SEMA4D antibody, preparation method and application thereof
A technology of antibody and antibody conjugate, applied in the field of SEMA4D antibody and its preparation, can solve problems such as single method, inability to effectively inhibit tumor growth, and poor effect of in vitro activity experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0243] The preparation method of the nucleic acid is a conventional preparation method in the art. Preferably, it includes the following steps: obtaining the nucleic acid molecule encoding the above-mentioned protein by gene cloning technology, or obtaining the nucleic acid molecule encoding the above-mentioned protein by artificial full-sequence synthesis .
[0244] Those skilled in the art know that substitutions, deletions, alterations, insertions or additions can be appropriately introduced into the base sequence encoding the amino acid sequence of the above protein to provide a polynucleotide homologue. The homologue of the polynucleotide in the present invention can be prepared by replacing, deleting or adding one or more bases in the gene encoding the protein sequence within the scope of maintaining antibody activity.
[0245] carrier
[0246] The invention also provides a recombinant expression vector comprising the nucleic acid.
[0247] The recombinant expression v...
Embodiment 1
[0321] Embodiment 1 hybridoma technology prepares SEMA4D antibody
[0322] (1) Preparation of immunogen
[0324] The nucleotide sequence (as shown in sequence table SEQ ID No.421) containing the coding human SEMA4D protein extracellular region (hSEMA4D ECD) Met22-Arg734 was cloned into the pCP vector with His tag (the cloning step was provided by Shanghai Ruizhi Chemical Co., Ltd. Research Co., Ltd.), National Research Counceil Canada) and prepared plasmids according to established standard molecular biology methods. For specific methods, see [Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Second Edition (Plainview, New York: Cold Spring Harbor Laboratory Press)]. CHO-S cells (purchased from Gibco) were transiently transfected with polyetherimide (PEI, purchased from Polyscience), and expanded at 37° C. using CDFortiCHO medium (purchased from Gibco). After 8-10 days, the cell culture fluid is coll...
Embodiment 2
[0352] Example 2 Preparation of SEMA4D antibody by phage display technology
[0353] (1) Biotinylation of SEMA4D protein
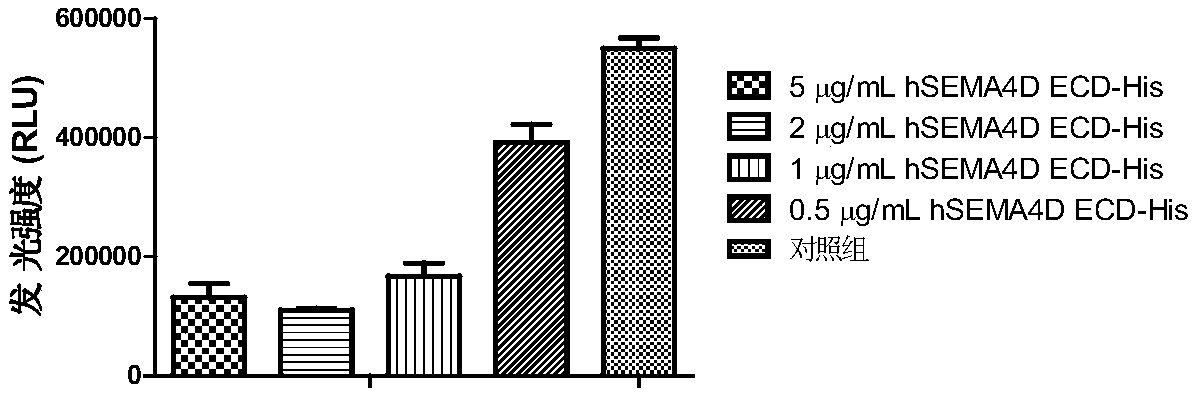
[0354] With 0.15M Na 2 HCO 3The protein immunogen prepared in Example 1 (ie hSEMA4D ECD-His) was dialyzed to a final concentration of 1 mg / mL. Biotin-NHS (purchased from Sigma Aldrich) was dissolved in DMF to a final concentration of 10 mg / mL. Mix Biotin-X-X-NHS and protein immunogen at a molar ratio of 8:1. After standing at room temperature for 30 minutes, add 1M NH 4 Cl terminates the reaction. Then, it was dialyzed against PBS phosphate buffer (pH 7.4) overnight at 4° C. to remove free biotin to obtain a biotinylated immunogen (ie, biotinylated hSEMA4D ECD-His). The concentration of biotinylated hSEMA4D ECD-His was measured with BCA protein concentration assay kit (purchased from Pierce).
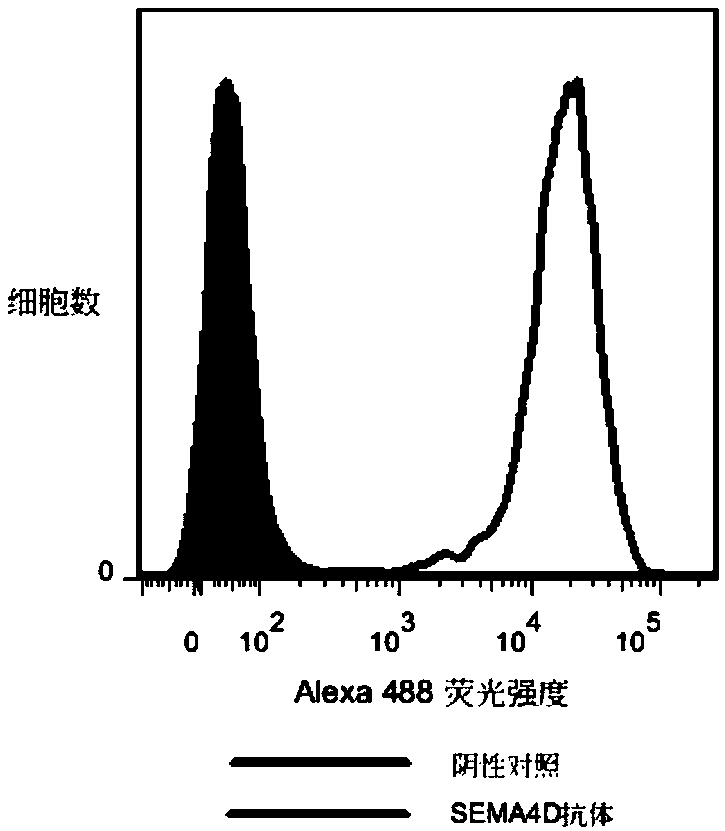
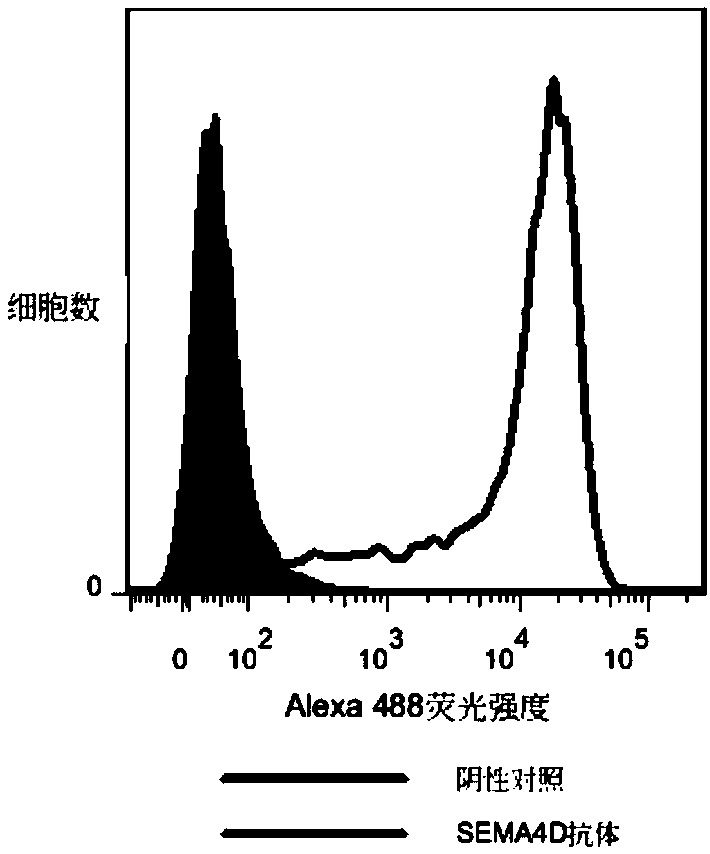
[0355] The activity of biotinylated hSEMA4D ECD-His was determined by FACS method. The stable transgenic cell line 293F hPlexin B1 expressing the human SEMA4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com