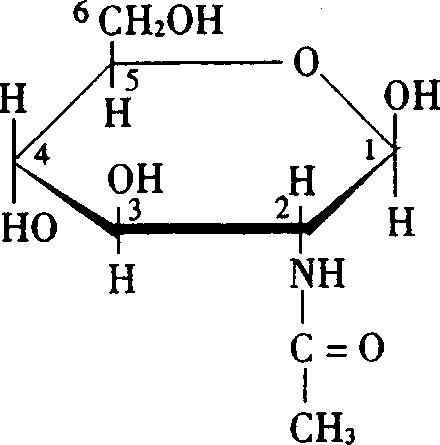
Application of N-acetly glucosamine in the preparation of medicine for treating local injury and full body syndrome due to virus or bacterial infestation
An acetamido, bacterial infection technology, applied in antibacterial, anti-infective, antiviral and other directions, to achieve good prognosis, rapid action, and the effect of relieving local damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1. The wave-promoting test of the compound of formula (I)
[0019] 1. Test materials and methods:
[0020] 1.1 Sample: pure compound of formula (I).
[0021] 1.2 Test material:
[0022] Strains: Proteus mirabilis should meet the following biochemical reaction characteristics: power (+), urease (+), lactose (-), glucose (+), H 2 S (-), phenylalanine deaminase (+).
[0023] Medium: modified LB medium (composition: 1% tryptone, 0.5% yeast extract, 1% sodium chloride, 0.1% glucose, 0.002% TTC, pH7.2-7.4).
[0024] 1.3 Test method:
[0025] Control sample: plant Proteus mirabilis in the center of the LB plate and culture at 37°C for 9 hours;
[0026] Test sample: Add the compound of formula (I) at a final concentration of 0.5% to the LB plate, plant Proteus mirabilis in the same way, and culture at 37° C. for 9 hours.
[0027] 2. Test results and evaluation:
[0028] In the control sample, there are concentric rings that continuously expand outwards at inte...
Embodiment 2
[0030] The toxicological test of embodiment 2. formula (I) compound
[0031] Carry out the toxicology test of formula (I) compound, comprise:
[0032] 1. Acute toxicology test: including oral, intravenous injection and maximum dose administration test;
[0033] 2. Ames test;
[0034] 3. Mouse bone marrow cell micronucleus test;
[0035] 4. Mouse sperm teratogenic test;
[0036] 5. Chromosomal aberration test of mouse testis;
[0037] 6. Chronic lethal test;
[0038] 7. Subchronic toxicity (90-day feeding) test;
[0039] 8. Traditional teratogenicity test;
[0040] Test conclusion shows: when formula (I) compound acute toxicity test dose exceeds 2g / kg, acute poisoning reaction does not yet occur; In long-term toxicity test, the highest dose has reached 1g / kg, through four weeks test observation, no toxic reaction occurs; In the reproduction test, mice were fed from a conventional dose of 7 mg / kg, and after three passages, it was proved that the compound of formula (I) ha...
Embodiment 3
[0041] Example 3. Cytological test for regulation of microheterogeneic changes
[0042] The conventional incomplete 1640 medium was used for cell culture, and B16 tumor cells (purchased from Shanghai Institute of Cell Biology Tumor Cell Bank) were inoculated. In the continuous culture for more than 48 hours, under the condition that the waste of cell metabolism affects the growth environment, the changes of the micro-heterogeneity of the cells and the control effect of N-acetylglucosamine on them were observed. After adding N-acetylglucosamine with a final concentration of 1g / 100ml in the culture medium, the number of cells increased steadily with the prolongation of culture time during the growth process. Control test cells could not proliferate in the same medium and under the same culture conditions in the absence of N-acetylglucosamine. The test shows that under the condition of the compound of formula (1), the cells can adjust the micro-heterologous changes of the cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com