Neutralizing monoclonal antibody resisting to tetanus toxin and application thereof
A monoclonal antibody and anti-tetanus technology, which is applied in the field of genetic engineering and immunoglobulin, can solve problems such as monoclonal antibodies that have not yet appeared, and achieve the effect of prolonging the death time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of anti-tetanus neutralizing monoclonal antibody
[0050] 1. Cloning of mouse monoclonal antibody gene
[0051] 1.1 Preparation of mouse monoclonal antibody
[0052] Using recombinant tetanus TeNT-Hc protein (CN200910135972.0) as immunogen, four strains of high-affinity and high-specificity mouse-derived anti-tetanus monoclonal antibodies TY1, TY5, TY8 and TY11 were obtained by conventional monoclonal antibody preparation methods Hybridoma cell line, the subtype of antibody molecule secreted is IgG1, and the light chain is Kappa subtype.
[0053] 1.2 Cloning of mAb variable region gene
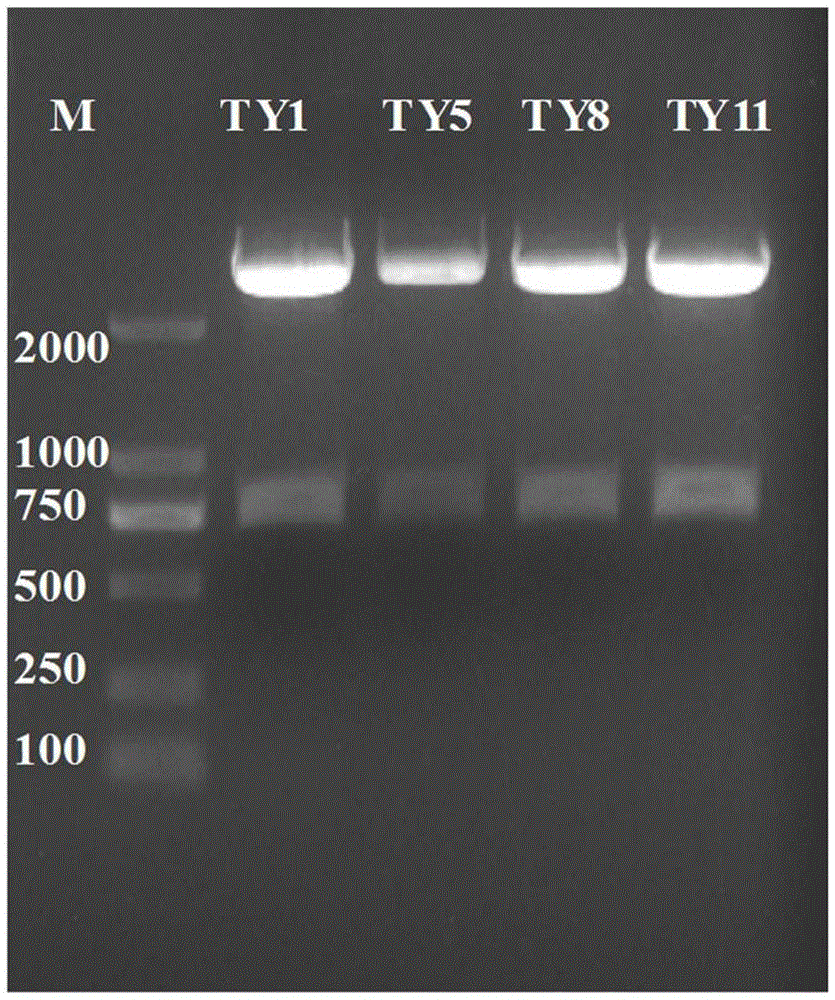
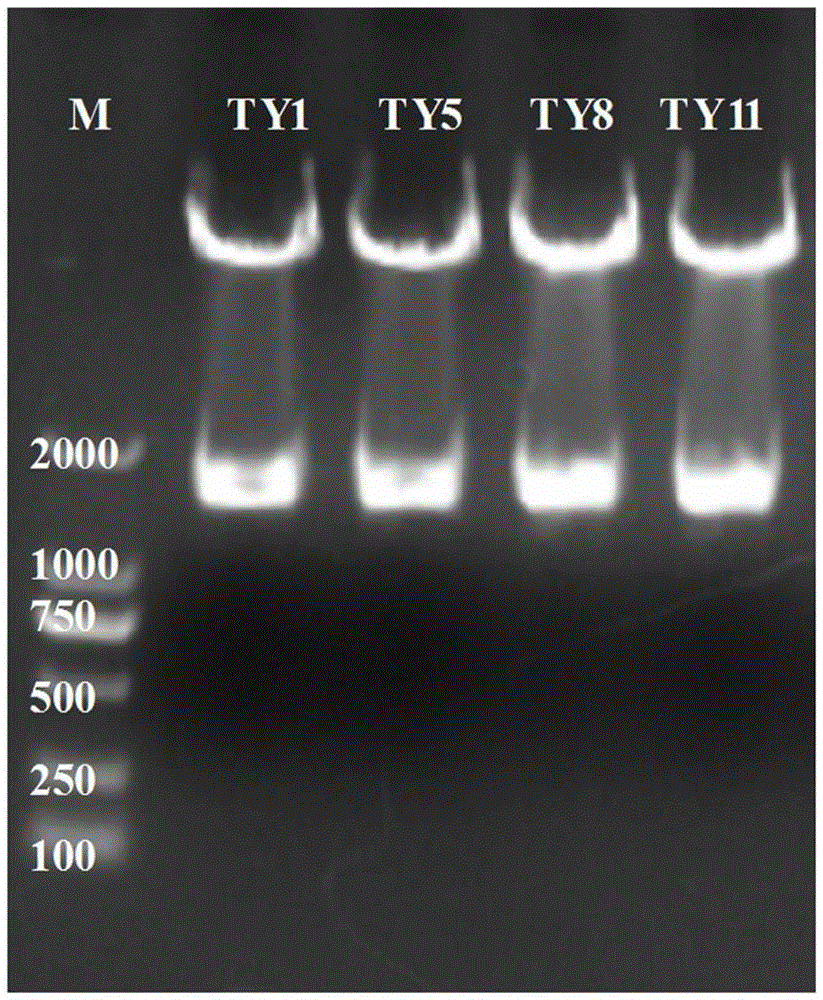
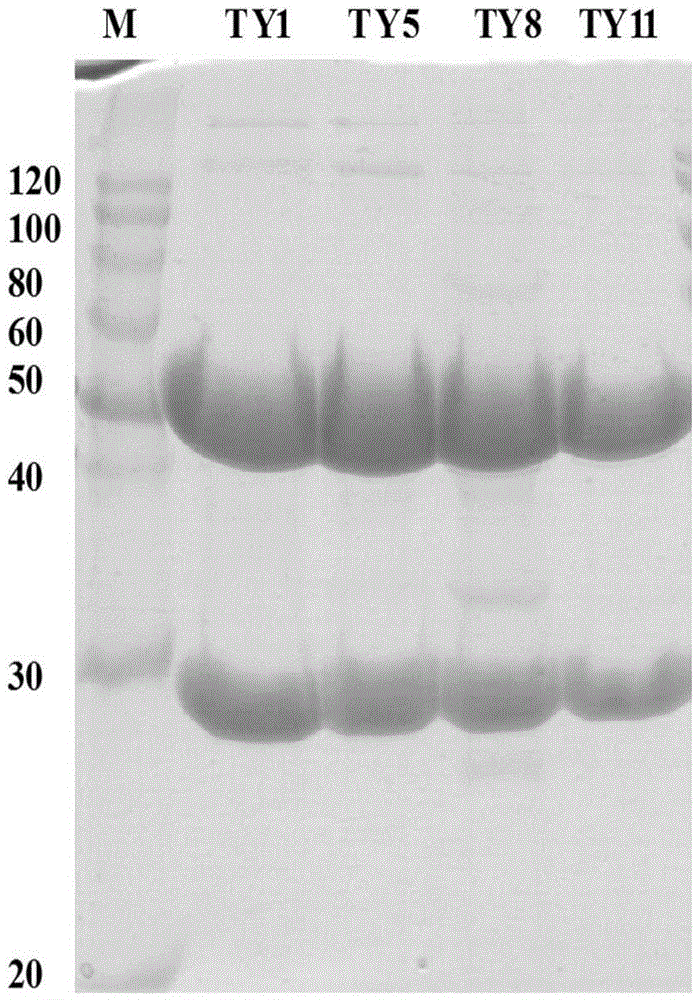
[0054] The monoclonal antibody is composed of a variable region and a constant region. Because the gene variation of the variable region of the mouse monoclonal antibody is large, and the gene sequence of the constant region is very conservative, the 5'-RACE method is used for cloning. A primer sequence was added to the 5' end, and PCR amplification was perform...
Embodiment 2
[0133] Embodiment 2: ELISA method detects the binding activity of monoclonal antibody and TeNT-Hc, TT protein
[0134] Recombinant TeNT-Hc protein or tetanus toxoid TT diluted to 2 μg / ml, 100ul / well coated with enzyme-linked plate, overnight at 4°C, blocked with blocking solution (20mMPB, 0.15M NaCl, 5% milk powder) at 37°C for 1h the next day After washing the plate, add the sample and incubate at 37°C for 1h to wash the plate, add 1:1000 diluted HRP-labeled goat anti-human Fc (Sigma, A0170), wash the plate after incubating at 37°C for 1h, develop color with TMB, and measure OD with a microplate reader 450 . The experimental results show that the four monoclonal antibodies all have good specificity and can specifically bind to TeNT-Hc and TT proteins, but have no binding activity to other proteins, see Figure 4 .
Embodiment 3
[0135] Example 3: Protective activity of monoclonal antibody in mice
[0136] Using BALB / c mice, female, 18-20g / mouse, 10 / group, mix different concentrations of TY1, TY5, TY8 and TY11 monoclonal antibodies and mixtures with 2 times the lethal dose of tetanus toxin, 37°C After incubation for 1 hour, it was injected into the abdominal cavity of mice, and the incidence and survival of mice were observed for 10 consecutive days. from Figure 5 It can be seen from the experimental results that monoclonal antibodies can significantly prolong the death time of mice and have partial protective activity. The toxin protection rates of TY1, TY5, TY8, and TY11 monoclonal antibodies to mice with double lethal doses are 50%, 30%, respectively. %, 40%, and 40%. from Image 6 It can be seen from the experimental results that when the four antibodies are used in combination with a dose of 50 μg or more, they can completely protect the mice from the toxin challenge of less than twice the let...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com