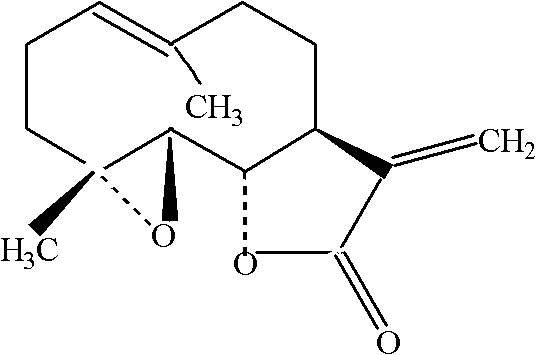
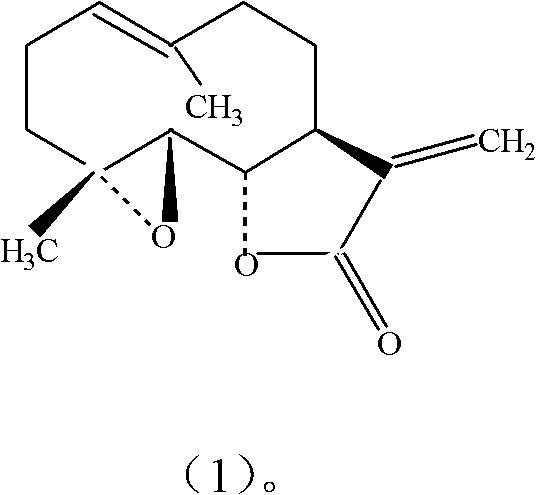
Application of parthenolide as platelet-activating factor (PAF) antagonist
A technology of parthenolide and activating factor, applied in antiviral agents, medical preparations containing active ingredients, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
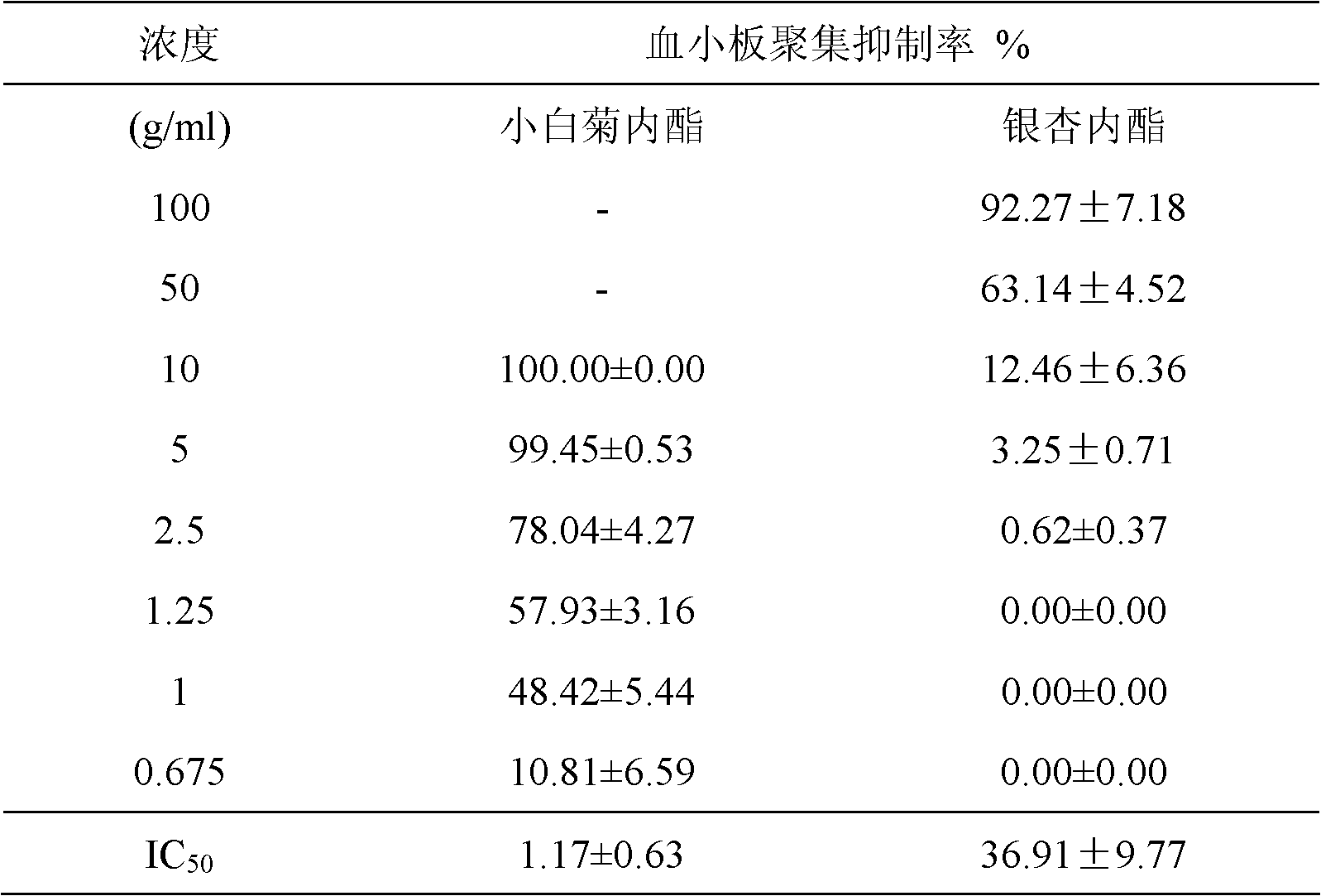
[0017] Experimental example 1. Parthenolide PAF antagonistic activity experiment:
[0018] 1. Experimental animals:
[0019] Male Japanese white rabbits weighing 2.0 to 2.2 kg.
[0020] 2. Drugs and reagents:
[0021] Test drug: parthenolide, homemade, chromatographic purity 98.33%.
[0022] Control drug: Ginkgolide, a product of Shanghai Ronghe Pharmaceutical Technology Development Co., Ltd., with a declared content of 97.81%.
[0023] Inducer: Rabbit Platelet Activating Factor (PAF), a product of Sigma
[0024] 3. Experimental method:
[0025] Preparation of platelet rich plasma (PRP) and platelet poor plasma (PPP): blood was collected from the common carotid artery of awake rabbits and anticoagulated with 3.8% sodium citrate 9:1 (v:v) Collected in a plastic centrifuge tube, centrifuged at 900 rpm for 8 minutes, the upper layer was taken as PRP, and the remaining blood was centrifuged at 3000 rpm for 5 minutes, and the upper layer was taken as PPP. The number of platelets in PRP was con...
experiment example 2
[0032] Experimental example 2: Experimental study on the acute toxicity of parthenolide
[0033] The purpose of the experiment: to observe the acute toxicity and death of mice within one week after the test substance parthenolide was administered once by gavage, and calculate the median lethal dose (LD) of parthenolide according to the number of deaths. 50 )
[0034] 1. Test substance:
[0035] Parthenolide
[0036] 2. Preparation method:
[0037] Accurately weigh 3750mg of parthenolide, add a little Tween-80 to a mortar, grind it, slowly add distilled water to the top 30ml, and then dilute to 100mg / ml, 80mg / ml, 64mg / ml, 51.2mg in the same ratio of 0.8 / ml, five concentration emulsions are stored in the refrigerator for later use.
[0038] 3. Experimental animals:
[0039] Source: KM mice, provided by the Animal Room of Jiangsu Cancer Institute
[0040] Weight: 20±2g
[0041] Gender: each group is half male and half male
[0042] Number of animals: 10 animals per group, calculate LD 50 .
[...
Embodiment 1
[0060] Weigh 20g parthenolide and grind it through a 100-mesh sieve, add 100g starch, 20g lactose, 15g sodium carboxymethyl cellulose, 10g microcrystalline cellulose, 10g magnesium stearate, and pass the 100-mesh sieve. Mix uniformly by increasing method, granulate with proper amount of 10% starch slurry, press to make 1000 tablets or fill into 1000 capsules to make tablets or capsules. Clinically used in the treatment, adjuvant treatment and prevention of thrombosis, atherosclerosis, ischemic cardiovascular disease, cerebral ischemia, asthma, liver fibrosis and cirrhosis, nerve damage, gastrointestinal ulcer and necrosis, psoriasis, etc. . The effective rate of clinical treatment can reach over 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com