Matrix metalloproteinase-9 polypeptide inhibitor 4 and its application
A technology of peptide inhibitors and matrix metals, applied in peptide/protein components, peptides, anti-inflammatory agents, etc., can solve the problems of lack of specificity and side effects of small molecule inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Chemical Synthesis of Peptides
[0024] The peptides were synthesized using Fmoc chemistry. The synthesis reaction is carried out from the C-terminus to the N-terminus. There are free amino groups on the Rink medium (available from Advanced ChemTech Company), and Glu, Gly, Arg, Bip, D-Cys, D-Pyr and Pro are connected sequentially. During each ligation step, the amino acid residues are activated, and the activation mixture contains 4 times as many HBTU, HOBt, DIEA and Fmoc-amino acids as there are free amino groups on the medium. After each amino acid linking reaction, a mixture of pyridine / acetic acid / N-methylimidazole (4:1:0.5) was used to block the unlinked free amino group for 10 minutes. After each amino acid linking reaction and before the next amino acid linking, the Fmoc-group on the medium should be removed, and the Fmoc-group should be removed using dimethylformamide containing 20% piperidine, which takes 15 minutes. Finally, after all amino acid residues a...
Embodiment 2
[0027] IC50 values of peptide inhibitors against several target enzymes (matrix metalloproteinase-3, -8, -9 and tumor necrosis factor releasing enzyme) in vitro.
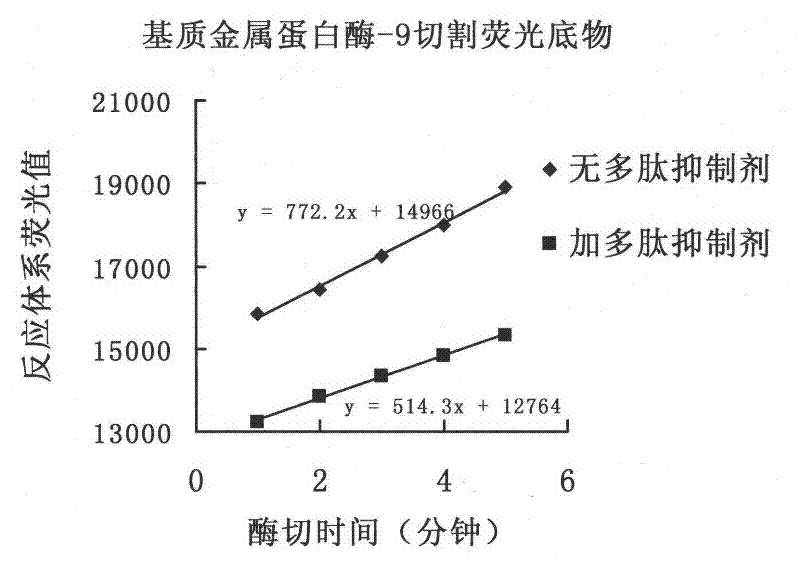
[0028] Recombinant human matrix metalloproteinase-9 was expressed by Sf9 insect cells. The enzyme was activated with 0.01 μM matrix metalloproteinase-3 active site, and the buffer used was 100 mM Tris / HCl, pH 7.4, 100 mM NaCl, 10 mM CaCl 2 and 0.01% Tween-20. The concentration of matrix metalloproteinase-9 during activation was 92ng / μl (1μM). Enzyme activity is detected by cleaving the fluorescently generated peptide substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH 2 And detect the generated fluorescence value (excitation wavelength = 328nm, detection wavelength = 392nm). All the detection is carried out in 100μl reaction system at 37°C.
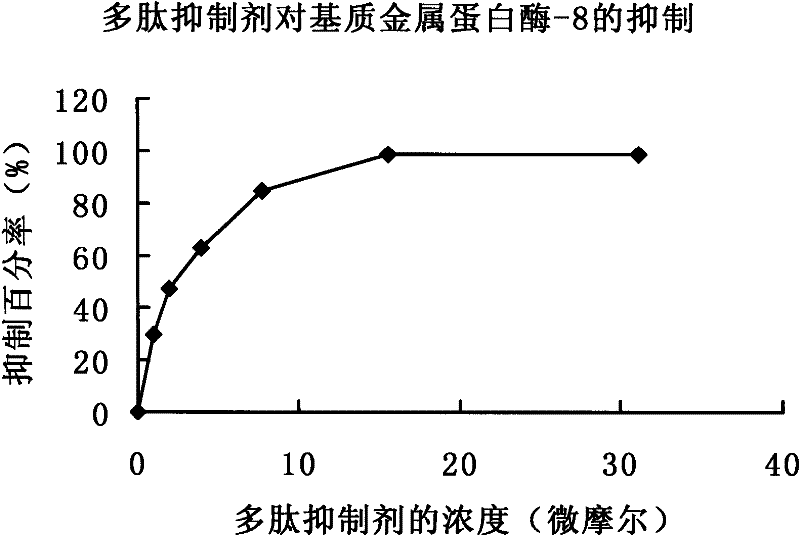
[0029] Recombinant human matrix metalloproteinase-8 is detected similarly to -9 and uses the same fluorogenic substrate. The reaction was also carried out in a 100 μl reaction sy...
Embodiment 3
[0033] Testing the Viability of Peptide Inhibitors in Vivo Using an Endotoxic Shock Model
[0034] Before establishing the endotoxic shock model, we first determined the LD50 of LPS (E.coli 0111: B4) mice as 50 μg per mouse, and we used 100 μg per mouse in the experiment, so that the mice in the control group Rats can all die. In our previous scientific research work, we found that Regasepin2 (another peptide inhibitor, published) can improve the survival rate of C57BL / 6 mice during endotoxin shock. In order to establish endotoxin shock model in our mice, we did a positive control experiment with Regasepin2. The mice in the control group were injected with 100 μg of LPS, while the mice in the Regasepin2 experimental group were injected with 0.7 mg of polypeptide 5 minutes after the injection of LPS. By making the Kaplan-Meier survival curve, it was found that Regasepin2 can effectively protect the mice injected with 100 μg and improve the survival rate ( Figure 7 ). After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com