inflammationOrganic active ingredientsBiocideAdrenergicAdrenergic receptor agonists
The present invention concerns an injectable composition comprising a filler or a botulinum toxin and an adrenergic receptor agonist, and its use for diminishing, decreasing or avoiding skin reactions due to injection, specially redness, ecchymosis, bruising, bleeding, erythema, oedema, necrosis, ulceration, swelling and / or inflammation.
Owner:GALDERMA RES & DEV SNC
Matrix metalloproteinase-9 polypeptide inhibitor 1 and its application
InactiveCN102268068AImprove survival rateAvoid damagePeptide/protein ingredientsAntipyreticArginineFactor ii
The invention relates to the field of medicaments, in particular to a polypeptide which has the effects of inhibiting substrate metal prolease-9 and tumor necrosis factor liberase and relieving the damage of an acute inflammatory reaction to an organism. The sequence of the polypeptide is Pro-Arg-Cys-(D-Bip)-(D-Arg)-Gly-Glu which is a brand new sequence (D-Bip is D-type diphenyl alanine, and D-Arg is D-type arginine). The polypeptide can be used for inhibiting the activities of the substrate metal prolease-9 and the tumor necrosis factor liberase on 1 micromole level in vitro and increasing the survival rate of an endotoxic shock mouse in an in-vivo test, and has a potential new medicament development value.
Owner:CHINA PHARM UNIV
Preparing derivative of tetradotoxin and application for giving up drug habits and easing pain
InactiveCN1795863AQuality is easy to controlEasy to usePowder deliveryOrganic active ingredientsSulfonateCITRATE ESTER
The derivatives of tetrodotoxin, including the tartarate, maleate, sulfonate, hydrochloride, sulfate, hydroborate, methanseulfonate, acetate and citrate of tetrodotoxin, their forms and preparing processes, and their application in dropping drugs and relaxing pain are disclosed.
Owner:林文翰 +2
Treatment of Pain With Resiniferatoxin and Related Analogs
InactiveUS20090209633A1Avoid permanent damagePreventing significant regenerationOrganic active ingredientsBiocideTRPV Cation ChannelsTRPV
A method of treating inflammatory pain conditions is provided that involves administering an effective amount of a TRPV1 agonist, such as resiniferatoxin, tinyatoxin and related potent agonists and their analogs, to a patient to selectively induce nerve terminal depolarization block and / or nerve terminal death in select TRPV1-containing neurons, to provide the desired pain relief without significant permanent damage to cell bodies of the select TRPV-1 containing neurons.
Owner:SOUTHERN ILLINOIS UNIVERSITY
Application of group of cobra neurotoxin molecules having high affinity with nicotinic acetylcholine receptors for rapid acting on pain easing
The cobra neurotoxin is capable of binding to nicotinic acetylcholine receptors after nerve synapses to block neuronal caudal ion flow to realize analgesic effect, however, the product on the market takes 2 hours to be effective and the curative effect is unstable, which cannot meet clinical requirement. The neurotoxins need to pass a blood cerebral barrier in the brain to play an analgesic effect, and the product on the market has no clear components, including various molecular weight proteins, so the speed for permeating the blood cerebral barrier is slow; and at the same time, affinity with the nicotinic acetylcholine receptors is also inconsistent. The application screens a group of neurotoxin molecular monomers having high affinity with the nicotinic acetylcholine receptor, can quickly pass the blood cerebral barrier, and has analgesic effect and stable therapeutic effect in 30 minutes. The neurotoxin molecular monomer overcomes the defects that a mixture cannot specify which neurotoxin is present, the protein primary structure is not existed, the quality cannot be precisely controlled; according to the invention, the quality is controlled, clinical safety and effectiveness are better guaranteed.
Owner:祁展楷
Conus lividus neurotoxin and coding sequence and use thereof
InactiveCN102154300AInhibition of contractionBlock transmitter deliveryNervous disorderPeptide/protein ingredientsCDNA libraryNeural biology
The invention provides a Conus lividus neurotoxin gene 1v1.3 and a polypeptide 1v1c coded thereby and use of the polypeptide 1v1c in the preparation of tool medicines for neurobiological study and analgesic medicines. In the invention, a process of constructing a cDNA library is adopted to clone the conotoxin gene 1v1.3 in the toxin canal of Conus lividus, and through analysis on a base sequence with a tool software SEQTOOL, the mature peptide amino acid sequence of the polypeptide 1v1c coded by the Conus lividus neurotoxin gene 1v1.3 is determined to be represented by a sequence 400(2) in a sequence table. The polypeptide 1v1c provided by the invention can block the transmission of a transmitter of neuromuscular junction, and according to a mouse hot-plate method, the polypeptide 1v1c has pain relieving effect and can be used for preparing the tool medicines for neurobiological study and analgesic medicines.
Owner:SUN YAT SEN UNIV
Toxin peptides with extended blood halflife
InactiveCN101232903APeptide/protein ingredientsPharmaceutical non-active ingredientsInflammatory Bowel DiseasesHalf-life
Disclosed is a composition of matter of the formula (X<1>)a-(F<1>)d-(X<2>)b-(F<2>)e-(X<3>)c and multimers thereof, in which F<1> and F<2> are half-life extending moieties, and d and e are each independently 0 or 1, provided that at least one of d and e is 1; X<1>, X<2>, and X<3> are each independently -(L)f-P-(L)g-, and f and g are each independently 0 or 1; P is a toxin peptide of no more than about 80 amino acid residues in length, comprising at least two intrapeptide disulfide bonds; L is an optional linker; and a, b, and c are each independently 0 or 1, provided that at least one of a, b and c is 1. Linkage to the half-life extending moiety or moieties increases the in vivo half-life of the toxin peptide, which otherwise would be quickly degraded. A pharmaceutical composition comprises the composition and a pharmaceutically acceptable carrier. Also disclosed are a DNA encoding the inventive composition of matter, an expression vector comprising the DNA, and a host cell comprising the expression vector. Methods of treating an autoimmune disorder, such as, but not limited to, multiple sclerosis, type 1 diabetes, psoriasis, inflammatory bowel disease, contact-mediated dermatitis, rheumatoid arthritis, psoriatic arthritis, asthma, allergy, restinosis, systemic sclerosis, fibrosis, scleroderma, glomerulonephritis, Sjogren syndrome, inflammatory bone resorption, transplant rejection, graft-versus-host disease, and lupus and of preventing or mitigating a relapse of a symptom of multiple sclerosis are also disclosed.
Owner:AMGEN INC
NOVEL CADHERIN RECEPTOR PEPTIDE FOR POTENTIATING Bt BIOPESTICIDES
ActiveUS20090175974A1Increased toxicityBiocidePeptide/protein ingredientsBacillus thuringiensisBiopesticide
Disclosed is a novel cadherin peptide that enhances the toxicity of Cry proteins. A novel insecticide composition comprising an effective amount of cadherin peptide having SEQ. ID. NO:2 and an effective amount of Bacillus thuringiensis Cry protein wherein the cadherin peptide comprises a Cry3Aa toxin binding region from the full-length T. molitor cadherin and has synergistic characteristics of a binary toxin potentiating Cry3 and Cry1 toxins against coleopterans and lepidopteran species, respectively
Owner:US SEC AGRI +1
Immunogenic conjugates of low molecular weight hyaluronic acid with polypeptide toxins
InactiveCN1525869ACross-reactiveAntibacterial agentsImmunoglobulins against bacteriaDiseaseImmunogenicity
The present invention provides antigenic compositions and methods for treatment and prevention of infection and disease caused by group A and group C streptococci. In particular, the invention provides low molecular weight hyaluronic acid, low molecular weight hyaluronic acid linked to a carrier and compositions comprising them. The compositions elicit antibodies to low molecular weight hyaluronic acid which are cross-reactive with group A and C streptococci and which are minimally cross-reactive with native hyaluronic acid. The invention is particularly useful for providing both active and passive immunogenic protection for those infected wiht or at risk infection with group A and group C streptococci. Additionally, the present invention provides methods and compositions useful for diagnosing infections and diseases caused by group A and group C streptococci.
Owner:BAXTER INT INC +1
Application of G protein coupling receptor 18 agonist in preparation of infection drugs
InactiveCN106344922AInhibit inflammationInhibitory reactivityAntibacterial agentsHydroxy compound active ingredientsImmunologic disordersDisease
The invention provides application of G protein coupling receptor 18 agonist in the preparation of infection drugs; an endotoxin-induced rat is treated with the G protein coupling receptor 18 agonist, lung tissues of the rat are taken on first, third, fifth and seventh days, pathogenic condition of tissue inflammation is observed, it can be seen from pictures that the G protein coupling receptor 18 agonist can significantly inhibit inflammation and immunity symptom reaction of the endotoxin-induced rat, and therefore, the G protein coupling receptor 18 agonist is applicable to the treatment of infection and immunity diseases; the G protein coupling receptor 18 agonist is significantly effective in inhibiting endotoxin-induced lung tissue inflammation reaction and is applicable to the preparation of drugs for treating sepsis infections; scientific evidences are provided for the clinical application of the G protein coupling receptor 18 agonist in treating sepsis infections.
Owner:安徽济人药业股份有限公司
Application of benzoylated phaeophyceae polysaccharide for preparing medicines for treating Parkinson disease
InactiveCN101940588ASignificant neuroprotective effectImprove securityOrganic active ingredientsNervous disorderOxygenLAMINARIA ANGUSTATA
The invention relates to application of benzoylated phaeophyceae polysaccharide for preparing medicines for treating Parkinson disease, which is characterized in that 1mg / ml of the benzoylated phaeophyceae polysaccharide has the action on protecting nerves and can resist the cell damage induced by neurotoxin 6-hydroxy dopamine and high ferrum and reduce the generation of reactive oxygen substances in the cells induced by the neurotoxin 6-hydroxy dopamine and high ferrum; the benzoylated phaeophyceae polysaccharide is prepared from natural phaeophyceae polysaccharide by benzoylation; the natural phaeophyceae polysaccharide comes from other phaeophyceaes of kelp seaweeds or gulfweeds, sea mustards, sargassum fusiforme, sargassum thunbergii kuntze, sargassum kjellmanianum, kelp, and the like; the phaeophyceae polysaccharide has the advantages of rich sources and high safety; and the phaeophyceae polysaccharide has the protection action on the cell damage induced by neurotoxin and high ferrum. The invention has an important application value on developing a novel I-class natural medicine for treating Parkinson disease in China.
Owner:QINGDAO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com