Chromone derivatives as matrix metalloproteinase inhibitors
a matrix metalloproteinase inhibitor and chromone derivative technology, applied in the field of chromone derivatives, can solve the problems of lack of specificity for any particular enzyme, use of currently known mmp inhibitors, and no selective or non-selective inhibitor of mmp-13 has been approved and marketed for the treatment of any disease in any mammal
Inactive Publication Date: 2005-06-21
WARNER-LAMBERT CO
View PDF39 Cites 28 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
"This patent describes a new group of compounds called chromone derived compounds that have various uses. These compounds can be used as medicines, as well as in other fields such as electronics and sensors. The patent also describes different types of chromone compounds that can be made using different methods. Overall, this patent provides a new way to create and use new compounds for various applications."
Problems solved by technology
A major limitation on the use of currently known MMP inhibitors is their lack of specificity for any particular enzyme.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 1
4-(6-Benzylcarbamoyl-4-oxo-4H-benzo[e][1,3]oxazin-3-ylmethyl)-benzoic acid
example 2
4-[6-(4-Fluoro-benzyl)-carbamoyl-4-oxo-4H-benzo[e][1,3]oxazin-3-ylmethyl]-benzoic Acid
example 3
3-(4-Fluoro-benzyl)-4-oxo-3,4-dihydro-2H-benzo[e][1,3]oxazine-6-carboxylic Acid Benzylamide
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Login to View More
Abstract
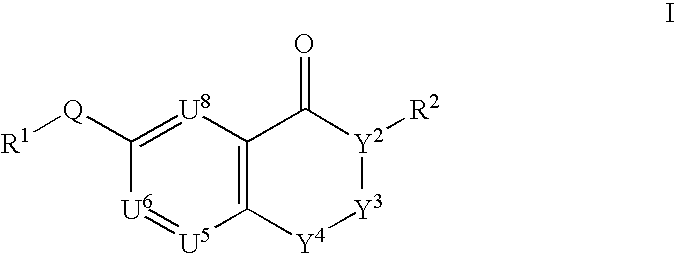
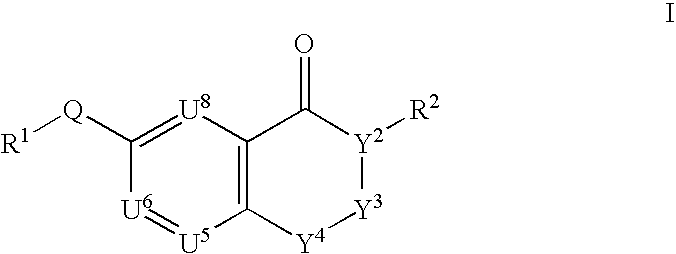
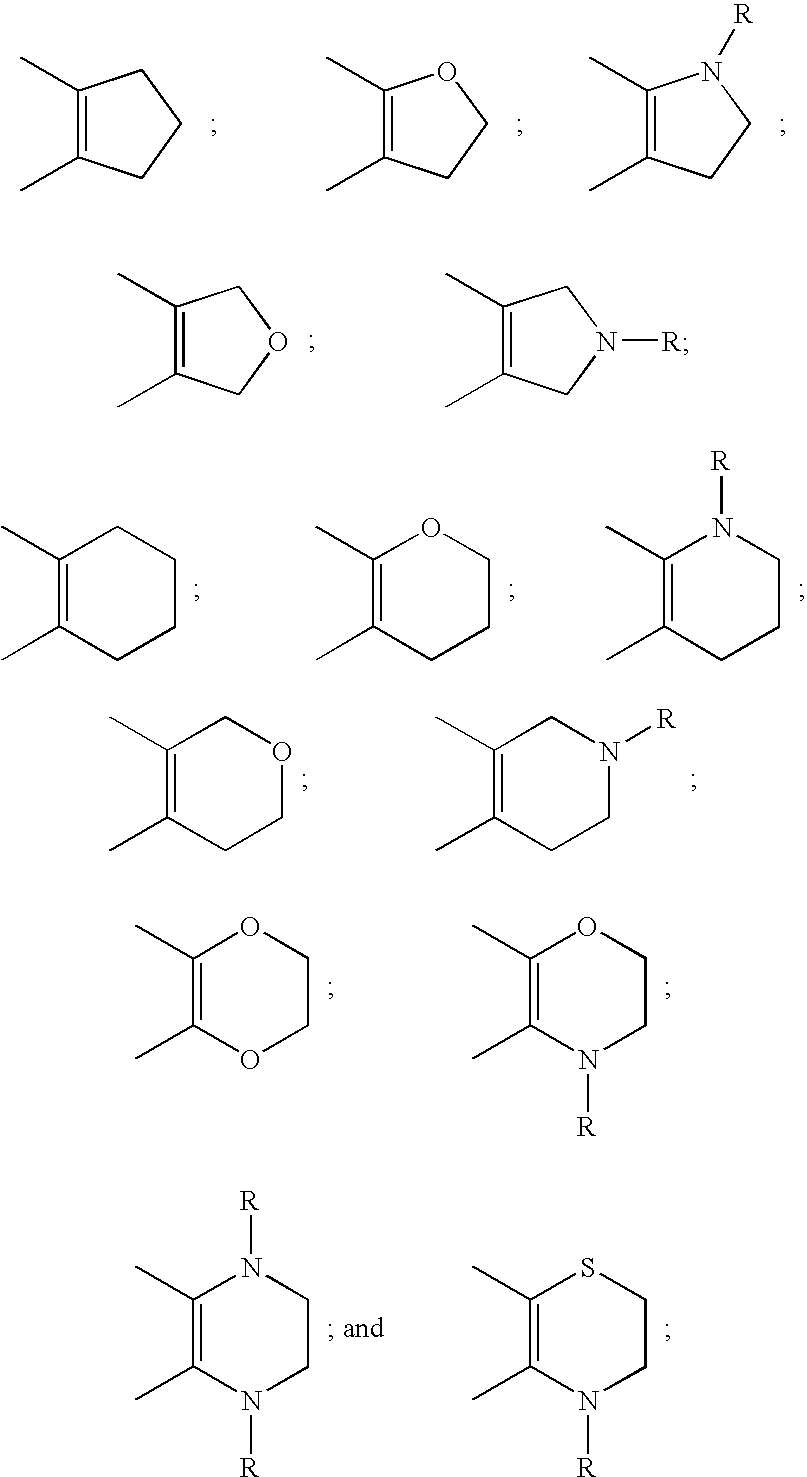
This invention provides compounds defined by Formula I or a pharmaceutically acceptable salt thereof,wherein R1, Q, Y2, Y3, Y4, U5, U6, U8, and R2 are as defined in the specification. The invention also provides pharmaceutical compositions comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, as defined in the specification, together with a pharmaceutically acceptable carrier, diluent, or excipient. The invention also provides methods of inhibiting an MMP-13 enzyme in an animal, comprising administering to the animal a compound of Formula I, or a pharmaceutically acceptable salt thereof. The invention also provides methods of treating a disease mediated by an MMP-13 enzyme in a patient, comprising administering to the patient a compound of Formula I, or a pharmaceutically acceptable salt thereof, either alone or in a pharmaceutical composition. The invention also provides methods of treating diseases such as heart disease, multiple sclerosis, osteo- and rheumatoid arthritis, arthritis other than osteo- or rheumatoid arthritis, cardiac insufficiency, inflammatory bowel disease, heart failure, age-related macular degeneration, chronic obstructive pulmonary disease, asthma, periodontal diseases, psoriasis, atherosclerosis, and osteoporosis in a patient, comprising administering to the patient a compound of Formula I, or a pharmaceutically acceptable salt thereof, either alone or in a pharmaceutical composition. The invention also provides combinations, comprising a compound of Formula I, or a pharmaceutically acceptable salt thereof, together with another pharmaceutically active component as described in the specification.
Description
CROSS-REFERENCE TO RELATED APPLICATIONS[0001]This application claims benefit of priority from U.S. Provisional Patent Application No. 60 / 403,094, filed Aug. 13, 2002.FIELD OF THE INVENTION[0002]This invention relates to chromone derivatives which inhibit matrix metalloproteinase enzymes and thus are useful for treating diseases resulting from MMP-mediated tissue breakdown such as heart disease, cardiac insufficiency, inflammatory bowel disease, multiple sclerosis, osteo- and rheumatoid arthritis, arthritis other than osteo- or rheumatoid arthritis, heart failure, age-related macular degeneration, chronic obstructive pulmonary disease, asthma, periodontal diseases, psoriasis, atherosclerosis, and osteoporosis.BACKGROUND OF THE INVENTION[0003]Matrix metalloproteinases (sometimes referred to as MMPs) are naturally occurring enzymes found in most mammals. Over-expression and activation of MMPs, or an imbalance between MMPs and inhibitors of MMPs, have been suggested as factors in the pa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(United States)
IPC IPC(8): C07D311/00C07D265/22C07D311/22C07D265/00A61K31/353A61K31/536A61P1/02A61P1/04A61P9/04A61P9/10A61P11/06A61P17/06A61P19/02A61P19/10A61P25/00A61P29/00
CPCC07D311/22C07D265/22A61P1/02A61P1/04A61P11/06A61P17/06A61P19/02A61P19/10A61P25/00A61P29/00A61P9/04A61P9/10
Inventor ORTWINE, DANIEL FRED
Owner WARNER-LAMBERT CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com