Fully human monoclonal antibody against tetanus toxin and derivative thereof, and preparation method and application thereof
A monoclonal antibody, anti-tetanus technology, applied in biochemical equipment and methods, botanical equipment and methods, microorganism-based methods, etc., can solve problems that are limited to the laboratory stage and have not yet seen clinical applications, etc. , to achieve the effect of eliminating allergic reactions, prolonging the half-life in the body, and eliminating virus pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1 Preparation of anti-tetanus toxin monoclonal antibody hybridoma cell line
[0088] 5 x 10 cells from healthy blood donors immunized with tetanus toxoid vaccine were purchased from a commercial company (www.AllCells.com). 8 Peripheral blood mononuclear cells (trade name LeukoPak). Then on ice, the cells were incubated with anti-CD19-FITC and anti-CD27-PE antibodies (purchased from eBioscience) for 20 minutes, and after the cells were washed twice with PBS containing 1% FBS, the AriaII (BD Biosciences) flow cytometer Screen out CD19+CD27+ memory B cells. At the same time, Sp2 / 0-Agl4 mouse myeloma cells (purchased from ATCC, USA) in logarithmic growth phase in RPM1640+10% FBS culture medium were taken, washed three times with serum-free RPM1640 culture medium, and counted for later use. The memory B cells sorted above were mixed with Sp2 / 0 cells at a ratio of 10:1, and centrifuged at 1500 rpm for 7 minutes. Wash away the supernatant and prepare for fusion...
Embodiment 2
[0093] Example 2 Cloning of anti-tetanus toxin monoclonal antibody full-length heavy chain, light chain gene and variable region gene thereof
[0094] First, the total RNA of the tetanus toxin-resistant hybridoma (3B10) obtained in Example 1 was extracted using Qiagen's RNeasyPlusMini kit. then use IIFirstStrandcDNASynthesis Kit (NewEnglandBiolabs) used Oligo(dT)18 as a primer to reverse transcribe mRNA into cDNA. Using this cDNA as a template, design and synthesize degenerate primers (where W=A / T, K=G / T, R=A / G, Y=C / T, M =A / C, S=C / G, N=C / G / T, V=A / C / G).
[0095] The following are the upstream and downstream primers used to clone the antibody heavy chain and light chain genes respectively in Example 2:
[0096] Heavy chain upstream primer SEQ ID NO: 11
[0097] 5'-TGATCAGSACTGMACACAGAGRACTCACCATG-3'
[0098] Heavy chain downstream primer SEQ ID NO: 12
[0099] 5'-CTGACTCGAGTCATTTACCCGGAGACAGGGAGAGG-3'
[0100] Light chain upstream primer SEQ ID NO: 13
[0101] 5'-ATGGA...
Embodiment 3
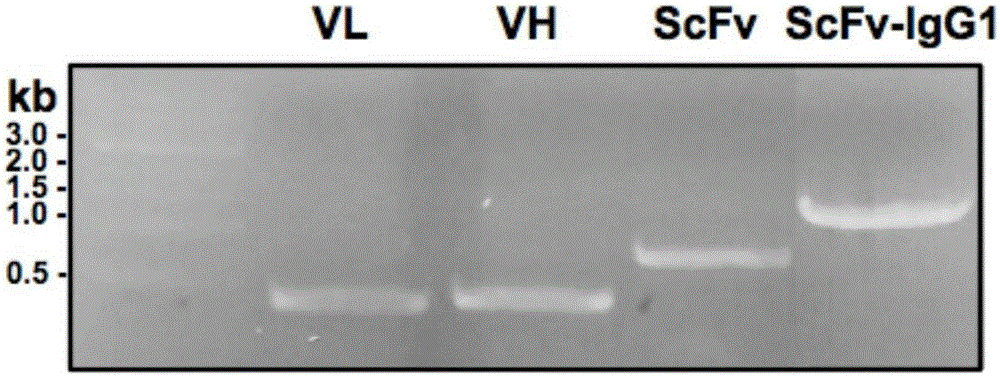
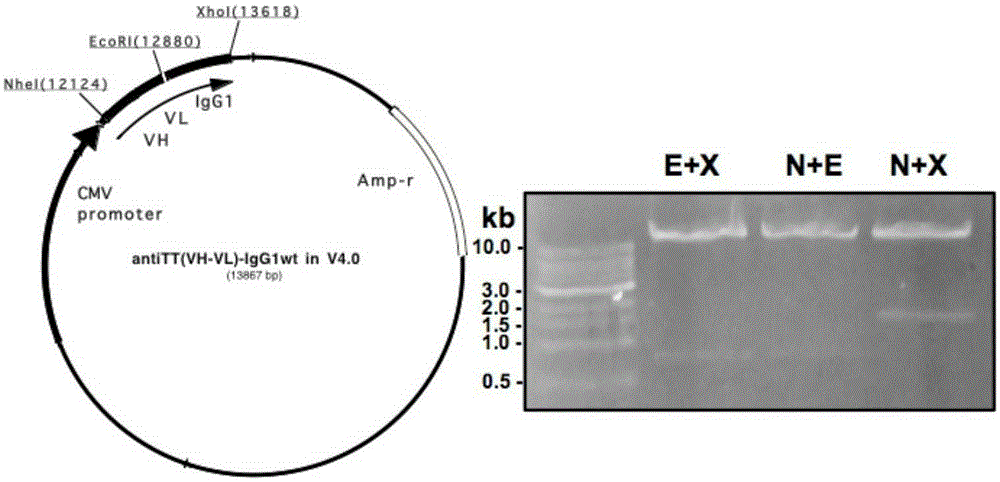
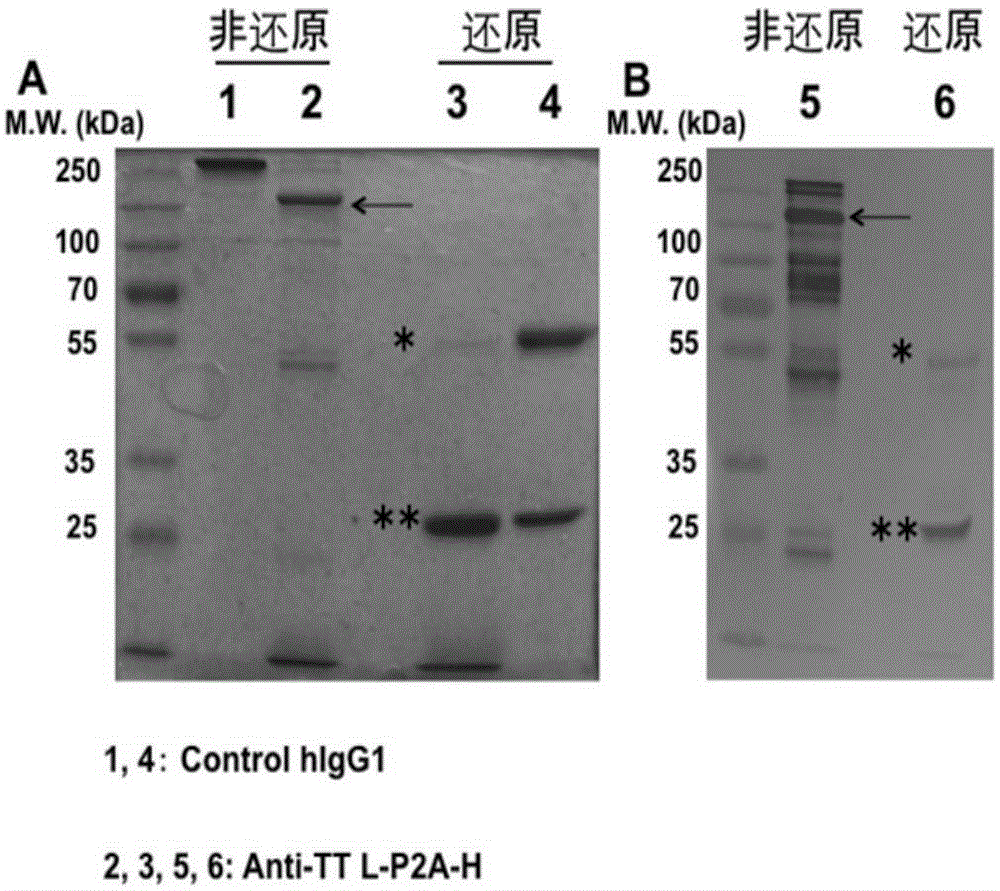
[0128] Example 3 Construction of Anti-Tetanus Toxin Monoclonal Antibody L-P2A-H Form and ScFv-IgG1 Form Expression Vector
[0129] The L-P2A-H format is an antibody light chain (L) and heavy chain (H) linked by a self-cleaving P2A peptide. Its construction adopts the method of overlappingPCR. First, in the same PCR reaction system as above, the light chain portion was amplified with the following primers:
[0130] Anti-TTlightUPNheI.primer:
[0131] SEQ ID NO: 195'-GTGGGCTAGCGACTACCAGATGACCCAGTCTCC-3'
[0132] P2A-TTlightantisense.primer:
[0133] SEQ ID NO: 205'-CCGGCCTGCTTCAGCAGGCTGAAGTTGGTGGCTCCGCTGCCACACTCTCCCCTGTTGAAG-3'
[0134] The heavy chain portion was amplified with the following primers:
[0135] P2A-TT Theavysense.primer:
[0136] SEQ ID NO: 215'-CTGCTGAAGCAGGCCGGCGATGTGGAGGAGAATCCTGGCCCCATGGAGTTTGGGCTGAGC-3'
[0137] Anti-TTheavyASXhoI.primer:
[0138] SEQ ID NO: 225'-CTGACTCGAGTCATTTACCCGGAGACAGGGAGAGG-3'
[0139] The 750bp light chain and the 1.5kb h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com