Toxin peptides with extended blood halflife
A toxin peptide, half-life technology, applied in the field of biochemistry, can solve the problems of expensive synthesis and refolding production process, short in vivo half-life and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
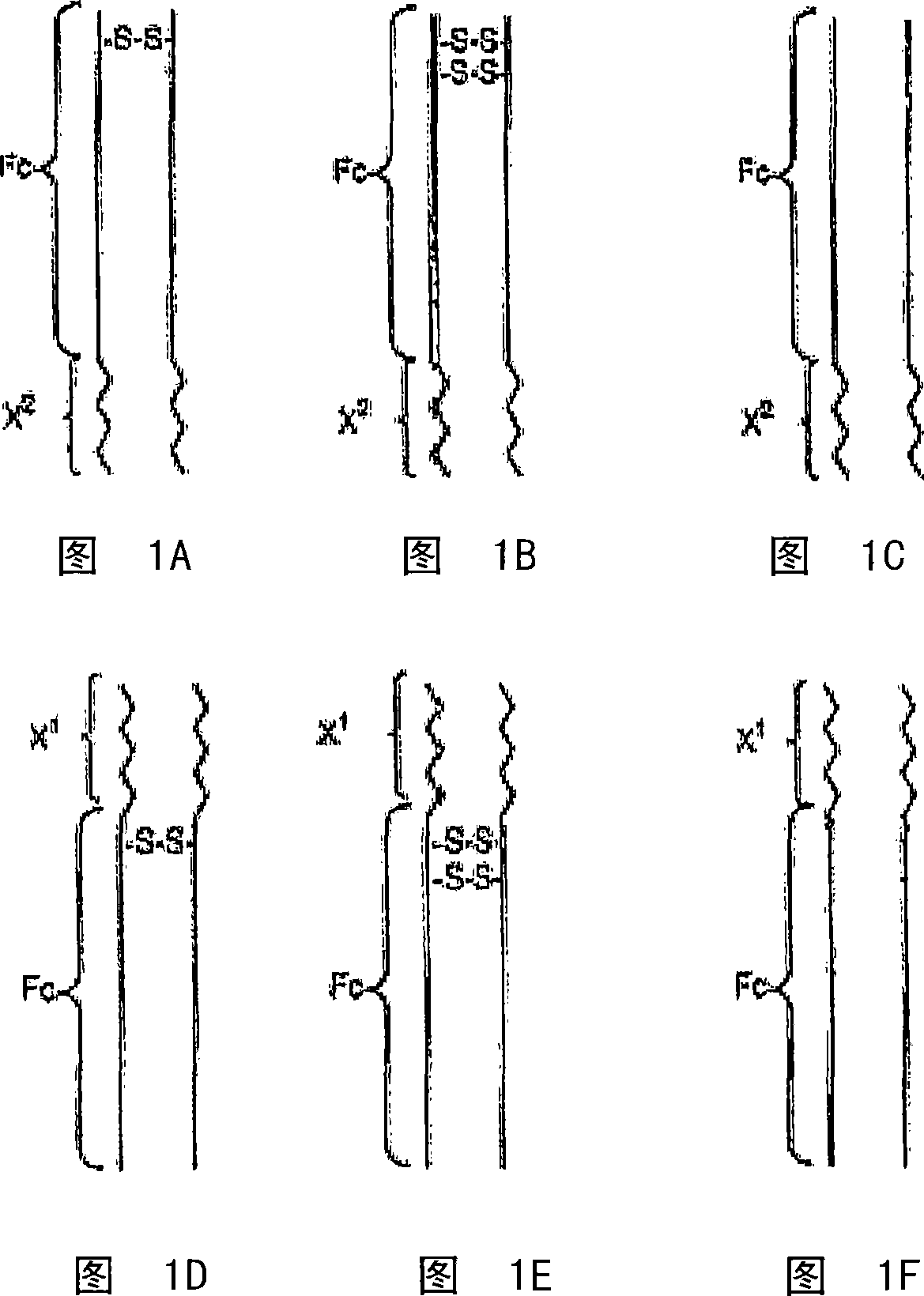
[0655] Mammalian expression of Fc-L10-ShK[1-35]
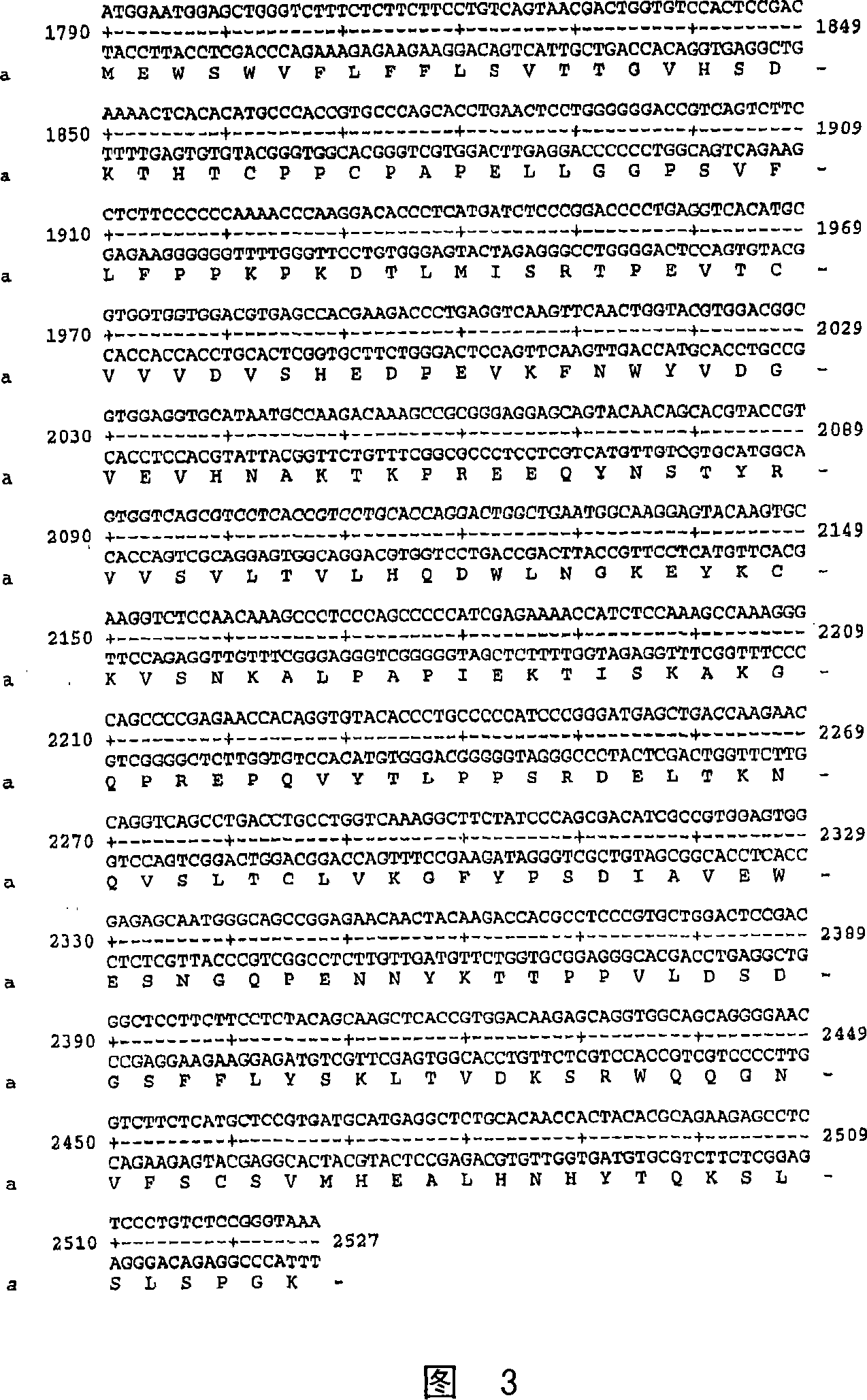
[0656] Fc-L10-ShK[1-35], also known as "Fc-2xL-ShK[1-35]", is an inhibitor of Kv1.3. The DNA sequence encoding the Fc region of human IgG1 was constructed as described below, which was fused in-frame with the linker sequence and the monomer of the Kv1.3 inhibitor peptide ShK[1-35]. Disclosed herein are methods for expressing and purifying peptide antibodies from mammalian cells (HEK 293 and Chinese hamster ovary cells).
[0657]The expression pcDNA3.1(+)CMVi was constructed and expressed by replacing the CMV promoter between MluI and HindIII in pcDNA3.1(+) with CMV promoter plus intron (Invitrogen) (Figure 13A). The expression vector pcDNA3 was prepared by cloning the PCR product of the HindIII-NotI digested human Fc-linker-ActivinRIIB fusion protein containing the 5'Kozak sequence, the signal peptide, and a large fragment of pcDNA3.1(+)CMVi digested with HindIII-NotI. 1(+)CMVi-hFc-ActivinRIIB (Figure 13B). The nucleotide and amin...
Embodiment 2
[0678] Mammalian expression of Fc-L-ShK[2-35]
[0679] The DNA sequence encoding the Fc region of human IgG1 fused in-frame with the monomer of the Kv1.3 inhibitor peptide ShK[2-35] was constructed using standard PCR techniques. The Fc-2xL-ShK[1-35] in the original pcDNA3.1(+)CMVi was used as a template (Example 1, Figure 14) to prepare ShK[2-35] and molecules of 5, 10 or 25 amino acid linker portion. All ShK constructs should have the following amino acid sequence
[0680] SCIDTIPKSRCTAFQCKHSMKYRLSFCRKICGIC
[0681] (SEQ ID NO: 92)
[0682] The first amino acid of the wild-type sequence is removed.
[0683] The sequence of the primer used to prepare Fc-L5-ShK[2-35], also called "Fc-1xL-ShK[2-35]" is as follows:
[0684] cat gga tcc agc tgc atc gac acc atc / / SEQ ID NO: 661;
[0685] cat gcg gcc gct cat tag c / / SEQ ID NO: 662;
[0686] The sequence of the primer used to prepare Fc-L10-ShK[2-35], also called "Fc-2xL-ShK[2-35]" is as follows:
[0687] cat gga tcc gga gga gga...
Embodiment 3
[0705] Bacterial expression of Fc-L-ShK[1-35]
[0706] Description of bacterial peptide antibody expression vector and procedures for cloning and expressing peptide antibody . The cloning vector used for bacterial expression (Examples 3-30) is based on pAMG21 (originally described in US Patent 2004 / 0044188). It has been modified to replace the kanamycin resistance component with ampicillin resistance. This is accomplished by the following steps: excise the DNA between the unique BstBI and NsiI sites of the vector and use the PCR primer CCA ACA CAC TTC GAAAGA CGT TGA TCG GCA C (SEQ ID NO: 667) and CAC CCA ACAATG CAT CCT TAA AAA AAT TAC GCC C (SEQ ID NO: 668), using pUC19 DNA as the β-lactamase conferring resistance to ampicillin The template source of the gene, replace the excised DNA with a suitably digested PCR fragment carrying the β-lactamase gene. The new version is called pAMG21ampR.
[0707]Description of the cloning vector pAMG21ampR-Fc-Pep used in Examples 3-30 (except E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com