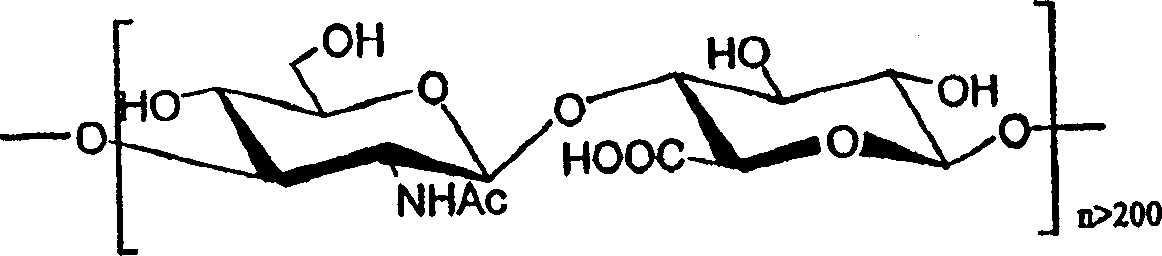
Immunogenic conjugates of low molecular weight hyaluronic acid with polypeptide toxins
An immunogenic, hyaluronic acid technology, applied in the field of LMW-HA/polypeptide conjugate molecules, hyaluronic acid/polypeptide conjugate molecules, can solve problems such as unpredictable HA or HA conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
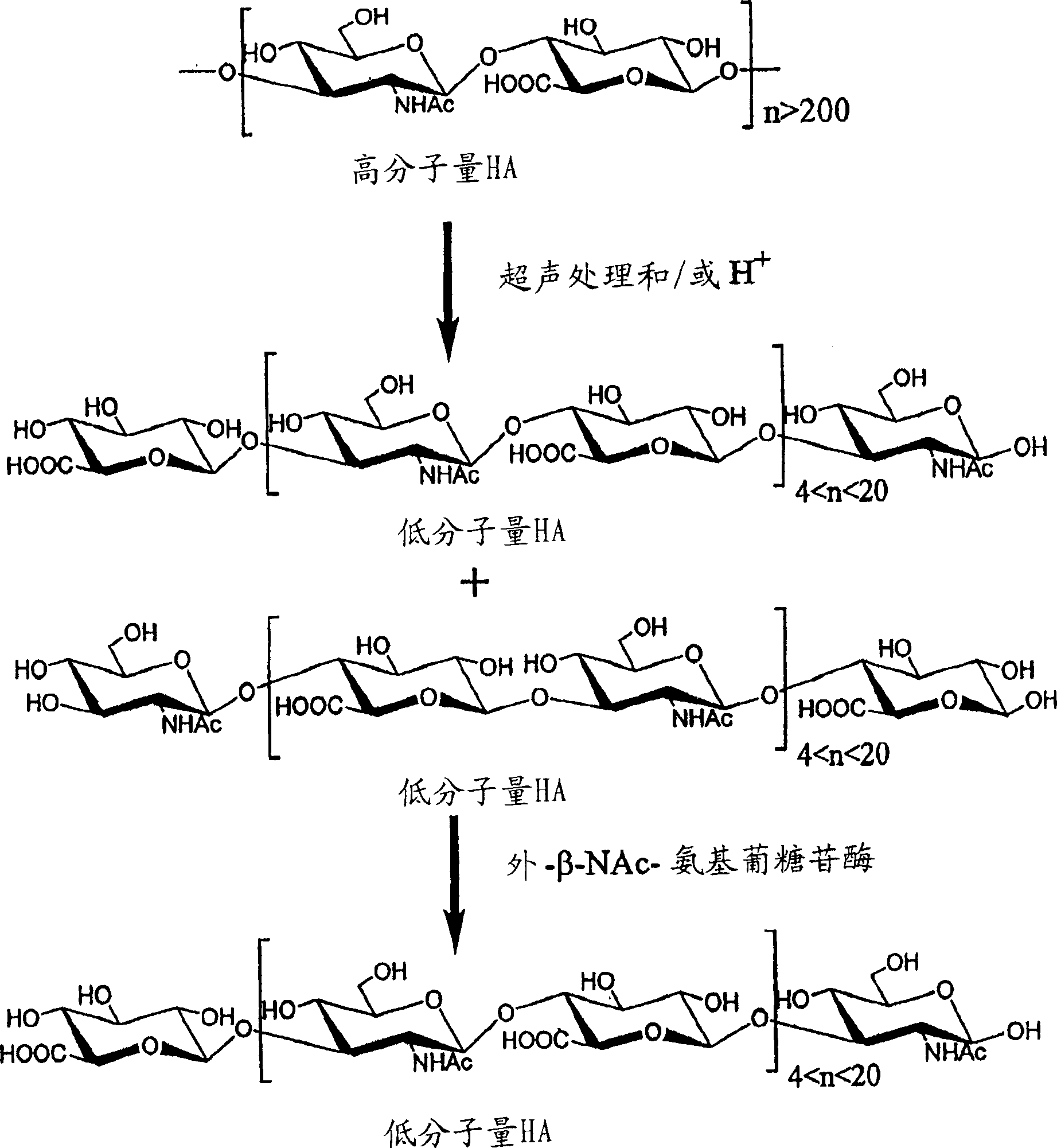
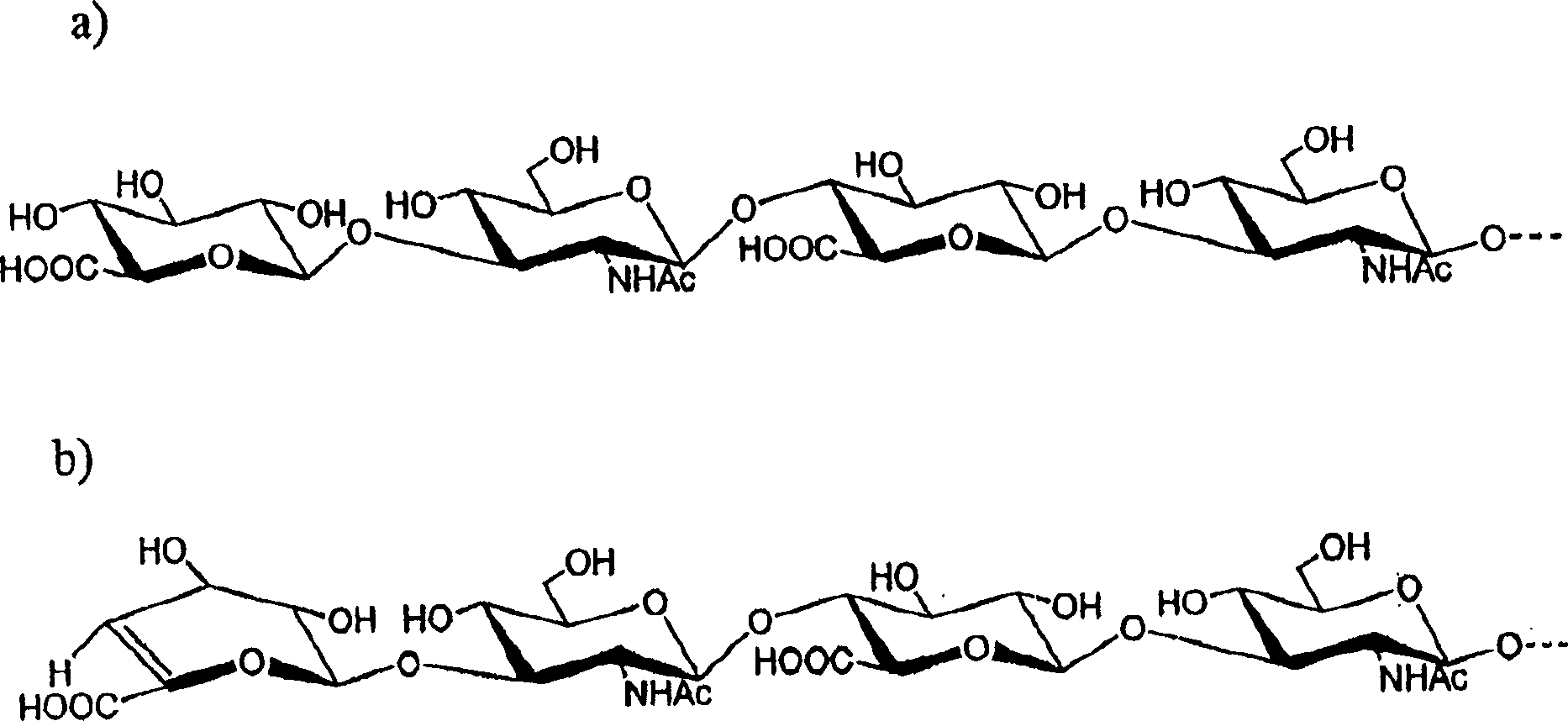
[0052] Preparation of LMW-HA
[0053] Acid hydrolytic depolymerization of hyaluronic acid
[0054] Hyaluronic acid (100 mg, Lifecore lot 1-9062-5) was added to 10 ml of 0.05N HCl solution. The mixture was heated at 80°C for 2 hours with stirring to dissolve all solids. The sample was heated at 100°C for an additional 1.5 hours. Aliquots from the reaction mixture were taken at various times and analyzed on a Bio-Rad system (Biologic) equipped with a Superose(R) 12 HR 10 / 30 column (Pharmacia) to monitor depolymerization. The solution was neutralized with 0.5N NaOH, followed by dialysis against a Diaflo(R) membrane with a molecular weight cut-off (MWCO) of 3,500, and then lyophilized. The product was size fractionated by a Superdex(R) 200PG (Pharmacia) column to obtain 65 mg of solid product. Samples were tested at 500MHz 1 H-NMR analysis confirmed the disaccharide repeating unit structure of hyaluronic acid (HA). The average molecular weight of the resulting fragments wa...
Embodiment 2
[0060] Preparation of LMW-HA conjugates
[0061] Hyaluronic acid treated with NaBH4 reducing acid
[0062] Hydrochloric acid depolymerized hyaluronic acid (65 mg) was dissolved in 6.5 ml deionized water. Adjust the pH to 10 with 0.5N NaOH and add NaBH 4 into this solution. The reaction mixture was kept at room temperature for 2 hours. Destruction of excess NaBH with 1M acetic acid 4 . After dialysis against water with a Diaflo(R) membrane of MWCO 3,500, followed by lyophilization, 35 mg of solid product were obtained. Periodate oxidation of reduced acid-treated hyaluronic acid
[0063] Two 18mg samples of the reduced and fractionated polysaccharide were dissolved in 1.3ml and 0.87ml of 10mM NaIO 4 in aqueous solution, thereby achieving degrees of oxidation (d.o.) of 10% and 20%, respectively. The reaction was stirred for 2 hours at room temperature in the dark and two samples were quenched with 20 μl of ethylene glycol. The reaction mixture was then dialyzed and lyoph...
Embodiment 3
[0075] Isolation of hyaluronic acid oligosaccharide inhibitors
[0076] Contents of 3 ampoules, hyaluronate lyase (EC 4.2.2.1) from Streptomyceshyalurolyticus (Sigma Biochemicals) dissolved in 10 mM PBS was added to hyaluronic acid (60 mg) which had been sonicated and incubated at 37 Incubate for 1.5 hours at °C. The reaction was terminated by boiling the reaction mixture at 100° C. for one minute in a water bath. By taking an aliquot of the reaction mixture and analyzing it using a BIO-RAD system (Biologic) equipped with a Superdex® peptide column (Pharmacia) at a flow rate of 0.75 ml / min using 10 mM PBS as the eluent, monitoring The process of enzymatic digestion. The solution was stored at 4°C until further purification.
[0077] Separation of oligosaccharides was performed by anion exchange chromatography with Mono-QHR5 / 5 column (Pharmacia) with HPLC1090 (Hewlett Packard 1090 Series II) system equipped with diode array detector, programmable autosampler, fraction col...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com