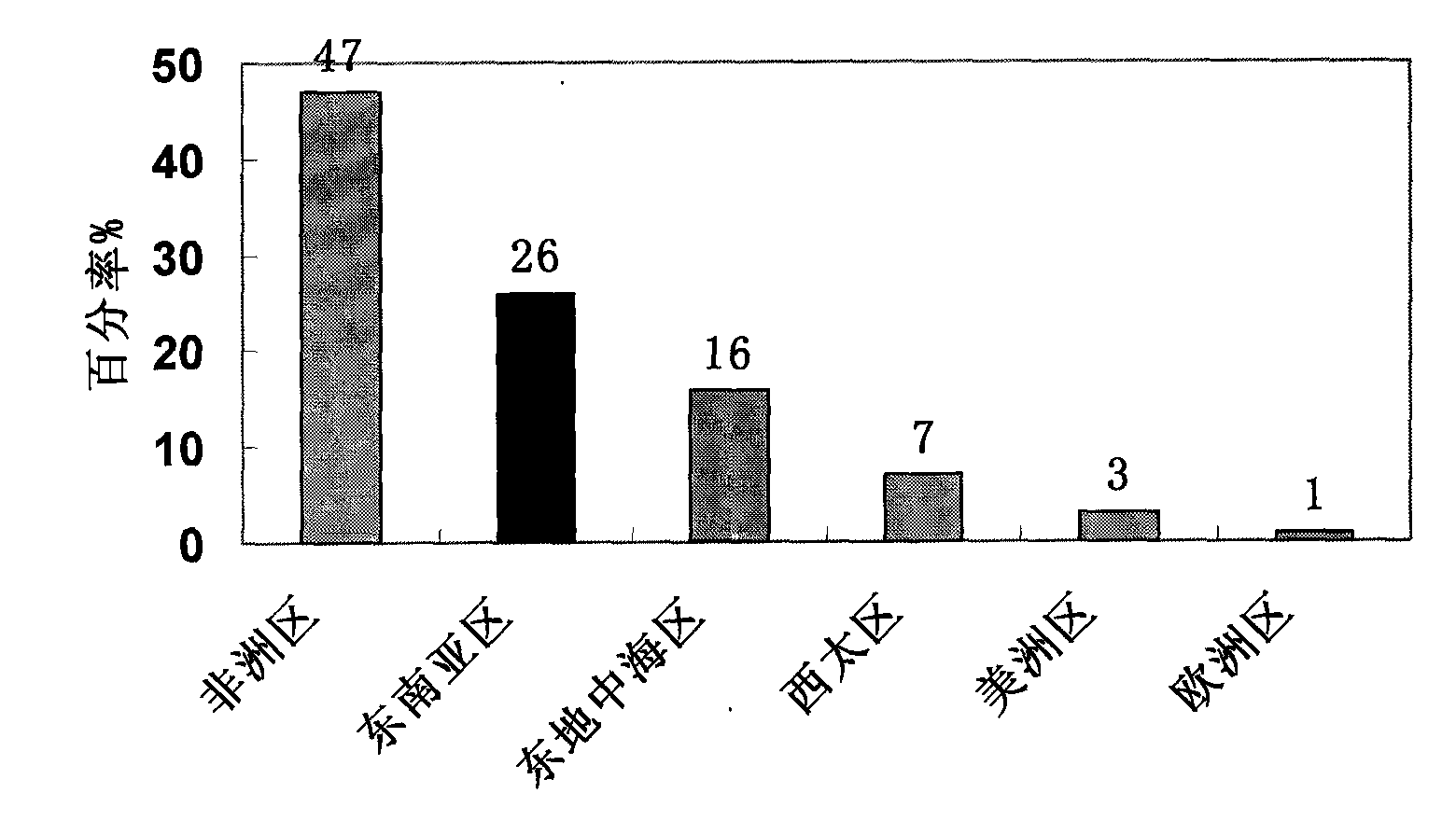
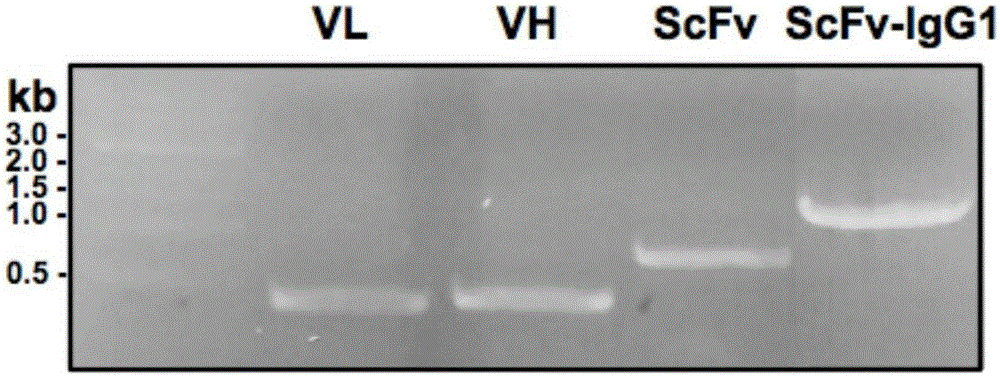
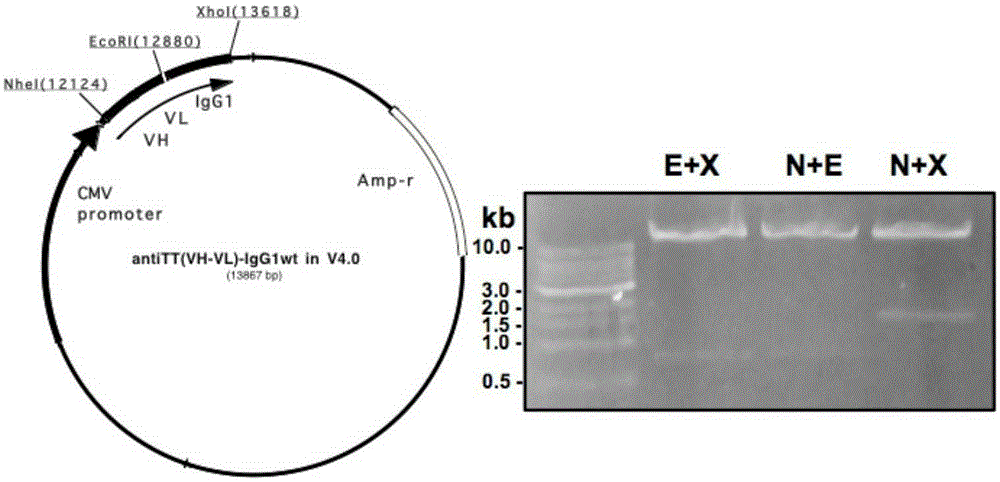
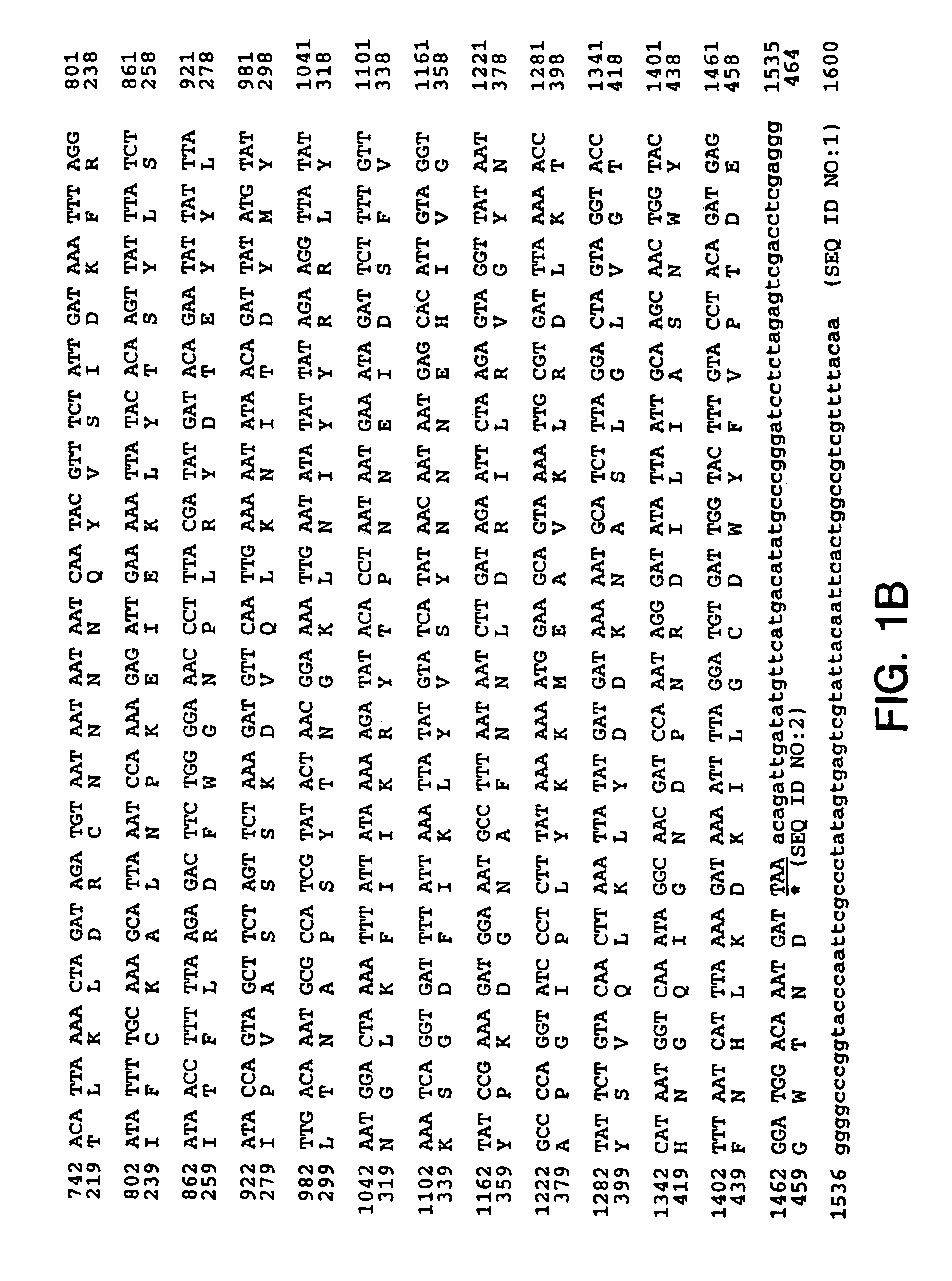
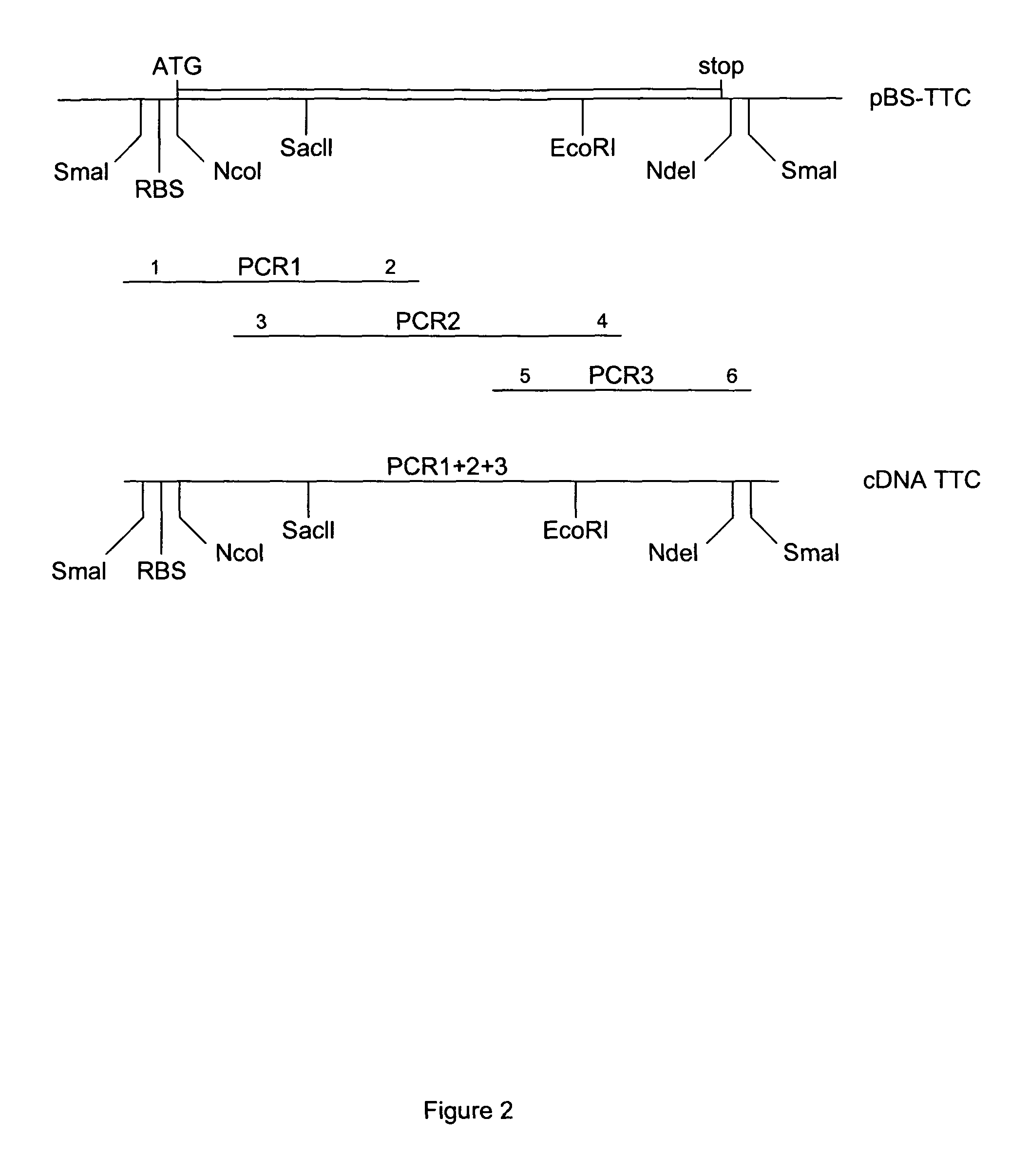
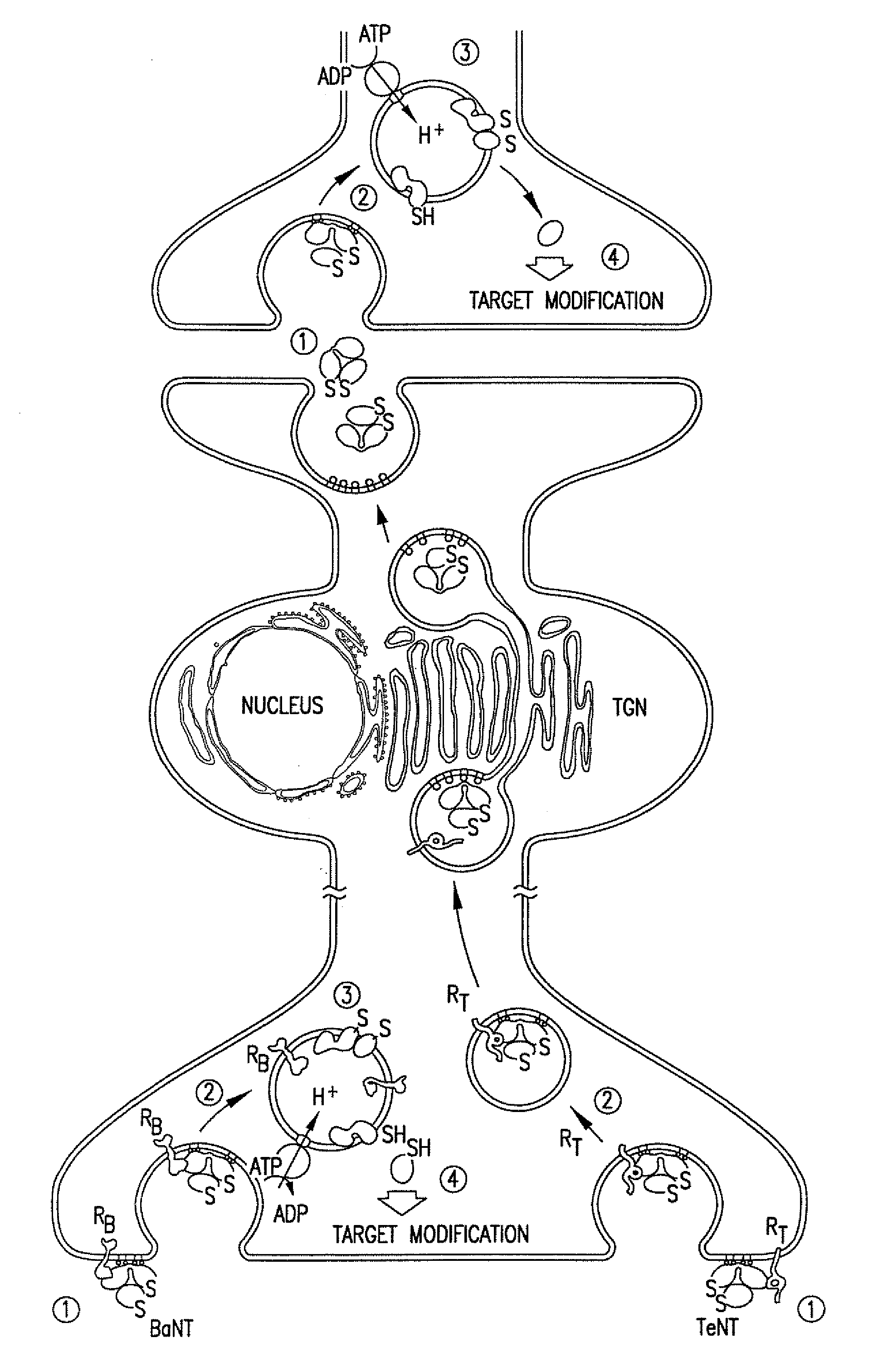
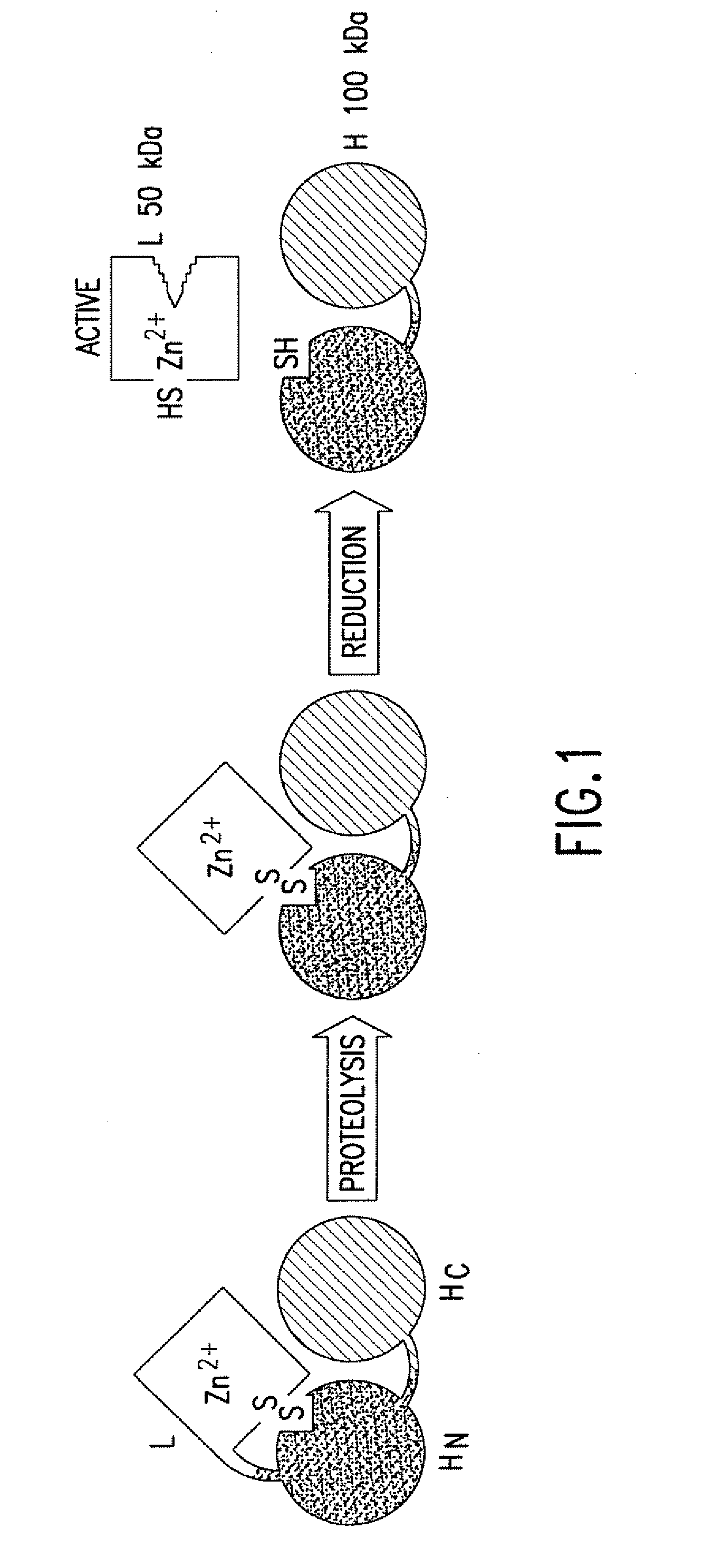
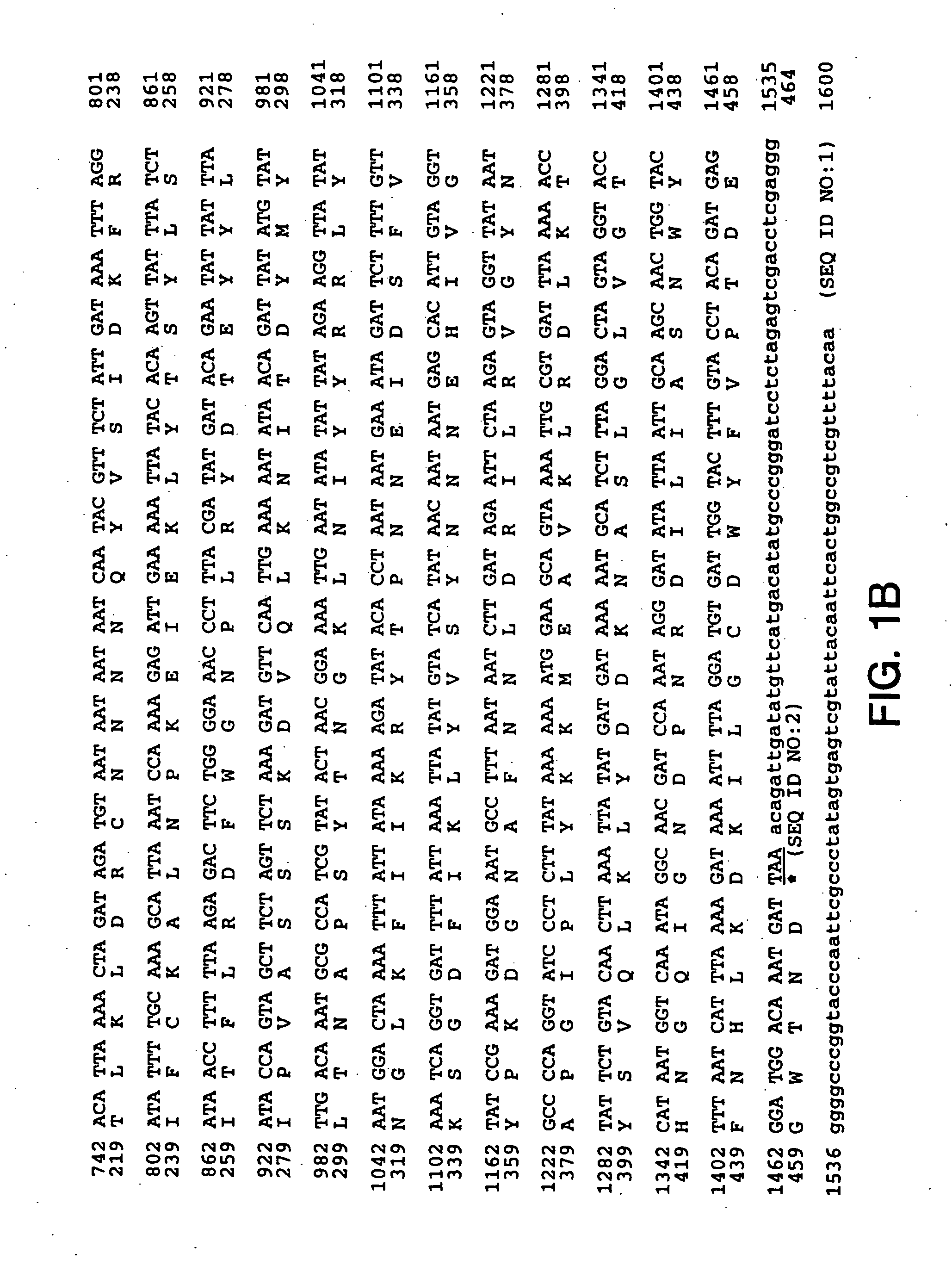
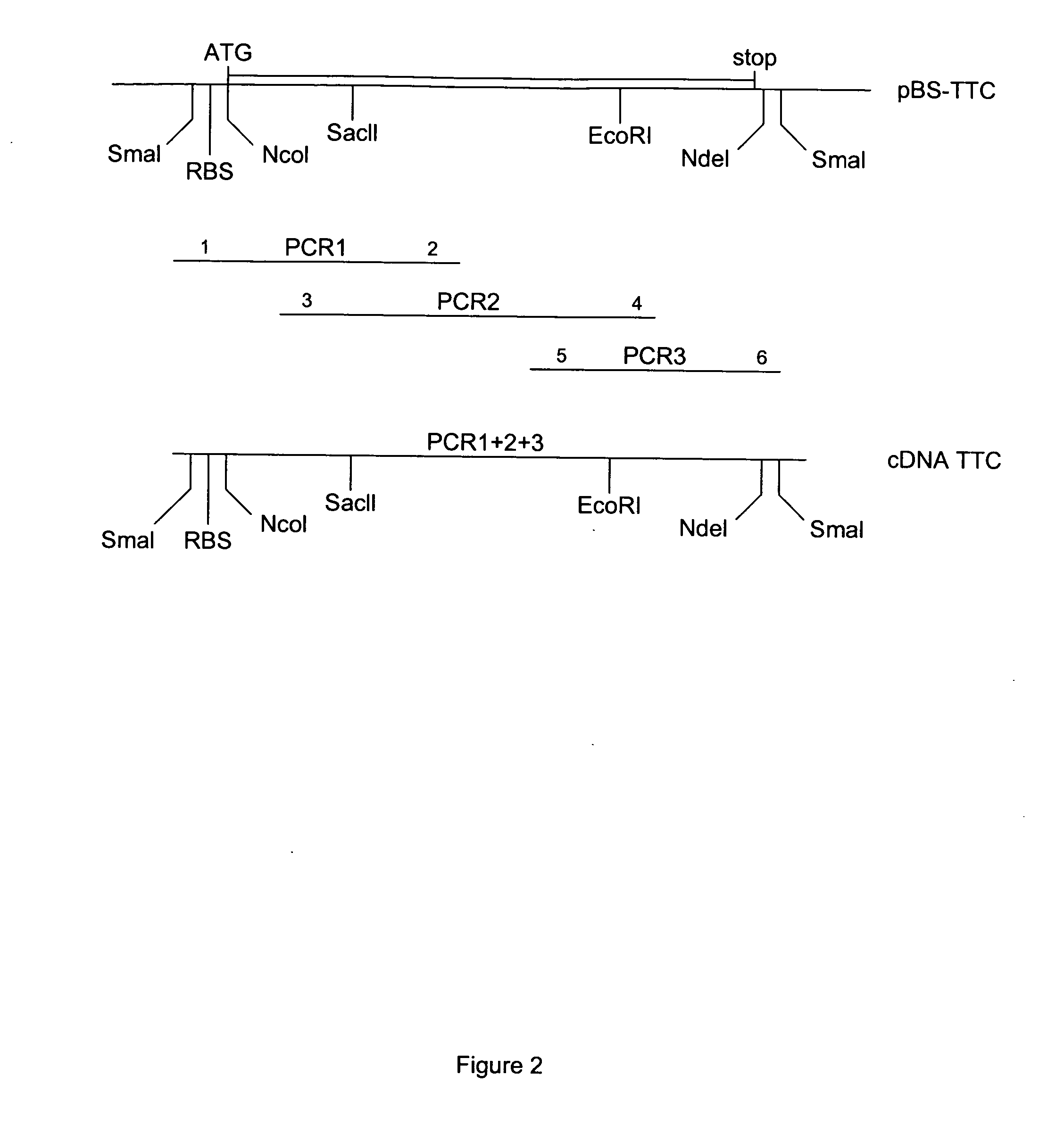
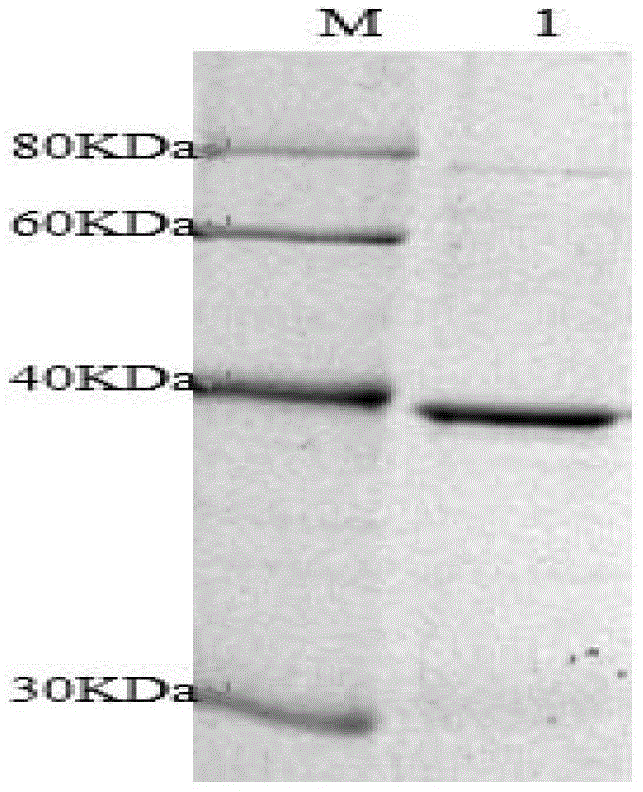
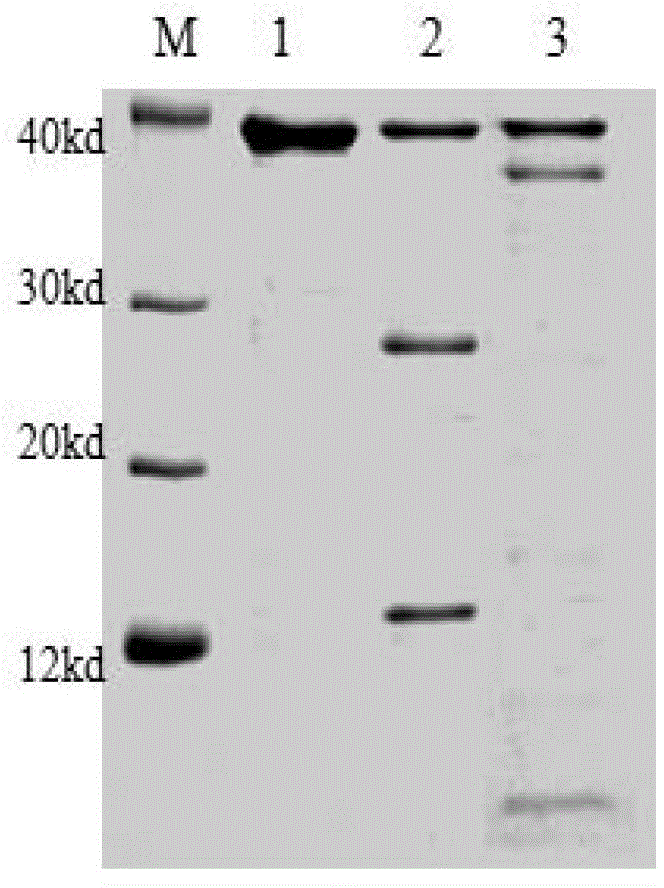
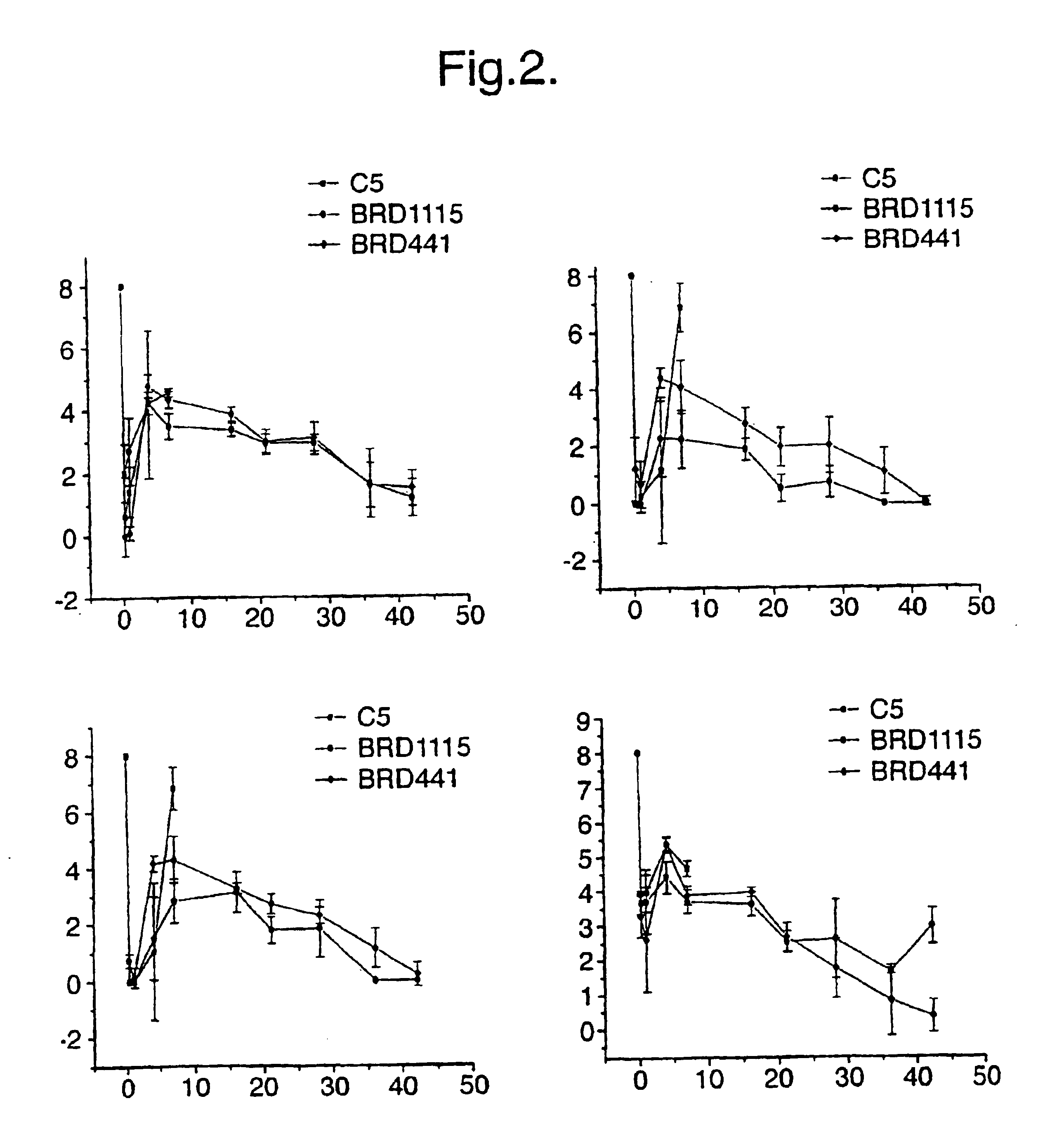
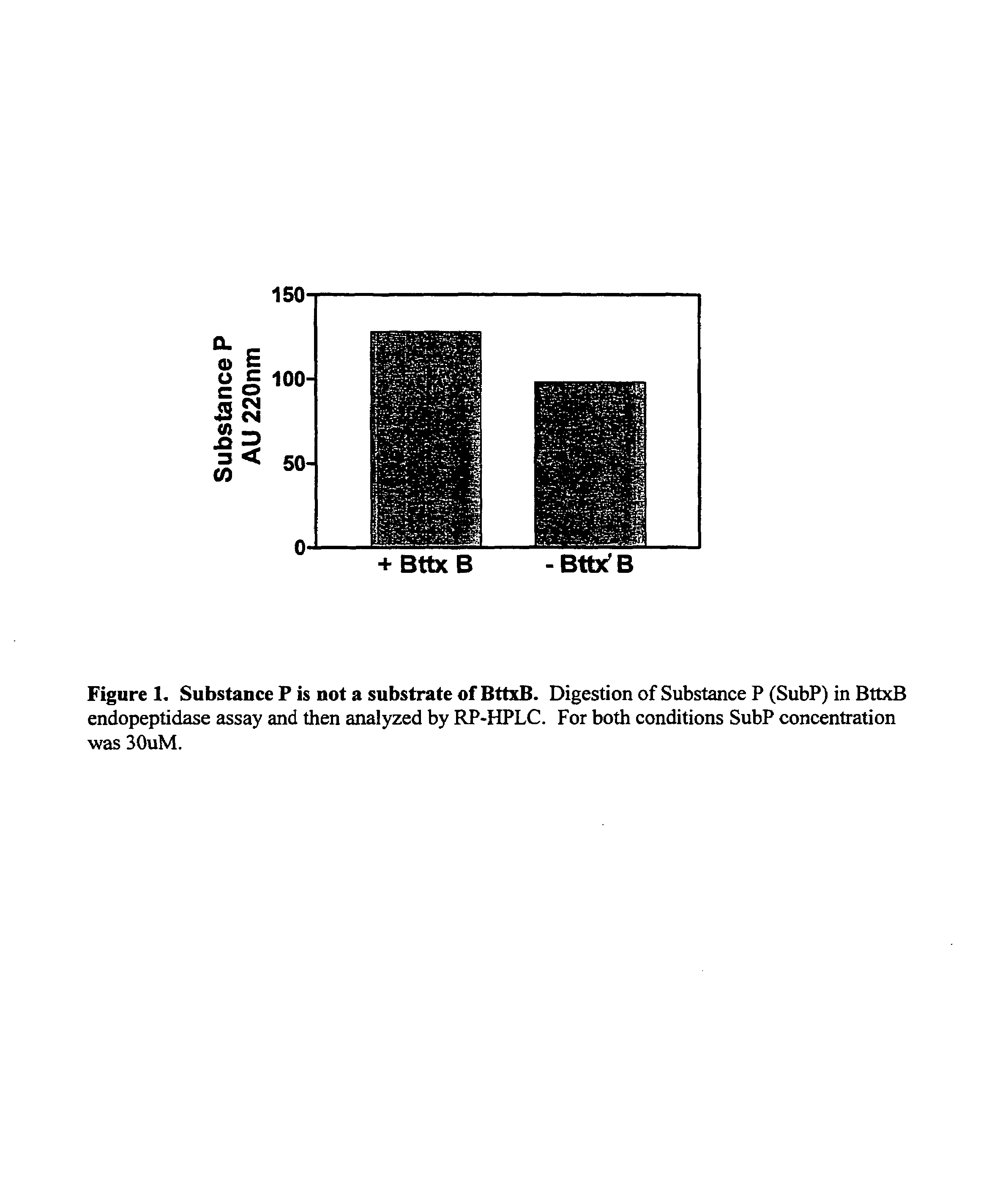
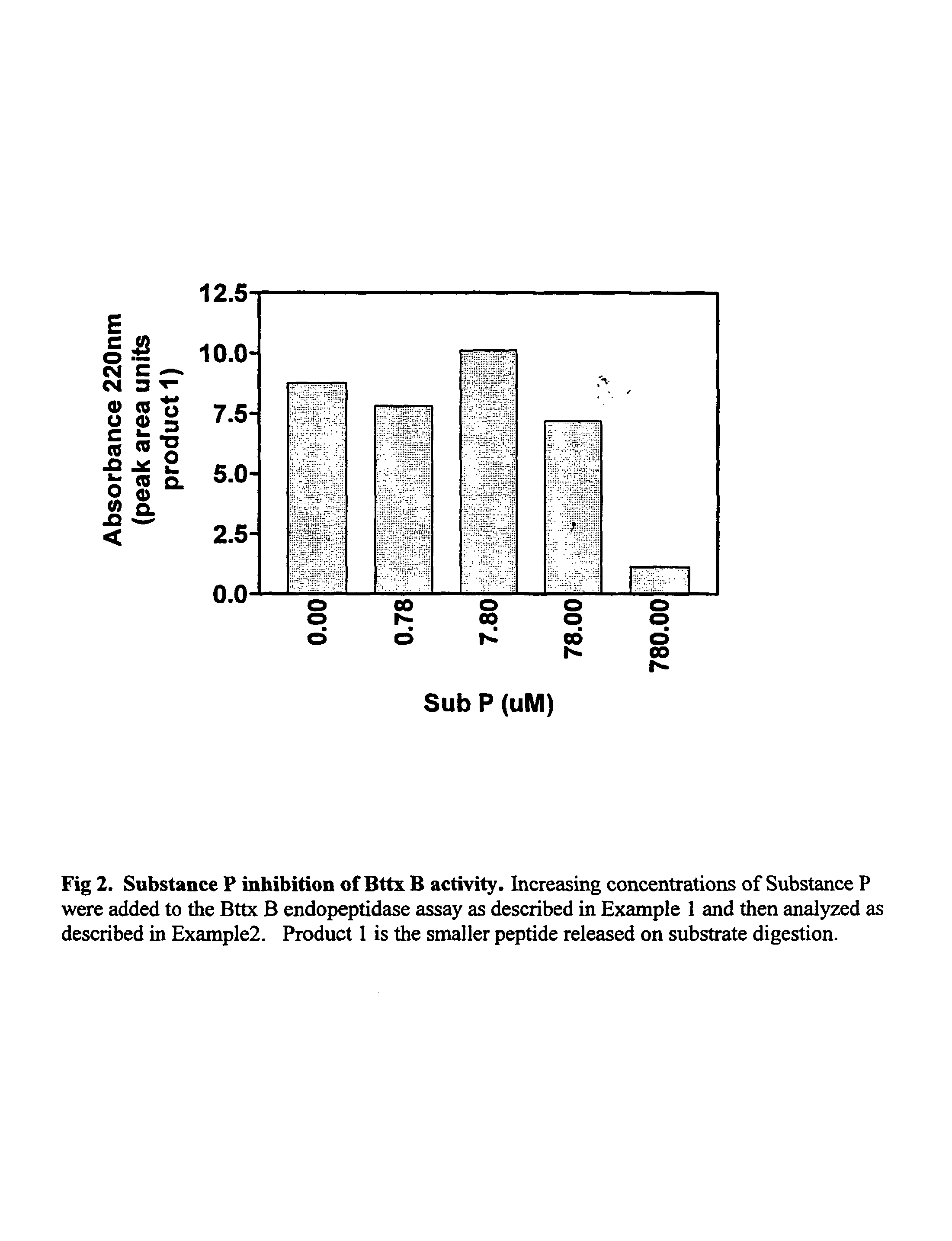
Patents
Literature
111 results about "Tetanus Toxins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
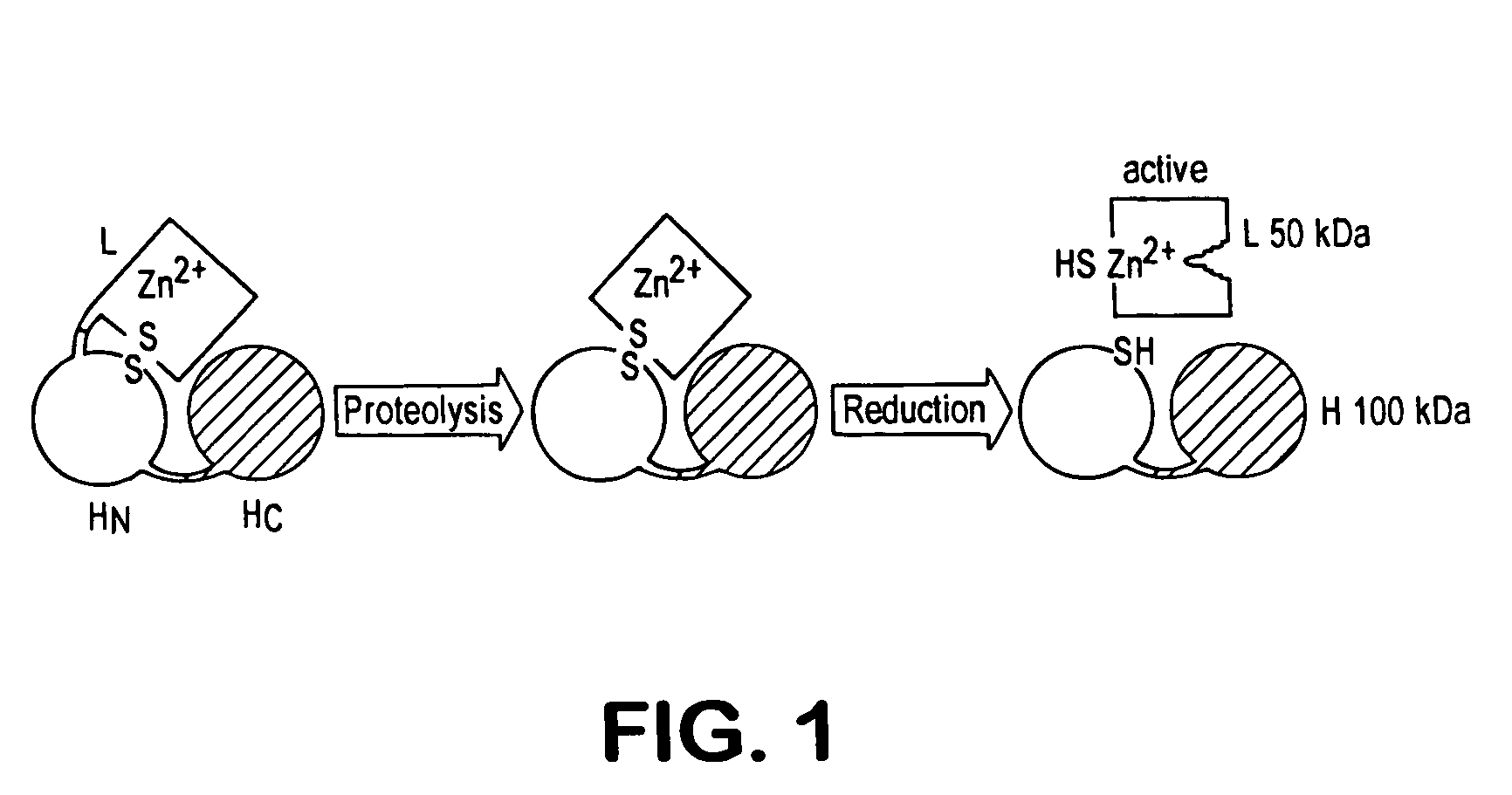
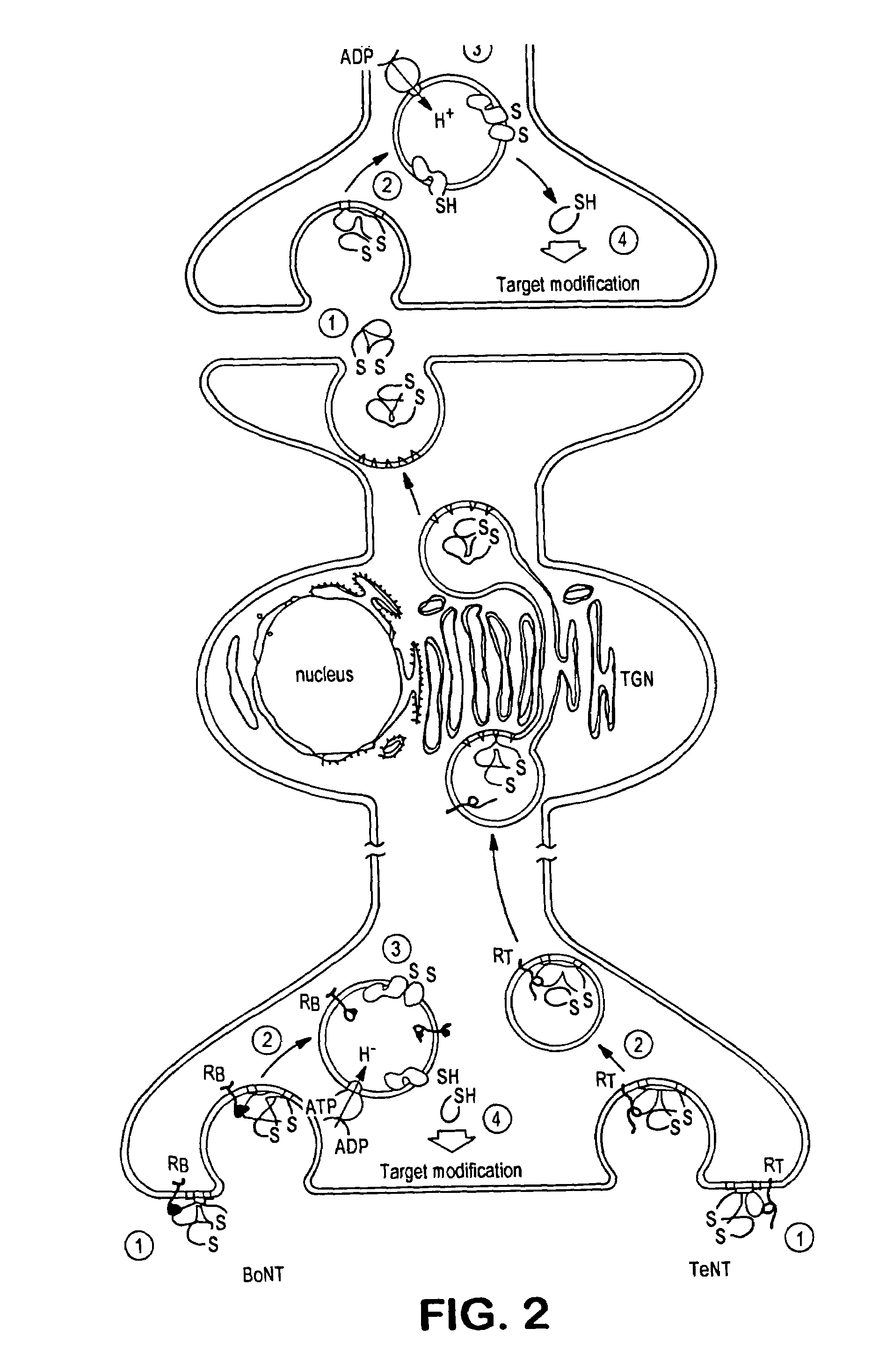
Tetanus toxin is an extremely potent neurotoxin produced by the vegetative cell of Clostridium tetani in anaerobic conditions, causing tetanus. It has no known function for clostridia in the soil environment where they are normally encountered. It is also called spasmogenic toxin, or TeNT.
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Methods for using tetanus toxin for beneficial purposes in animals (mammals)
Methods of using tetanus toxin to modulate or control neural functions or nonneural cellular activities at selected sites in animals, particularly in mammals, and more particularly in humans, are provided. Pharmaceutical formulations to modulate neural functions or non-neural cellular activities of an animal at selected sites in animals, particularly in mammals, and more particularly in humans are also provided. Uses of tetanus toxin in preparation of medicaments for methods of treating clinical disorders or symptoms of animals, particularly mammals and more particularly humans are also provided.
Owner:SANDERS
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Fret protease assays for botulinum serotype A/E toxins
InactiveUS7208285B2Used to determineDecreased acceptor fluorescence intensityBacteriaSugar derivativesFluorophoreReceptor for activated C kinase 1
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
High-efficiency 14-valent pneumococcal conjugate vaccine
InactiveCN101590224AEffective protectionAntibacterial agentsBacterial antigen ingredientsCoccidiaHaemophilus
The invention relates to a high-efficiency 14-valent pneumococcal conjugate vaccine, which is formed by coupling and combining capsular polysaccharide extracted from fourteen types of serotype pneumococcuses and carrier protein; the fourteen types of the serotype pneumococcuses are 1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F; and the carrier protein is diphtherin non-toxic mutated protein CRM197, tetanus toxin protein and influenza haemophilus protein D. Compared with the prior pneumococcal conjugate vaccine, the high-efficiency 14-valent pneumococcal conjugate vaccine has the advantages that: (1) the cross-protection rate of the pneumococcuses of fourteen serotypes (1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F) reaches more than 90 percent; and (2) the high-efficiency 14-valent pneumococcal conjugate vaccine can generate effective protection on senior citizens which are susceptible to pneumonia and is suitable for children under two years old.
Owner:广州精达医学科技有限公司
Toxin compounds with enhanced membrane translocation characteristics
InactiveUS20060024331A1Prevent steric hindrancePolypeptide with localisation/targeting motifBacterial antigen ingredientsProtein transduction domainMembrane translocation
The present invention relates to a compound comprising a toxin linked to a translocator. Non-limiting examples of toxins of the present invention are botulimum toxin, butyricum toxin, tetani toxins and the light chains thereof. In some embodiments, the translocator of the present invention comprises a protein transduction domain.
Owner:ALLERGAN INC
Methods for using tetanus toxin for benificial purposes in animals (mammals)
InactiveUS20040248188A1Strong specificityHigh activitySenses disorderNervous disorderMammalTreated animal
Methods of using tetanus toxin to modulate or control neural functions or normeural cellular activities at selected sites in animals, particularly in mammals, and more particularly in humans, are provided. Pharmaceutical formulations to modulate neural functions or non-neural cellular activities of an animal at selected sites in animals, particularly in mammals, and more particularly in humans are also provided. Uses of tetanus toxin in preparation of medicaments for methods of treating clinical disorders or symptoms of animals, particularly mammals and more particularly humans are also provided.
Owner:SANDERS
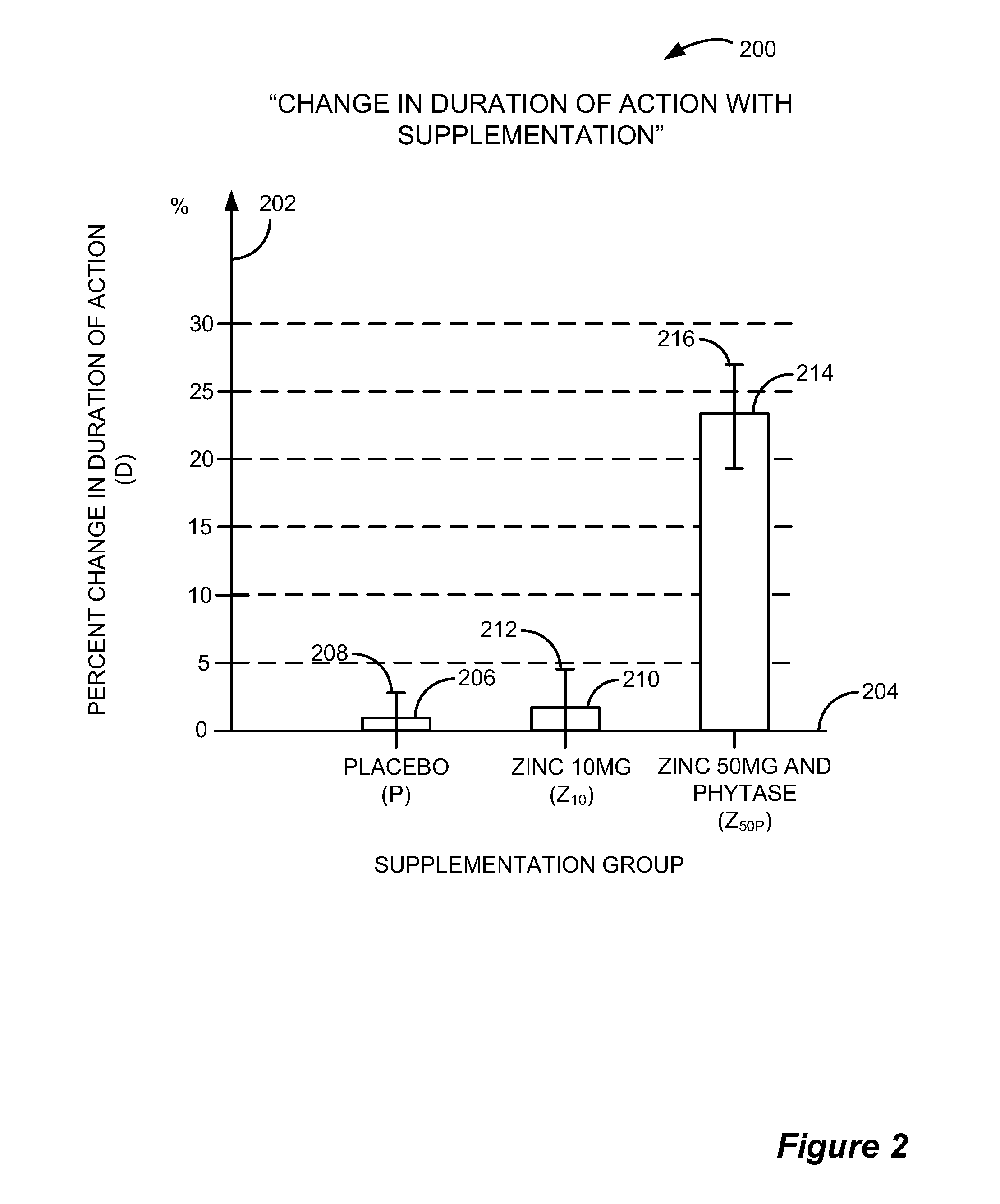
Compositions and Methods for Enhancing Metal Ion Dependent Drug Therapies
ActiveUS20100330163A1Safely and effectively rapidly increaseImproved therapeutic outcomeBiocideOrganic active ingredientsPhytaseMedicine
Methods and compositions are provided for increasing responsiveness to therapeutic metalloproteases including increasing and / or maximizing responsiveness and preventing botulinum and tetanus toxin resistance due to a functional deficiency of zinc. Also provided are methods for zinc replacement or supplement in lacking individuals comprising the administration of a zinc supplement for a loading period and / or administration of a phytase supplement together with the zinc supplement. Also provided are methods for standardization of botulinum toxin potency assays that provide for greater certainty and margins of safety in the use of products from different manufacturers.
Owner:CNSS IP HLDG
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS6822076B2Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsTetanusBasophilia
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-lIe-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Fully human monoclonal antibody against tetanus toxin and derivative thereof, and preparation method and application thereof
ActiveCN105153305AResist attackHigh school and tetanus toxin capacityAntibacterial agentsImmunoglobulins against bacteriaHeavy chainHalf-life
The invention provides a fully human monoclonal antibody against tetanus toxin. The amino acid sequences of a heavy-chain variable region and a light-chain variable region of the monoclonal antibody are SEQ ID No. 3 and SEQ ID No. 4, respectively; preferably, the amino acid sequences of a heavy chain and a light chain of the monoclonal antibody are SEQ ID No. 1 and SEQ ID No. 2, respectively. The invention further provides a preparation method and application of the fully human monoclonal antibody against tetanus toxin and a derivative thereof. The fully human monoclonal antibody against tetanus toxin provided by the invention can eliminate the biological risks of anaphylactic reaction and virus contamination, has a sufficiently long half life and high titer and in-vivo activity, can be applied to large-scale industrial production.
Owner:ANTAGEN BEIJING BIOTECH CO LTD
Methods for direct visualization of active synapses
InactiveUS7923015B2Easy to transportEasy to operateNervous disorderPeptide/protein ingredientsCell biologyTetanus Toxins
A method for visualizing an active synapse wherein said method comprises: (a) exposing cells forming the active synapse to a biomarker comprising at least fragment C of tetanus toxin and a reporter protein; and (b) visualizing the biomarker; wherein the accumulation of the biomarker into dendritic spines of the cells allows visualization of an active synapse. Also, a method for screening molecules capable of modulating synapse activity is provided. A kit useful for the early diagnosis of neurodegenerative disease comprises a biomarker comprising at least fragment C of tetanus toxin and a reporter protein.
Owner:INST PASTEUR +1
Human anti-tetanotoxin monoclonal antibody, method for preparation and application
InactiveCN1634991ADoes not induce allergic reactionsProtection attackAntibacterial agentsImmunoglobulins against animals/humansHigh titerTetanus Toxins
The invention discloses a human originated anti tetanus toxin monoclonal antibody, a preparation process and uses thereof. The human originated antibody comprising an antibody variable region with gene and protein sequences as the sequence 1 has the capability of neutralizing the tetanus toxin. It can not induce obvious anaphylactic response, and has a higher titer and long action.
Owner:龚小迪
Fret protease assays for clostridial toxins
InactiveUS20080032318A1Used to determineDecreased acceptor fluorescence intensityPeptide-nucleic acidsMaterial analysis by observing effect on chemical indicatorSerotypeFluorophore
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
Preparation and application of humanized anti-spasmotoxin monoclone antibody
InactiveCN101220096ADoes not induce allergic reactionsProtection attackImmunoglobulins against animals/humansAntibody ingredientsAnimal virusHypersensitive response
The invention provides a human McAb to tetanus toxin, which is a human antibody comprising a variable region of the antibody. The antibody consists of or not consists of a constant region of the antibody and has the ability of neutralizing the tetanus toxin, with the variable region gene and protein sequence indicated as the sequence table 1. The antibody is characterized in that the antibody can not induce obvious allergic reaction, has higher titer and longer effect without any animal virus pollution, and can be produced in an unlimited quantity. The antibody also provides the relating biological functions, the testing methods, the manufacturing method and the application.
Owner:龚小迪
Hybrid protein for inhibiting the degranulation of mastocytes and the use thereof
InactiveUS20030059912A1Avoid allergic reactionsAvoid symptomsHydrolasesPeptide/protein ingredientsADAMTS ProteinsPotassium
A hybrid protein contains a protein that binds to a receptor of mastocytes and basophils and is endocyted by them. The protein can be IgE; IgE fragment; IgE Fc fragment; antibody against IgE receptor of mastocytes and basophils; fragment of the antibody against the IgE receptor of mastocytes and basophils; antibody against mastocyte specific potassium channel; and mast cell degranulating peptide. The hybrid protein also contains a protease cleaving proteins of the secretion process of the mastocytes and basophils so as to inhibit the secretion process without killing the mastocytes and basophils. The protease can be light chain Clostridium botulinum toxin; proteolytically active fragment of the light chain of a Clostridium botulinum toxin containing an amino acid sequence His-Xaa-Xaa-Xaa-His-Xaa-Xaa-His wherein Xaa is an amino acid; light chain of the tetanus toxin; proteolytically active fragment of the light chain of the tetanus toxin containing His-Asp-Leu-Ile-His-Val-Leu-His; IgA protease of Neisseria gonorrhoeae; and proteolytic domain of the IgA protease of Neisseria gonorrhoeae.
Owner:MERZ PHARMA GMBH & CO KGAA
Human rotavirus Delta VP8* subunit recombinant protein and application thereof
ActiveCN103319604AImprove immune efficiencyFast titerBacteriaViral antigen ingredientsCross neutralizationRotavirus RNA
The invention relates to human rotavirus Delta VP8* subunit recombinant protein and application thereof. The human rotavirus Delta VP8* subunit recombinant protein comprises a T cell epitope P2 in tetanus toxin and a rotavirus Delta VP8* subunit. By the recombinant protein disclosed by the invention, the immune efficacy of a Delta VP8* subunit vaccine can be greatly improved; faster and stronger neutralization antibody titer can be induced; moreover, anti-p[4] genotype specific rotavirus cross neutralization antibody of high titer can be induced; simultaneously, the potential risk of inducing intussusception by taking attenuated rotavirus vaccine orally can be overcome; therefore, the recombinant protein is applicable to preparing a rotavirus vaccine.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Porcine rotavirus delta VP8* subunit recombinant protein and applications thereof
InactiveCN103304642AStrong immune responseFast titerViral antigen ingredientsVirus peptidesVp4 geneInclusion bodies
The invention relates to a porcine rotavirus delta VP8* subunit recombinant protein and an encoding gene of the protein. The invention further provides a recombinant protein formed after increasing tetanus toxin T cell epitope P2 into the recombinant protein, and an encoding gene. The delta VP8* protein is 64th-site to 223th-site amino acid in the VP8* and can effectively stimulate an organism to produce specific serum antibody, humoral immune response is good, the problem that the VP4 gene can not conduct prokaryotic expression due to overlarge fragment can be overcome, the protein can be expressed as a soluble protein in vitro, so that the problem that the VP8* is expressed as an inclusion body in vitro can also be overcome; the T cell epitope P2 (830th-site to 844th-site amino acid of TT) in the tetanus toxin is induced into the delta VP8* subunit recombinant protein, so that the immunity efficacy of the protein can be greatly improved, the faster and stronger neutralizing antibody titer can be induced, and high-titer rotavirus cross neutralizing antibody can also be induced.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Anti-tetanotoxin antibody, and preparation method and application thereof
ActiveCN102875674AEffective neutralizationLess side effectsAntibacterial agentsBacterial antigen ingredientsBiotechnologyHeavy chain
The invention discloses an anti-tetanotoxin antibody. In the heavy chain variable region, the amino acid sequence of CDR1 is disclosed as SEQ ID NO.5, the amino acid sequence of CDR2 is disclosed as SEQ ID NO.6, and the amino acid sequence of CDR3 is disclosed as SEQ ID NO.7; and in the light chain variable region, the amino acid sequence of CDR1 is disclosed as SEQ ID NO.8, the amino acid sequence of CDR2 is disclosed as SEQ ID NO.9, and the amino acid sequence of CDR3 is disclosed as SEQ ID NO.10. The invention also discloses a nucleotide sequence for coding the anti-tetanotoxin antibody, and a corresponding recombinant plasmid and recombinant expression vector. The invention also discloses a preparation method and application of the anti-tetanotoxin antibody. The anti-tetanotoxin antibody disclosed by the invention is a full human antibody, has the advantages of low side reaction, high affinity and simple preparation method, and has wide industrial application prospects.
Owner:CHENGDU RONGSHENG PHARMA
Fusion protein of tetanus toxin T cell expressing bit polypeptide and human bata lymph cell stimulating factor and preparing thereof
InactiveCN1793178AImprove protectionOvercome the limitations of self-toleranceHybrid peptidesVector-based foreign material introductionEscherichia coliDisease
The invention discloses tetanus toxin T cell epi position peptide and human B lymphocyte activate and gene interfusing albumen. It could activate CD4+T cell to take cell action, promote body liquid immunity level. It adopts step clone to construct TT and BLyS interfusing gene and gain high efficiency expression in coliform bacteria. It conquers the immunity tolerate of the body.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
High-level expression of tetanus toxin receptor binding domain Hc in Escherichia coli and application
The invention relates to a method for a tetanus toxin receptor binding domain Hc to be subjected to high-level soluble expression in Escherichia coli through nucleotide sequence optimization. According to the sequencing result of a domestic C.Tetani virulent strain CMCC64008, the tetanus toxin receptor binding domain Hc sequence is analyzed and optimized, the optimized sequence is SEQ ID No.1 and the coded protein sequence is SEQ ID No.2. The synthesized Hc gene is linked into an expression vector pET32a(+) after undergoing double enzyme digestion, the recombinant Hc is subjected to high soluble expression in Escherichia coli and the target protein accounts for about 46% of the total protein in the supernatant undergoing bacteriociasis. After QFF column purification, phenyl hydrophobic column purification and SP column purification, the purity of the target protein can be more than 95% and the yield thereof is more than 300mg / L. The recombinant protein prepared by the method of the invention has good immunogenicity, can induce the mice to produce high-titre protective antibodies and can resist attack of high-dose lethal toxins. The method has extensive application prospect in large-scale high-level preparation of the tetanus toxin recombinant subunit vaccine Hc.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Human vaccine for preventing hydrophobia and tetanus
ActiveCN102671194AIncreased potencyImprove immunityAntibacterial agentsBacterial antigen ingredientsRabiesTetanus toxoids
The invention discloses a human vaccine for preventing hydrophobia and tetanus, which belongs to the field of vaccines. The vaccine consists of a hydrophobia vaccine and a tetanus toxin, wherein the hydrophobia vaccine is obtained by inoculating a CDKHBP-1 strain onto a human diploid cell WI-38 and purifying. By using the hydrophobia vaccine and the tetanus toxin prepared with the preparation method disclosed by the invention together, immunity to hydrophobia and tetanus can be realized; and moreover, the tetanus toxin can be used for remarkably enhancing valence effect of the hydrophobia vaccine, plays a role in enhancing immunity, and is convenient for administration.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Fret protease assays for botulinum serotype a/e toxins
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
Fret protease assays for clostridial toxins
InactiveUS20080038756A1Peptide-nucleic acidsMaterial analysis by observing effect on chemical indicatorSerotypeFluorophore
The present invention provides clostridial toxin substrates useful in assaying for the protease activity of any clostridial toxin, including botulinum toxins of all serotypes as well as tetanus toxins. A clostridial toxin substrate of the invention contains a donor fluorophore; an acceptor having an absorbance spectrum overlapping the emission spectrum of the donor fluorophore; and a clostridial toxin recognition sequence that includes a cleavage site, where the cleavage site intervenes between the donor fluorophore and the acceptor and where, under the appropriate conditions, resonance energy transfer is exhibited between the donor fluorophore and the acceptor.
Owner:ALLERGAN INC
Methods for direct visualization of active synapses
InactiveUS20050060761A1Easy to transportEasy to operateNervous disorderPeptide/protein ingredientsBiologic markerFragment C tetanus toxin
A method for visualizing an active synapse wherein said method comprises: (a) exposing cells forming the active synapse to a biomarker comprising at least fragment C of tetanus toxin and a reporter protein; and (b) visualizing the biomarker; wherein the accumulation of the biomarker into dendritic spines of the cells allows visualization of an active synapse. Also, a method for screening molecules capable of modulating synapse activity is provided. A kit useful for the early diagnosis of neurodegenerative disease comprises a biomarker comprising at least fragment C of tetanus toxin and a reporter protein.
Owner:INST PASTEUR +1
Immune responses to fusion proteins
InactiveUS6936464B1Improve aspectEnhance vaccine efficacyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsFusion Protein ExpressionIn vivo
The invention relates to a nucleic acid construct for delivery into living cells in vivo for inducing an immune response in a patient to an idiotypic determinant present on a malignant B cell in the patient; the construct directing the expression of a fusion protein, said fusion protein comprising the idiotypic determinant and at least one T helper cell epitope from tetanus toxin. The invention further relates to a method of making the nucleic acid construct, a method of treating a patient, and to a composition comprising the nucleic acid construct.
Owner:CANCER RES TECH LTD
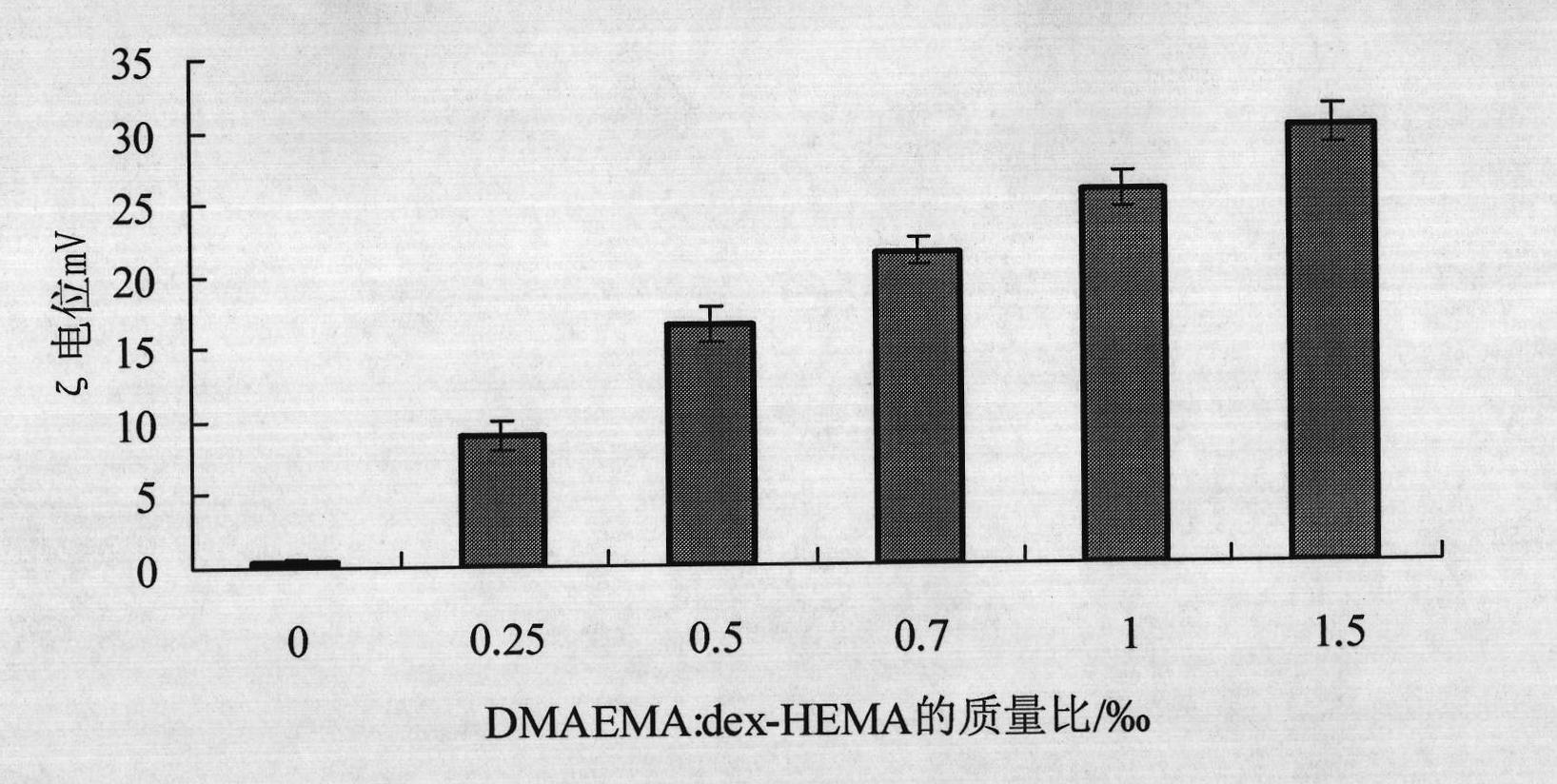
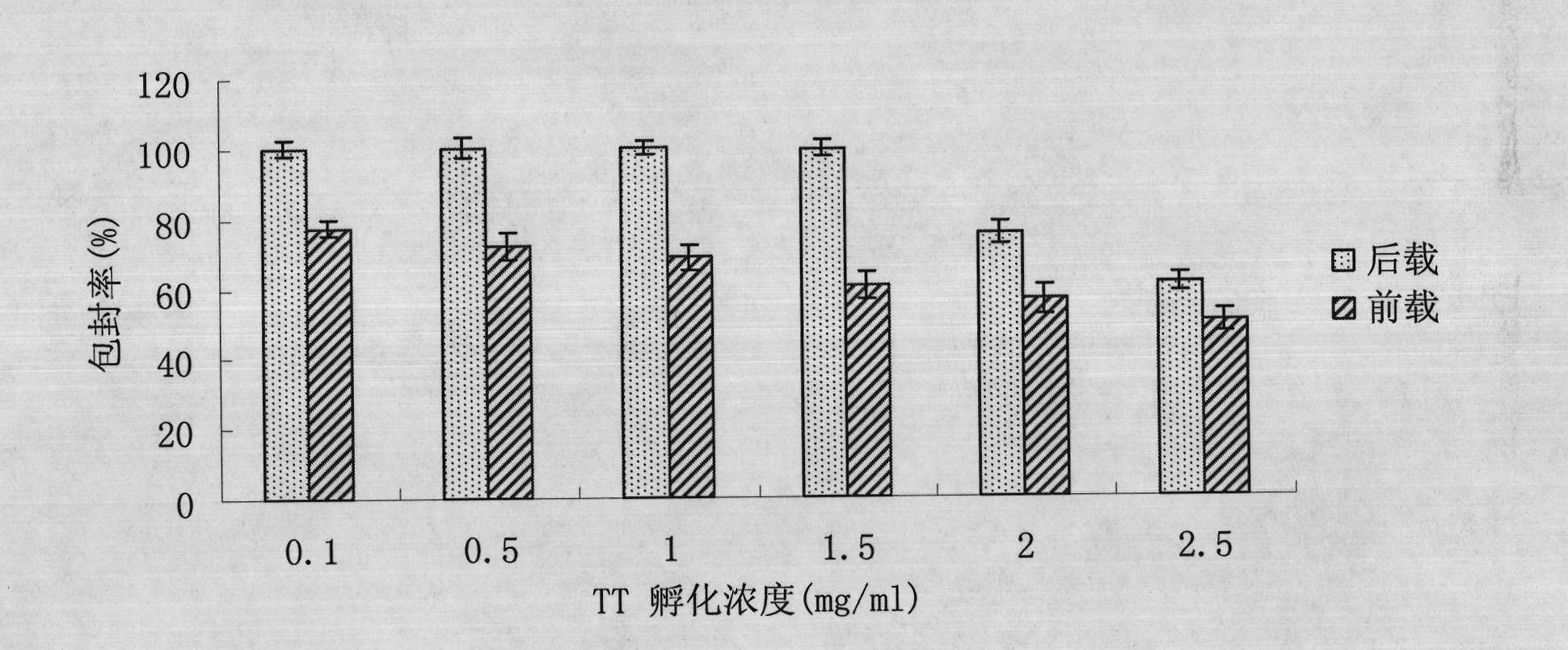
Single dose immunization against tetanus toxin cation dextran microspheres and preparation method thereof
InactiveCN101869704AReduce the number of injectionsImprove vaccination coverageAntibacterial agentsBacterial antigen ingredientsControlled releaseTetanus
The invention relates to the filed of pharmaceutical preparation, in particular to single dose immunization against tetanus toxin cation dextran microspheres and a preparation method thereof. The single dose immunization against tetanus toxin cation dextran microspheres are prepared by carrying tetanus toxin after electrostatic interaction on cation hydroxyethyl acrylate dextran microspheres. The tetanus toxin controlled release microspheres can reduce the injection frequency of tetanus vaccine, improve the vaccination coverage and reduce the drop-out rate, thereby effectively preventing tetanus and providing a single dose tetanus toxin controlled release vaccine preparation with long-term effect and realizing the whole course immunity by one injection.
Owner:CHINA PHARM UNIV
Substrate protein SNVP, and coding gene and application thereof
The invention discloses a clostridial neurotoxin substrate protein, and a coding gene and an application thereof. The clostridial neurotoxin substrate protein is a protein (a) composed of an amino acid sequence represented by sequence 2 in a sequence table, or is a protein (b) obtained through substituting and / or deleting and / or adding one or more amino acid residues to the amino acid sequence represented by the sequence 2, related to the clostridial neurotoxin detection and derived from the sequence 2. The substrate protein SNVP provided by the invention has seven toxin serotype botulinum and tetanus toxin enzyme hydrolysis sites, can specifically identify A, B, E, C, D, F and G type botulinum toxins and tetanus toxins, and can be used for the detection and the parting discrimination of the botulinum toxins and tetanus toxins as a core detection reagent.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Vaccines containing attenuated bacteria
InactiveUS6905691B1Easy to foldIncidenceBacterial antigen ingredientsBacteriaMucosal Immune ResponsesBacteroides
The invention relates to a vaccine comprising a bacterium attenuated by a non-reverting mutation in a gene encoding a protein which promotes folding of extracytoplasmic proteins. Such mutations were initially identified as being useful in vaccines from a bank of randomly inserted, transposon mutants in which attenuation was determined as a reduction in virulence of the organism in the mouse model of infection. Site directed mutation of the gene results in a strain which shows at least 4 logs of attenuation when delivered both orally and intravenously. Animals vaccinated with such a strain are protected against subsequent challenge with the parent wild type strain. Finally, heterologous antigens such as the non-toxic and protective, binding domain from tetanus toxin, fragment C, can be delivered via the mucosal immune system using such strains of bacteria. This results in the induction of a fully protective immune response to subsequent challenge with native tetanus toxin.
Owner:CELLTECH PHARMA EURO
Neutralizing monoclonal antibody resisting to tetanus toxin and application thereof
ActiveCN105542004AStrong specificityProlonged deathAntibacterial agentsImmunoglobulins against bacteriaLethal doseComplete antibody
The invention discloses a neutralizing monoclonal antibody resisting to tetanus toxin. Hybridoma cells are obtained from tetanus toxin heavy chain C fragment immune mice, light chain and heavy chain variable region genes of the antibody are taken, human-mouse chimeric complete antibody expression vectors are built, CHO cell transfection is carried out, purification is carried out, and then the antibody is obtained. The antibody has the activity of specific binding to tetanus toxin, the monoclonal antibody can partially protect mice against attack of tetanus toxin, and four antibodies can completely protect mice against a twofold lethal dose of tetanus toxin in combination.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Previns as specific inhibitors and therapeutic agents for botulinum toxin B and tetanus neurotoxins
InactiveUS7235521B1Suppression problemCompound screeningApoptosis detectionCrystallographyTetanus neurotoxin
The compounds of the invention are generally described by the formula (1): B1Z*2B3Z*4X*5Q6F7X8X9X10X11, (2): B1X2X3X4X5Q6F7X8X9X10X11, or (3): B1X2B3X4Z5Q6F7Z8X9X10X11 and the salts, esters, amides, and acyl forms thereof. Each position represented by a letter indicates a single amino acid residue: B is a basic or polar / large amino acid or a modified form thereof; X is a small or hydrophobic amino acid or a modified form thereof; X* is a small or polar / large amino acid or a modified form thereof; Z is a polar / large or hydrophobic amino acid or a modified form thereof; Z* is Proline or a polar / large or hydrophobic amino acid or a modified form thereof. As described below, one or more of the peptide linkages between the amino acid residues may be replaced by a peptide linkage mimic. These compounds may be used as molecular building blocks to create compounds that are optimized for inhibiting the protease activity of Botulinum B and tetanus toxins.
Owner:ARMY GOVERNMENT OF THE UNITED STATES DEPT OF THE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com